Abstract
Background
Breast cancer is the most common malignancy in women and the underlying mechanism of breast cancer cell metastasis is still far from uncover. Integrin subunit alpha 7 (ITGA7) is a functioning protein. It has been detected in many malignancies. But the function of ITGA7 in breast cancer is not clear. Our aim is to explore ITGA7 expression and its role in breast cancer.
Methods
Real-time PCR was performed to determine ITGA7 expression in BC tissues and normal adjacent tissues. The specific functions of ITGA7 in breast cancer cell lines (MDA-MB-231 and BT-549) transfected with small interfering RNA were determined through migration, invasion assays. Western blot assays were performed to determine the expression of c-met and vimentin.
Results
ITGA7 was down-regulated in breast cancer tissues compared to the adjacent normal tissues (T:N =7.68±27.38: 41.01± 31.47, P<0.001) and this observation was consistent with the TCGA cohort (T:N =4.51±0.45:5.40±0.61, P<0.0001). In vitro experiments showed that knocking down ITGA7 significantly inhibited the migration and invasion of the breast cancer cell lines (MDA-MB-231 and BT-549). Meanwhile, knockdown of ITGA7 promoted c-met and vimentin expression, which may induce invasion and migration.
Conclusion
ITGA7 plays an important tumorigenic function and acts as a suppress gene in breast cancer. Our findings indicate that ITGA7 was the gene associated with breast cancer.
Keywords:
Introduction
Breast cancer is the most common malignancy in women. As the reason for cancer-related death, breast cancer ranks second after lung cancer.Citation1,Citation2 Many studies have proven that invasion of cancer cells was the major reason for cancer-related death.Citation3,Citation4 With acknowledging development of medical technology, doctors can treat breast cancer patients by surgery, chemotherapy, endocrine therapy, or targeted therapies.Citation5 But the treatment is not always satisfactory. So, it is still important to explore the mechanism of breast cancer and find new biomarkers at an early stage.
C-met is a key regulator of cancer progression, and it has been reported that upregulated c-met is related with poor survival rates and malignant activities of breast cancer.Citation6,Citation7 During cancer progression, cancer cells can experience a feature change from an epithelial to a mesenchymal phenotype, which is called epithelial–mesenchymal transition (EMT).Citation8,Citation9 Hung et alCitation10 found that osthole suppresses HGF-induced EMT via repression of the c-Met/Akt/mTOR pathway in human breast cancer cells.
Integrin subunit alpha 7 (ITGA7) belongs to the integrin alpha chain family, and the coding gene is located on chromosome 12q13.2.Citation11 It has been found that ITGA7 is expressed in many cancers including malignant melanoma, prostate and liver carcinomas, and glioblastoma.Citation12,Citation13 Low levels of ITGA7 mediate cell adhesion migration on specific laminin isoforms and influence tumors growth and motility.Citation14 Ziober et alCitation12 found that upregulation of ITGA7 could reduce melanoma cell tumor growth, motility, and metastasis.Citation12 Ren et alCitation13 found that downregulation of ITGA7 expression increased the rate of migration in lung cancer cells. However, the relationship between ITGA7 and breast cancer is not exactly clear.
In order to clarify the expression and effect of ITGA7 in breast cancer, our study examined ITGA7 expression in breast cancer tissues and paired adjacent nontumor tissues by using real-time quantitative PCR (RT-qPCR), and we verified our clinical specimen data by using The Cancer Genome Atlas (TCGA) database. Besides, we characterized the function of ITGA7 in breast cancer cell lines. We found that downregulated ITGA7 could promote breast cancer cell migration and invasion.
Materials and methods
Patients and breast tissue samples
In this present study, we obtained 36 breast cancer tissues and paired adjacent nontumor tissues from the Department of Thyroid and Breast Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China. All patient-derived specimens and information were collected and recorded based on the protocols provided by the Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University. These 36 fresh tissues were snap-frozen in liquid nitrogen immediately and stored at −80°C. Breast cancer mRNA expression data were downloaded from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/). Gene expression data were available for 1,100 breast cancer samples compared to 113 normal samples.
Ethical approval
Ethical approval for this study was obtained from the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Cell cultures and growth conditions
MDA-MB-231, BT-549, SK-BR-3, MDA-MB-468, MCF-7, and MCF-10A cells were used in this study. These cells were obtained from Shanghai Cell Biology, Institute of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). MDA-MB-231, MCF-7, and SK-BR-3 were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco). BT-549 were cultured in Roswell Park Memorial Institute-1640 medium (Gibco) supplemented with 10% FBS (Gibco). MDA-MB-468 cells were cultured in L-15 medium (Gibco) supplemented with 10% FBS (Gibco). MCF-10A cells were cultured in DMEM-F12 (Gibco) supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, 2 mM of l-glutamine, 20 ng/mL of epidermal growth factor, and 10% FBS (Gibco) MDA-MB-468 cells were incubated in a standard cell culture incubator (Thermo, Waltham, MA, USA) at 37°C without CO2. The others were incubated in a standard cell culture incubator (Thermo) at 37°C with 5% CO2.
Cell transfection
MDA-MB-231 and BT-549 were transfected using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) by following the manufacturer’s protocol. About 100,000 cells were plated 1 day before transfection. ITGA7 was silenced by transfecting 10 nM siRNA for 48 h. The siRNA sequences used in the study were the ITGA7 siRNAs that targeted the following sequences: ITGA7 siRNA-1, forward 5′-GCAUCAAGAGCUUCGGCUATT-3′and reverse 5′-UAGCCGAAGCUCUUGAUGCTT-3′; ITGA7 siRNA-2, forward 5′-GCUGCCCACUCUACAGCUUTT-3′ and reverse 5′-AAGCUGUAGAGUGGGCAGCTT-3′; ITGA7 siRNA-3, forward 5′-GGUCAUCCUCCUGGCUGUATT-3′and reverse 5′-UACAGCCAGGAGGAUGACCTT-3′. Both siRNAs were provided by Genepharma (Shanghai, People’s Republic of China) company.
RNA extraction and RT-qPCR
Total RNA was lysed using TRIzol reagent according to the manufacturer’s instructions (Invitrogen). The purity of the isolated RNA was measured at 260/280 nm by spectrophotometry (Thermo). After measurement, RNA samples were stored at −80°C. Real-time reactions were run and analyzed by using a real-time PCR system (Applied Biosystems 7500, ThermoFisher Scientific). The relative expression of mRNA was calculated using the comparative cycle threshold (CT) (2−ΔΔCT) method with GAPDH as the endogenous control to normalize the data. The sequences of the primers used were as follows:
ITGA7 forward: 5′-GCTGTGAAGTCCCTGGAAGT GATT-3′ and reverse: 5′-GCATCTCGGAGCATCAAGTTC TT-3′; GADPH forward: 5′-GTCTCCTCTGACTTCA ACAGCG-3′ and reverse: 5′-ACCACCCTGTTGCTGTA GCCAA-3′.
Invasion and migration assay
For cell Transwell assays, cells were trypsinized with trypsin and collected in the medium containing 10% FBS. Invasion of cells was measured in Matrigel (BD, Franklin Lakes, NJ, USA)-coated Transwell inserts (6.5 mm, Costar, Manassas, VA, USA) containing polycarbonate filters with 8 μm pores. The inserts were coated with 50 μL of 1 mg/mL Matrigel matrix according to the manufacturer’s recommendations. A total of 40,000 cells (~250 μL) were transferred into the upper chamber. About 700 μL medium containing 20% FBS was filled in the lower chamber. Then the plate was placed into the incubator. After 24 h, the membrane was fixed with 4% paraformaldehyde and stained with 0.4% crystal violet solution for 15 min. Motility assays were similar to invasion assay except that the Transwell insert was not coated with Matrigel. Cell migration and invasion ability were assessed by counting the cells that had migrated and invaded through the membrane. Five random fields of view were selected and images captured under a microscope at a magnification of 20×.
Western blot analysis
Whole cell lysates were separated by 10% sodium dodecyl sulfate–polyacrylamide gels electrophoresis (BioRad, Berkeley, CA, USA) and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% nonfat milk for 2 h at room temperature. According to the manufacturer’s protocol, the membranes were probed with polyclonal antibody overnight at 4°C. The membranes were then incubated with the anti-mouse IgG or anti-rabbit IgG as secondary antibody (Abcam, Cambridge, MA, USA) for 1 h at room temperature. The primary antibodies used were as follows: Vimentin (Abcam, ab92547), ITGA7 (Abcam, ab203254), and human GAPDH (Sigma, St. Louis, MO, USA).
Statistical analysis
All statistical analyses were performed using SPSS 23.0 software (IBM Corporation, Armonk, NY, USA). Data are presented as mean ± standard error. The differences were considered to be statistically significant at P<0.05. Student’s t-test (2-tailed) was performed to analyze differences between groups.
Result
ITGA7 was downregulated in breast cancer tissues
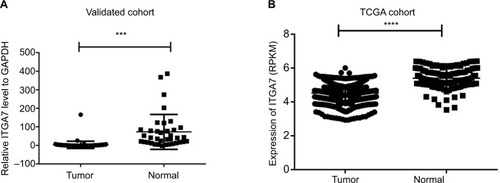
In order to investigate the function of ITGA7 in breast cancer tumorigenesis, the expression levels of ITGA7 were investigated in 36 breast cancer tissues and paired adjacent nontumor tissues by using RT-qPCR. We found that ITGA7 expression was significantly lower in breast cancer tissues, compared to the adjacent normal tissues (T:N =7.68±27.38:41.01±31.47, P<0.001) (). TCGA also showed that ITGA7 was downregulated in breast cancer compared to the adjacent normal tissues (T:N =4.51±0.45:5.40±0.61, P<0.001) (). In a word, these results implied that ITGA7 might function as a tumor suppressor in breast cancer.
Figure 1 ITGA7 expression in breast cancer in validated cohort and TCGA cohort.
Notes: (A) ITGA7 expression was examined by RT-qPCR in 36 paired human breast cancer tissues and adjacent noncancerous tissues (paired t-test, P<0.001). A logarithmic scale of 2−ΔΔCt is used to represent the fold change in quantitative real-time PCR detection. (B) The TCGA cohort contained 1,100 breast tumor tissues and 113 normal tissues. RPKM is used to represent expression of ITGA7. The analysis was done using the Mann–Whitney U-test. ***P<0.001: ****P<0.0001.
Abbreviations: ITGA7, integrin subunit alpha 7; RPKM, reads per kilobases per million reads; RT-qPCR, real-time quantitative PCR; TCGA, The Cancer Genome Atlas.
The relationship between ITGA7 expression and clinical features
To understand the relation between ITGA7 and breast cancer, we investigated the relationship of ITGA7 with clinicopathological features. In the TCGA cohort, we divided the patients into low-expression group and high-expression group according to the median value. The results revealed that lymph node metastasis (P=0.030) and tumor size (P=0.024) were significantly related to the ITGA7 expression (). In the validated cohort, we divided all patients into the low-expression group (n=18) and high-expression group (n=18) according to the median value as same. The results showed that age (P=0.169) and lymph node metastasis (P=0.075) were not related to the expression of ITGA7 negatively (). These results indicated that low ITGA7 expression may influence the ability of migration of breast cancer cells and was associated with unfavorable prognosis in breast cancer.
Table 1 The relationship between ITGA7 and clinicopathologic characteristics in TCGA cohort
Table 2 The relationship between ITGA7 and clinicopathologic characteristics in the validated cohort
ITGA7 regulates migration and invasion of breast cancer lines
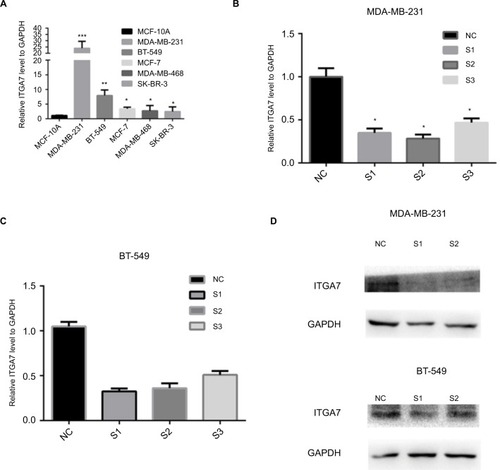
To confirm the role of ITGA7 in breast cancer, we assessed ITGA7 expression level in several breast cancer cell lines and normal breast cell lines by using RT-qPCR. We found that expression level of ITGA7 was higher in MDA-MB-231 and BT-549 than in other breast cell lines (). So, we selected MDA-MB-231 and BT-549 for further experiments. To further examine whether ITGA7 functions in breast cancer progression, we knocked down ITGA7 expression in MDA-MB-231 and BT-549 using siRNA. As can be seen in , both mRNA and protein levels of ITGA7 were significantly reduced.
Figure 2 The expression level of ITGA7 in breast cancer cell lines.
Notes: (A) The relative expression of ITGA7 gene (compared with the GAPDH gene) using RT-qPCR. Compared to the other cell lines, ITGA7 was expressed at a higher level in MDA-MB-231 and BT-549. (B–D) MDA-MB-231 and BT-549 cells were transfected with siRNA-1, siRNA-2, or si-NC for 48 h, and both mRNA and protein levels of ITGA7 were significantly reduced. Compared with corresponding control group, the expression of ITGA7 gene in S1 and S2 group was lower. We defined ITGA7 siRNA-1 as S1, ITGA7 siRNA-2 as S2, and ITGA7 siRNA-3 as S3. *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: ITGA7, integrin subunit alpha 7; NC, negative control; RT-qPCR, real-time quantitative PCR.
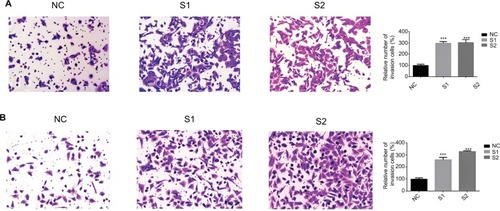
Previous studies have proven that a cancer cell’s abilities of migration and invasion were correlated with tumor progression.Citation15,Citation16 We next examined whether knocking down ITGA7 affected the functions of breast cancer cell lines. Our results showed that downregulated ITGA7 significantly enhanced migration capacity of MDA-MB-231 and BT-549 compared with the control groups (). The invasion assays also showed that downregulated ITGA7 effectively enhanced invasion capacity of MDA-MB-231 and BT-549 ().
Figure 3 Downregulation of ITGA7 gene expression in MDA-MB-231 and BT-549 cells inhibits migration.
Notes: These experiments were done at least 3 independent times. (A) In MDA-MB-231, Transwell migration assays in downregulation ITGA7 cells and their corresponding control cells. (B) In BT-549, Transwell migration assays in downregulation of ITGA7 cells and their corresponding control cells. Quantitative results of migration assays. The stained cells were then counted manually from 5 randomly selected fields and normalized with cell proliferation. **P<0.01 in comparison with the NC group using Student’s t-test. We defined ITGA7 siRNA-1 as S1 and ITGA7 siRNA-2 as S2.
Abbreviations: ITGA7, integrin subunit alpha 7; NC, negative control.
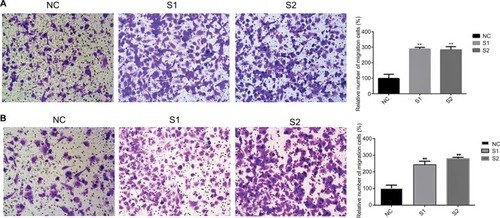
Figure 4 Downregulation of ITGA7 gene expression in MDA-MB-231 and BT-549 cells inhibits invasion.
Notes: These experiments were done at least 3 independent times. (A) Transwell invasion assays in downregulation ITGA7 cells and their corresponding NC cells in MDA-MB-231. (B) Transwell invasion assays in downregulation ITGA7 cells and their corresponding negative control cells in BT-549. Quantitative results of invasion assays. The stained cells were manually counted from 5 randomly selected fields and normalized with cell proliferation. ***P<0.001 in comparison with the NC group using Student’s t-test. We defined ITGA7 siRNA-1 as S1 and ITGA7 siRNA-2 as S2.
Abbreviations: ITGA7, integrin subunit alpha 7; NC, negative control.
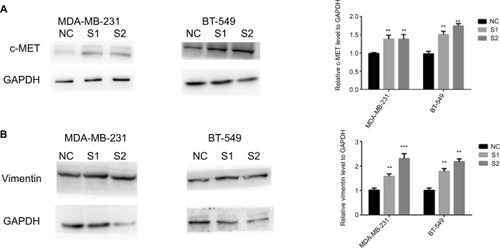
ITGA7 regulates migration and invasion via addition to c-met-regulated vimentin
We found that knockdown of ITGA7 led to significantly increased c-met in MDA-MB-231 and BT-549 (). In a previous study,Citation10,Citation28 repression of the c-Met/Akt/mTOR pathway was found to suppress EMT. Vimentin is important in EMT and tumor progression.Citation17,Citation18 In order to explore the potential mechanism of ITGA7 in breast cancer, we measured the expression level of epithelial markers and mesenchymal markers in MDA-MB-231 and BT-549 using Western blotting. The result showed that cells with knocked down ITGA7 had significantly higher vimentin expression level (). Our result showed that knockdown of ITGA7 may regulate vimentin via enhancing c-met.
Figure 5 Downregulation of ITGA7 regulates migration and invasion via addition to c-met-regulated vimentin.
Notes: These experiments were done at least 3 independent times. We defined ITGA7 siRNA-1 as S1 and ITGA7 siRNA-2 as S2. (A) The influence of ITGA7 expression on c-MET in MDA-MB-231 and BT-549 cells by Western blot. (B) The influence of ITGA7 expression on vimentin in MDA-MB-231 and BT-549 cells by Western blot. **P<0.01 and ***P<0.001 in comparison with the NC group using Student’s t-test. We defined ITGA7 siRNA-1 as S1 and ITGA7 siRNA-2 as S2.
Abbreviations: ITGA7, integrin subunit alpha 7; NC, negative control.
Discussion
Breast cancer represents the highest health burden in the world, which is consistent with recently published data which reports that breast cancer is a significant part of the cause of cancer-related death in women worldwide.Citation2 Although much progress in medical research had been made in breast cancer,Citation19–Citation21 there is still much left unknown about the molecular mechanisms of breast cancer. Some studies have demonstrated that ITGA7 gene was associated with malignant melanoma, prostate and liver carcinomas, and glioblastoma.Citation12 However, there is still known little about its function in breast cancer.
In our study, the main aim was to prove the potential role of ITGA7 in breast cancer. We found that ITGA7 in breast cancer tissues was downregulated compared to paired adjacent nontumor tissues by RT-qPCR. This result was further identified in TCGA cohort. We analyzed the clinical features of breast cancer patients from TCGA and found that lymph node metastasis (P=0.030) and tumor size (P=0.024) were significantly related with the ITGA7 expression. These findings suggest that ITGA7 may play an important role in breast cancer and encouraged us to proceed with the next step to study the ITGA7 gene in cell lines. We found that ITGA7 was expressed at a higher level in MDA-MB-231 and BT-549 than in other breast cell lines. Then, using cellular and molecular technology, we found that knockdown of ITGA7 led to an increase in migration and invasion abilities, which is consistent with ITGA7 being associated with breast cancer.
In recent decades, the development of c-met has been increasingly recognized to play pivotal roles in promoting tumor process.Citation22–Citation24 Jia et alCitation25 found that inhibiting c-MET could enhance the response of the colorectal cancer cells to irradiation in vitro and in vivo. Our study proved that knocking down ITGA7 could enhance c-met. EMT is a developmental multistep molecular process which may induce tumor process.Citation26,Citation27 Recently, EMT was found that facilitate the progression of breast cancer. It has been reported that repression of the c-Met/Akt/mTOR pathway could suppress EMT in breast cancer.Citation10 So, we tested the EMT-related epithelial marker and the mesenchymal markers after ITGA7 knockdown. We found that the mesenchymal marker vimentin was induced after ITGA7 knockdown. Together, these results mean that knockdown of ITGA7 enhances breast cancer cell migration and invasion via c-met regulated vimentin.
However, our study still has several limitations. For one thing, gain-of-function experiments need to be conducted to further validate the results. For another, we need to expand the validation group to provide reliable clinicopathological features and findings.
Conclusion
By using bioinformatics analysis and cellular and molecular approaches, the role of ITGA7 gene as a suppressor gene in breast cancer was demonstrated. We found that knockdown of ITGA7 could enhance breast cancer cell migration and invasion. This study indicated that ITGA7 may act as a potential drug target in breast cancer.
Acknowledgments
This study was funded by Natural Science Foundation of Zhejiang Province (LY18H160053, LY17H160053, and LY18H160053) and the Science and Technology Project of Wenzhou (Y20170030). The authors thank Yizuo Chen and Zhiqiang Ye for assistance help with data analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- AhmedMIHarveyJRKirbyJAliSLennardTWJO-98 role of the chemokine receptor CXCR4 in breast cancer metastasisEur J Cancer Suppl20075330
- van ZijlFKrupitzaGMikulitsWInitial steps of metastasis: cell invasion and endothelial transmigrationMutat Res20117281–2233421605699
- PelkonenMLuostariKTengströmMLow expression levels of hepsin and TMPRSS3 are associated with poor breast cancer survivalBMC Cancer201515143126014348
- ZhaoXQuJHuiYClinicopathological and prognostic significance of c-Met overexpression in breast cancerOncotarget2017834567585676728915628
- GisterekILataEHalonAPrognostic role of c-met expression in breast cancer patientsRep Pract Oncol Radiother201116517317724376976
- AcloqueHAdamsMSFishwickKBronner-FraserMNietoMAEpithelial-mesenchymal transitions: the importance of changing cell state in development and diseaseJ Clin Invest200911961438144919487820
- TiwariNGheldofATatariMChristoforiGEMT as the ultimate survival mechanism of cancer cellsSemin Cancer Biol201222319420722406545
- HungCMKuoDHChouCHSuYCHoCTWayTDOsthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-Met/Akt/mTOR pathway in human breast cancer cellsJ Agric Food Chem201159179683969021806057
- SongWKWangWFosterRBielserDAKaufmanSJH36-a7 is a novel integrin α chain that is developmentally regulated during skeletal myogenesisJ Cell Biol199211736436571315319
- ZioberBLChenYQRamosDMWalehNKramerRHExpression of the α7β1 laminin receptor suppresses melanoma growth and metastatic potentialCell Growth Differ199910747949010437916
- RenBYuYPTsengGCAnalysis of integrin α7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcomaJ Natl Cancer Inst2007991186888017551147
- YaoCCZioberBLSquillaceRMKramerRHα7 integrin mediates cell adhesion and migration on specific laminin isoformsJ Biol Chem19962714125598256038810334
- LiuYSuCShanYYangSMaGTargeting Notch1 inhibits invasion and angiogenesis of human breast cancer cells via inhibition nuclear factor-κB signalingAm J Transl Res2016862681269227398151
- LeeHGoodarziHTavazoieSFAlarconCRTMEM2 is a SOX4-regulated gene that mediates metastatic migration and invasion in breast cancerCancer Res201676174994500527328729
- BrzozowaMWyrobiecGKolodziejIThe aberrant overexpression of vimentin is linked to a more aggressive status in tumours of the gastrointestinal tractPrz Gastroenterol201510171125960808
- ChenCZimmermannMTinhoferIKaufmannAMAlbersAEEpithelial-to-mesenchymal transition and cancer stem(-like) cells in head and neck squamous cell carcinomaCancer Lett20133381475622771535
- JungJHSonSHKimDHCONSORT-independent prognostic value of asphericity of pretherapeutic F-18 FDG uptake by primary tumors in patients with breast cancerMedicine (Baltimore)20179646e843829145250
- HromasRKimHSSidhuGThe endonuclease EEPD1 mediates synthetic lethality in RAD52-depleted BRCA1 mutant breast cancer cellsBreast Cancer Res201719112229145865
- QianKLiuGTangZThe long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2Arch Biochem Biophys20176151928034643
- HarunNCostaPChristophiCTumour growth stimulation following partial hepatectomy in mice is associated with increased upregulation of c-MetClin Exp Metastasis201431111423900501
- HarshmanLCChoueiriTKTargeting the hepatocyte growth factor/c-Met signaling pathway in renal cell carcinomaCancer J201319431632323867513
- LiYWangJGaoXc-Met targeting enhances the effect of irradiation and chemical agents against malignant colon cells harboring a KRAS mutationPLoS One2014911e11318625427200
- JiaYDaiGWangJc-MET inhibition enhances the response of the colorectal cancer cells to irradiation in vitro and in vivoOncol Lett20161142879288527073569
- LuoZLiYZuoMEffect of NR5A2 inhibition on pancreatic cancer stem cell (CSC) properties and epithelial-mesenchymal transition (EMT) markersMol Carcinog20175651438144827996162
- HuSHWangCHHuangZJmiR-760 mediates chemoresistance through inhibition of epithelial mesenchymal transition in breast cancer cellsEur Rev Med Pharmacol Sci201620235002500827981531
- ChenQYJiaoDMMiR-206 inhibits HGF-induced epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via c-Met/PI3k/Akt/mTOR pathwayOncotarget2016714182471826126919096
