Abstract
Background
Topotecan (TP) is an anticancer drug acting as topoisomerase I inhibitor that is used in the treatment of many types of cancers including leukemia, but it has significant side effects. Andrographolide, a compound extracted from Andrographis paniculata, was recently proven to inhibit the growth of cancer cells and can induce apoptosis. The aim of this study is to investigate the possible synergism between TP and andrographolide in acute myeloid cells in vitro.
Materials and methods
U937 acute myeloid leukemic cells were cultured using Roswell Park Memorial Institute (RPMI) medium and then treated for 24 h with TP and andrographolide prepared through the dilution of dimethyl sulfoxide (DMSO) stocks with RPMI on the day of treatment. Cell proliferation was assessed using cell proliferation assay upon treatment with both compounds separately and in combination. Cell-cycle study and apoptosis detection were performed by staining the cells with propidium iodide (PI) stain and Annexin V/PI stain, respectively, followed by flow cytometry analysis. Western blotting was used to assess the expression of various proteins involved in apoptotic pathways.
Results
Both TP and andrographolide showed an antiproliferative effect in a dose-dependent manner when applied on U937 cells separately; however, pretreating the cells with andrographolide before applying TP exhibited a synergistic effect with lower inhibitory concentrations (half-maximal inhibitory concentration). Treating the cells with TP alone led to specific cell-cycle arrest at S phase that was more prominent upon pretreatment combination with andrographolide. Using Annexin V/PI staining to assess the proapoptotic effect following the pretreatment combination showed an increase in the number of apoptotic cells, which was supported by the Western blot results that manifested an upregulation of several proapoptotic proteins expression.
Conclusion
The pretreatment of U937 with andrographolide followed by low doses of TP showed an enhancement in inducing apoptosis when compared to the application of each compound separately.
Introduction
Topotecan (TP) is an anticancer drug currently used in the treatment of many types of cancers, including leukemia.Citation1,Citation2 Being a camptothecin analog, TP acts as a topoisomerase I inhibitor by interfering with the enzymatic repair of nuclear DNA, thereby inducing cell death. Studies have shown the antiproliferative effect of TP on glioma cells, small cell lung cancer, prostate cancer cells, platinum-resistant ovarian cancers, neuroblastoma cells, and leukemic cells.Citation3–Citation7
However, TP exhibits several significant side effects, including hematologic toxicities like thrombocytopenia, anemia, and neutropenia, as well as nonhematologic toxicities such as fatigue and alopecia.Citation8–Citation11
To enhance the adequacy of TP use and addressing to its toxicity, different approaches have been considered. One of the ways is developing various formulations and methods of administration of TP. A novel approach is using the nanocarrier liposome to promote targeted delivery of TP.Citation12 Liposomal TP has improved the anticancerous efficacy of TP in Kunming mice with Hepatoma-22 tumor as well as in patients with neuroblastoma.Citation13,Citation14
Another method in improving TP’s efficacy is using it as an adjunct with radiotherapy, providing satisfactory results in targeting prostate cancer, small-cell lung carcinoma, recurrent primitive neuroectodermal tumors, recurrent platinum-resistant ovarian cancer, and cervical cancer.Citation15–Citation18
A more popular approach that both enhances the potency of TP and addresses its cytotoxicity is combination therapy. TP treatment has shown synergistic effects when combined with gemcitabin, gefitinib, and thymoquinone.Citation19–Citation21 As the combination of treatments involving TP have shown satisfactory and promising outcomes, further research has been directed to establish effective therapies involving TP and significant chemotherapeutic agents. TP, when administered with paclitaxel (Taxol) and bevacizumab (Avastin) in combination, has been proven to improve the overall survival and progression-free survival in patients with recurrent cervical small-cell neuroendocrine carcinoma as described by Frumovitz et al.Citation22
Another study tested pre exposure therapy on neuroblastoma cell lines with various inhibitors 24 h before treating with TP. Results showed a significant decrease in cell growth with three inhibitors (13-197, BI2536, and vismodegib) and TP as a single agent. When tested in vivo, the combined efficacy of vismodegib and TP resulted in a significant reduction of tumor size and increased survival of NSG mice. Interestingly, Western blots revealed that although TP alone has no effect on the expression of phosphorylated p65 (nuclear factor kappa B [NF-κB]) and S6K (mammalian target of rapamycin), in combination with the three inhibitors the expression levels of the phosphorylated p65 and S6K were significantly decreased in MYCN-amplified cells as stated by Chaturvedi et al.Citation23
Several plant-derived compounds have shown significant anticancer effects; andrographolide, a compound extracted from Andrographis paniculata, was recently proven to inhibit the growth of cancer cells through attenuating the NF-κB pathway.Citation24,Citation25 Andrographolide was also reported to induce apoptosis and autophagy in U937 (acute myeloid leukemia [AML]) cells, leading to cell death.Citation26
This led us to investigate the possible synergism between TP and andrographolide in vitro on acute myeloid cells, namely, U937. The present study aims to explore the possibility of enhancing the effect of low doses of TP in controlling cancer cells’ progression through administering them with different dosing schedules of andrographolide and to study the pathway involved in promoting cell death upon combination of both drugs.
Materials and methods
Cell culture
AML cell line, namely, U937 was obtained from American Type Culture Collection. The cells were maintained in suspension in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) in a humidified incubator at 37°C and 5% CO2. The cells were split every 3 days at a ratio of 1:2. Before any experiment, 10 µL of the cells was mixed with 10 µL of trypan blue and counted using a hemocytometer to check cell viability.
Drug preparation
TP (5 µM) and andrographolide (1000 µM) stock solutions were prepared by dissolving the compounds in dimethyl sulfoxide (DMSO) and then diluting them with RPMI on the day of treatment. The different working concentrations of the compounds (0.05–50 µM TP and 0.25–25 µM andrographolide) were obtained by serial dilutions of the DMSO stock solutions using RPMI. The concentration of DMSO to which the cells were exposed was always <0.5% (v/v).
Cell proliferation
The cells were plated in a 96-well plate at a density of 1×105 cells/well for 24 h. Different concentrations of TP and andrographolide were applied either separately or in combination. Co-treatment was performed by adding the two compounds simultaneously on cells for 24 h. Pretreatment was performed by exposing the cells to andrographolide for 24 h followed by TP treatment for another 24 h. RPMI alone was added to the control cells. WST-1 (Roche) and XTT (Sigma Aldrich) reagents were used, according to the manufacturer’s guide (10 µL of WST-1 reagent was added to each well while 40 µL of XTT was added to each well), to detect cell proliferation. Cell proliferation was assessed by recording the absorbance at a wavelength of 450 nm (using a Biotek ELx808 ELISA reader), which reflects the amount of formazan dye produced by metabolically active cells.
Cell-cycle analysis
Cells were seeded in six-well plates at a density of 0.5×105 cells/well. After incubation for 24 h and treatment with various compounds separately and in combination, the cells were fixed with ethanol, followed by propidium iodide (PI) staining. Cell DNA content was assessed by flow cytometry using Accuri C6 flow cytometer. The distribution of the cells into their respective cell-cycle phases was based on their DNA content: sub-G0/G1 (Pre-G) cells were <2n, G0/G1 cells were 2n, S cells were >2n but <4n, whereas M phase cells were 4n. Cell death was determined by an increase in the percentage of cells in the pre-G phase as compared to the control.
Apoptosis detection
Cells were plated in six-well plates at a density of 0.5×105 cells/well and incubated for 24 h. After treatment, Annexin V/PI staining was performed to detect and analyze apoptosis using flow cytometry. Cells were collected and centrifuged at 1500 rpm at 4°C for 5 min. The pellet was suspended in 500 µL suspension buffer, in addition to 5 µL Annexin and 5 µL PI (Annexin V–fluorescein isothiocyanate [FITC] Apoptosis Detection Kit, Abcam, Cambridge, UK) and immediately analyzed by the flow cytometer. Annexin V is a Ca2+-dependent phospholipid-binding protein that has a high affinity for phosphatidylserine, which is translocated from the cytoplasmic surface to the outer leaflet of the cell membrane upon apoptosis. The cell membrane is impermeable to PI and hence PI is excluded from living cells. Cells that are stained negative for FITC–Annexin V and negative for PI are considered living cells. Cells that are stained positive for both FITC-Annexin V and PI are either at the end stage of apoptosis, undergoing necrosis, or are already dead.
Western blots
Cells were plated in six-well plates at a density of 106 cells/mL to extract total proteins using the Q-proteome Mammalian Protein kit. Proteins were quantified using Lowry assay. Western blot analysis was done to measure the protein expression of p53, p21, Bax, Bcl2, cytochrome c, PARP, and caspase-3 and -9.
Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, proteins were separated on 10% gels and transferred to polyvinylidene difluoride membranes at 0.25 mA for 75 min. The membranes were then blocked with 5% skimmed dry milk in PBS containing 0.05% Tween 20 for a night at 4°C. Membranes were then incubated with primary antibody using anti-β-actin, anti-p53, anti-p21, anti-Bax, anti-bcl2, anti-caspase-3 and anti-caspase-9 antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) at a concentration of 1:500. After washing the membranes for 1 h using 1× PBS with 0.5% Tween 20, they were incubated with secondary antibody (obtained from Promega, Madison, WI, USA) at a concentration of 1:2500 for 1 h at room temperature. The membranes were then washed and the development was done using Western blotting chemiluminescent reagent enhanced chemiluminescence (GE Healthcare, Chicago, IL, USA) He and figures were taken using ChemiDoc XRS+ machine (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All the experiments were carried out in triplicate and each experiment was repeated three times. The results were reported as mean value±SD. The analysis was done using two-way analysis of variance. The level of significance upon comparing control versus treatment was set at P<0.05.
Results
Effect of andrographolide and TP on cell proliferation
When applied separately, both andrographolide and TP exhibited antiproliferative effects on U937 cell line in a dose-dependent manner with a half-maximal inhibitory concentration (IC50) of 22.25 µM with andro treatment and 0.65 µM with TP treatment at 24 h ( and ). Cell proliferation upon treatment with various combinations of TP and andrographolide was then assessed. Co-treating the cells with both andrographolide (10 µM) and TP simultaneously at various concentrations did not exhibit any significant changes in cell proliferation when compared to treating the cells with both drugs separately (). However, pretreating the cells with andrographolide (10 µM) for 24 h prior to TP addition (0.05, 0.1, 0.15, and 0.3 µM) led to a significant decrease in cell proliferation (P≤0.0001) as compared to treatment with the compounds separately. The optimal conditions that caused a significant reduction in proliferation were the pretreatment combination of 10 µM of andrographolide prior to 0.3 µM of TP, leading to a decrease in cell proliferation from 67% to 39% with P ≤0.0001 ().
Figure 1 Proliferation of U937 cells after 24 and 48 h of treatment with various concentrations of andrographolide alone (0–25 μM).
Note: U937 showed a decrease in cell proliferation reaching the half-maximal inhibitory concentration at 22.25 μM after 24 h and 18.9 μM after 48 h.
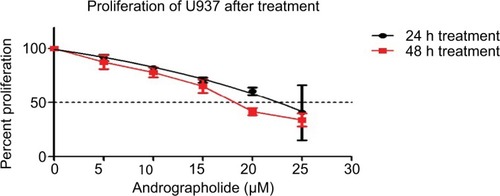
Figure 2 Proliferation of U937 cells after 24 h of cotreatment of 10 μM andrographolide with different TP concentrations (0–0.6 μM).
Note: U937 did not show any synergistic effect on decreasing cell proliferation.
Abbreviation: TP, topotecan.
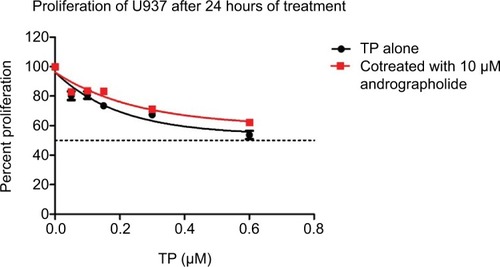
Figure 3 Proliferation of U937 cells after pretreating the cells with 10 μM of andrographolide for 24 h, followed by treatment with various concentrations of TP (0–0.6 μM) for another 24 h.
Notes: Pretreatment showed a significant decrease in cell proliferation. The optimal conditions were found to be 10 μM of andrographolide followed by 0.3 μM of TP, leading to a decrease in proliferation from 67% to 39%.
Abbreviation: TP, topotecan.
Effect of andrographolide and TP on cell-cycle arrest
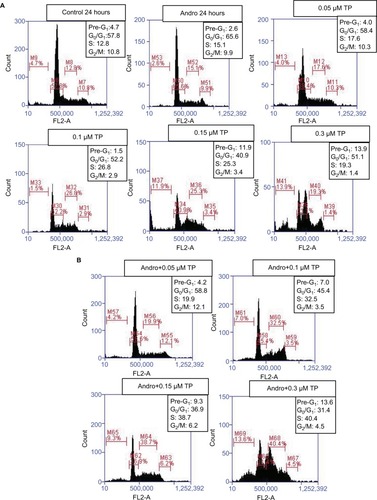
After determining the optimal combination range that has inhibited cell proliferation in U937 upon pretreatment with andrographolide before TP application, flow cytometry was used to assess the effect of this combination on cell-cycle arrest. Cells were pretreated with 10 µM of andrographolide followed by 0.05, 0.1, 0.15, and 0.3 µM of TP. After fixation with ethanol, the cells were stained with PI stain and then the DNA content of cells was analyzed using the C6 flow cytometer and cells were accordingly assigned to their respective phases: pre-G1 cells were <2n, G0/G1 cells were 2n, and S/M phase cells were >2n. When applied separately on the cells, TP caused a specific cell-cycle arrest at the S phase in addition to an increase in the percentage of cells in the pre-G1 phase at concentrations ≥0.3 µM (). These alterations in cell-cycle progression were further enhanced upon andrographolide pretreatment, even though andrographolide when applied alone did not show any significant change in the distribution of cells at the various stages of the cell cycle. Again, the pronounced synergistic effect was observed upon pretreatment with andrographolide followed by 0.3 µM of TP, which led to an increase in the percentage of cells in the S phase, reaching 40.4%, compared to 19.3% upon treatment with TP alone ().
Figure 4 Effect of andrographolide, TP separately (A), and TP after 24 h of pretreating with andrographolide (B), on cell-cycle progression using flow cytometry on U937 cells. Based on their DNA content, cells were distributed into the different phases of the cell cycle: pre-G1 cells were <2n, G0/G1 cells were 2n, and S/M phase cells were >2n.
Abbreviation: TP, topotecan.
Effect of andrographolide and TP on apoptosis
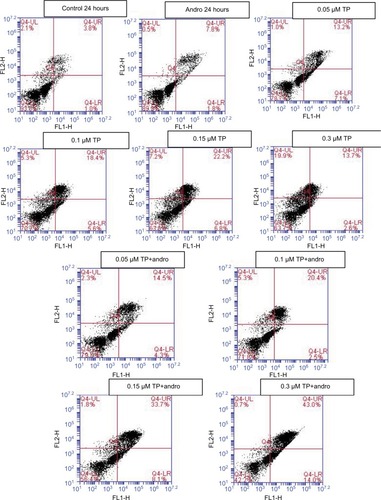
Annexin V/PI staining was used to determine if U937 cells died of apoptosis or necrosis upon the application of both compounds separately and after pretreatment using the same TP concentrations (0.05, 0.1, 0.15, and 0.3 µM). Upon combination of TP with andrographolide, the proapoptotic effect was further observed even though andrographolide alone did not show any alteration in cell survival. However, pretreating the cells with andrographolide prior to 0.3 µM of TP treatment resulted in a significant increase in the number of apoptotic cells from 16% to 57% ().
Figure 5 Effect of andrographolide and TP on apoptosis.
Notes: Cells were treated with andrographolide (10 μM), and with TP (0.05, 0.1, 0.15, and 0.3 μM) separately, and pretreated for 24 h with andrographolide followed by TP. The cells were stained with Annexin/propidium iodide, and then analyzed using flow cytometry. The lower left quadrant shows cells which are negative for both PI and annexin (normal cells). The upper left quadrant shows only PI positive cells (necrotic). The lower right quadrant shows annexin positive cells (early apoptotic). The upper right quadrant shows annexin and PI positive cells (late apoptotic cells).
Abbreviation: TP, topotecan.
Effect of andrographolide and TP on the proapoptotic and antiproliferative pathways
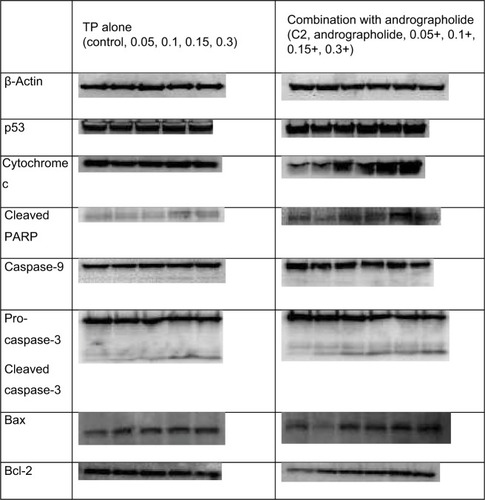
To determine the metabolic pathway through which the combination of andrographolide and TP is inhibiting the cell proliferation of U937, Western blot analysis was performed to assess the expression of a series of proteins related to different pathways. The cells were treated with either TP only at various concentrations (0.05, 0.1, 0.15, and 0.3 µM) or pretreated with andrographolide followed by the same concentrations of TP. Beta-actin was used to ensure equal loading. The expression of both p53 and cytochrome c did not change with TP alone, while it showed a dose-dependent upregulation upon the pretreatment with andrographolide. The proapoptotic effect of andrographolide and TP was assessed by measuring the expression of cleaved PARP, caspase-9, pro-caspase-3, and cleaved caspase-3. Cleaved PARP and caspase-9 showed a significant upregulation upon the separate treatment with TP and pretreatment with andrographolide, but the upregulation was more intense upon the combination of both compounds. Pro-caspase-3 appeared to be cleaved into its active form with increasing doses in both conditions with a more significant effect upon combination. Also, the upregulation in the expression of Bax was observed to be higher upon pretreating the cells with andrographolide; Bcl2 expression was downregulated with TP alone but upregulated with the combination. These results confirm that apoptosis was induced upon TP treatment through an intrinsic pathway and was further enhanced with the pretreatment of andrographolide ().
Figure 6 Western blot analysis for proapoptotic and antiproliferative proteins p53, p21, cleaved PARP, caspase-3, caspase-9, cytochrome c, Bax, and Bcl-2.
Notes: U937 cells were treated with andrographolide (10 μM), and with TP (0.05, 0.1, 0.15, and 0.3 μM) separately, and pretreated for 24 h with andrographolide followed by TP. β-Actin was used to ensure equal loading.
Abbreviation: TP, topotecan.
Discussion
As other camptothecin analogs, TP has shown a valuable antitumor effect in different cancer types clinically with the presence of some serious toxicities and side effects especially when used in high doses.Citation1,Citation12 Over years of use, many cancer types developed resistance against TP leading to advanced stages of malignancies and relapses that required shifting toward other chemotherapeutic derivatives.Citation27 However, due to its known efficacy, studies were directed to the application of TP in combination with different agents in order to study possible synergistic effects and decrease of TP toxicities.Citation3,Citation28 Treating U937 with gemcitabine prior to TP addition showed significant synergism at a lower IC50.Citation29 Similarly, cell growth inhibition appeared to increase when combining genistein, a natural derivative of soy, with TP treatment in prostate cancer cells.Citation28 In addition, andrographolide inhibited the growth of T-cell acute lymphoblastic leukemia cells as its analog did in U937 cells.Citation30,Citation31
The combination of TP with shinokin that is also a topoisomerase I inhibitor promoted cell-cycle arrest in G0/G1 and S phases with the induction of apoptosis.Citation3 Austrobailignan-1, another topoisomerase I inhibitor, retarded the cells upon treatment in G2/M phase in non-small-cell lung cancer cells, while the camptothecin derivative SN-38 arrested the cells of the same cancer type in the S- and G2 phases in vivo and in vitro.Citation32,Citation33 On the other hand, andrographolide induced cell-cycle arrest at G2/M phase in HepG2 cells.Citation34 The present study shows an S phase arrest in the cells treated with only TP, which is consistent with the predominant S phase arrest effect of many camptothecin derivatives.Citation27,Citation33 This S phase arrest appears to be increased around twofold upon the combination of TP and andrographolide.
Different camptothecin derivatives, including TP, are known to induce apoptosis in different cancer types through various metabolic pathways as described by Maurya et al.Citation33 TP had shown to exert its proapoptotic effects through p53 in some cancer types while it depends on an intrinsic pathway that involves caspases in others.Citation32,Citation35 Even though the possible role of p53 in causing apoptosis has been reported in many studies, its specific mechanism is still unclear, whereby it has led to resistance in some cell lines.Citation35,Citation36 p53 upregulation was also observed in Jurkat cells and melanoma cells upon treatment with andrographolide.Citation30,Citation37 Furthermore, topoisomerase I inhibitors have been proven to induce apoptosis by activation of caspase-2, -3, and -9 and PARP-inducing apoptosis.Citation32,Citation33 Andrographolide also induced apoptosis through the release of cytochrome c, cleavage of PARP, increase in the level of Bax, and activation of caspase-3, -8, and -9 in rheumatoid arthritis fibroblast-like synoviocytes and in lymphoma cells.Citation38,Citation39 Combining andrographolide with cisplatin showed an increase in the percentage of apoptotic cell death in ovarian cancer cells. This is similar to what is reported in the current study after the combination of andrographolide with TP on U937.Citation40 According to Uckun et al (1995),Citation41 expression of Bcl-2 was found to increase upon combination of TP with andrographolide, even though TP-induced apoptosis does necessarily require a decrease in bcl-2 expression. Pretreating the U937 cells with andrographolide prior to TP addition resulted in a significant increase in apoptosis through the upregulation of many proapoptotic proteins’ expression and was confirmed with Annexin/PI staining that showed an increase in the percentage of apoptotic cells rather than necrotic cells.
Conclusion
The pretreatment of U937 with andrographolide followed by low doses of TP showed an enhancement in inducing apoptosis when compared to the application of each compound separately. The synergistic effects of this combination appeared to involve more intrinsic mitochondrial pathways including the upregulation of Bax, cleavage of PARP with the release of cytochrome c, and the cleavage of different caspases into their active forms, especially caspase-3 and -9.
The new combination can be used as a new clinical strategy in chemotherapy to treat AML and improve TP treatment results with lower toxicities. Moreover, it might be used as an option in the treatment of other resistant cancer types after further studies and investigations.
Acknowledgments
This work was supported and funded by the School Research and Development Council of the Lebanese American University (SRDC-r-2016-6).
Disclosure
The authors report no conflicts of interest in this work.
References
- LondonWBFrantzCNCampbellLAPhase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children’s Oncology Group studyJ Clin Oncol201028243808381520660830
- PerkinsJBGoldsteinSCDawsonJLPhase I study of topotecan, ifosfamide, and etoposide (TIME) with autologous stem cell transplant in refractory cancer: pharmacokinetic and pharmacodynamic correlatesClin Cancer Res201117247743775322028494
- ZhangFLWangPLiuYHTopoisomerase I inhibitors, shikonin and topotecan, inhibit growth and induce apoptosis of glioma cells and glioma stem cellsPLoS One2013811e8181524303074
- AljuffaliIAMockJNCostynLJEnhanced antitumor activity of low-dose continuous administration schedules of topotecan in prostate cancerCancer Biol Ther201112540742021709443
- TsunetohSTeraiYSasakiHTopotecan as a molecular targeting agent which blocks the Akt and VEGF cascade in platinum-resistant ovarian cancersCancer Biol Ther201010111137114620935474
- TsangPSCheukATChenQRSynthetic lethal screen identifies NF-κB as a target for combination therapy with topotecan for patients with neuroblastomaBMC Cancer20121210122436457
- SrourSABordersEBCherryMAHolterJHermanTSelbyGBRole of topotecan, vinorelbine, thiotepa and gemcitabine chemotherapy in relapsed/refractory adult acute leukemiaLeuk Lymphoma201556102962296425721752
- ChintagumpalaMMFriedmanHSStewartCFA phase II window trial of procarbazine and topotecan in children with high-grade glioma: a report from the Children’s Oncology GroupJ Neurooncology2006772193198
- KlautkeGSchützeMBomborIBeneckeRPiekJFietkauRConcurrent chemoradiotherapy and adjuvant chemotherapy with topotecan for patients with glioblastoma multiformeJ Neurooncology2006772199205
- KollmannsbergerCMrossKJakobAKanzLBokemeyerCTopotecan – a novel topoisomerase I inhibitor: pharmacology and clinical experienceOncology19995611129885371
- ChangJZhangRMZhangYAndrographolide drop-pill in treatment of acute upper respiratory tract infection with external wind-heat syndrome: a multicenter and randomized controlled trialZhong Xi Yi Jie He Xue Bao20086121238124519063836
- SarafSJainAHurkatPJainSKTopotecan liposomes: a visit from a molecular to a therapeutic platformCrit Rev Ther Drug Carrier Syst201633540143227910741
- XingJQiXJiangYTopotecan hydrochloride liposomes incorporated into thermosensitive hydrogel for sustained and efficient in situ therapy of H22 tumor in Kunming micePharm Dev Technol2014918
- ChernovLDeyellRJAnanthaMDos SantosNGilabert-OriolRBallyMBOptimization of liposomal topotecan for use in treating neuroblastomaCancer Med2017661240125428544814
- TessonMRaeCNixonCBabichJWMairsRJPreliminary evaluation of prostate-targeted radiotherapy using (131) I-MIP-1095 in combination with radiosensitising chemotherapeutic drugsJ Pharm Pharmacol201668791292127139157
- KuboTFujiwaraKHottaKA phase II study of topotecan and cisplatin with sequential thoracic radiotherapy in elderly patients with small-cell lung cancer: Okayama Lung Cancer Study Group 0102Cancer Chemother Pharmacol201678476977427544764
- GhoshIMallickIRaySFeasibility and efficacy of salvage radiotherapy with concurrent weekly topotecan in recurrent primitive neuroectodermal tumorIndian J Cancer2015521252626837963
- SunWWangTShiFRandomized phase III trial of radiotherapy or chemoradiotherapy with topotecan and cisplatin in intermediate-risk cervical cancer patients after radical hysterectomyBMC Cancer20151535325935645
- GiovannettiEMeyVDanesiRInteraction between gemcitabine and topotecan in human non-small-cell lung cancer cells: effects on cell survival, cell cycle and pharmacogenetic profileBr J Cancer200592468168915700043
- CarcabosoAMElmeliegyMAShenJTyrosine kinase inhibitor gefitinib enhances topotecan penetration of gliomasCancer Res201070114499450820460504
- KhalifeREl-HayekSTarrasOHodrojMHRizkSAntiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemiaClin Lymphoma Myeloma Leuk201414SupplS46S5525486955
- FrumovitzMMunsellMFBurzawaJKCombination therapy with topotecan, paclitaxel, and bevacizumab improves progression-free survival in recurrent small cell neuroendocrine carcinoma of the cervixGynecol Oncol20171441465027823771
- ChaturvediNKMcGuireTRCoulterDWImproved therapy for neuroblastoma using a combination approach: superior efficacy with vismodegib and topotecanOncotarget2016712152151522926934655
- PengTHuMWuTTAndrographolide suppresses proliferation of nasopharyngeal carcinoma cells via attenuating NF-κB pathwayBiomed Res Int2015201573505625861643
- ZhangQQZhouDLDingYAndrographolide inhibits melanoma tumor growth by inactivating the TLR4/NF-κB signaling pathwayMelanoma Res201424654555525244079
- HossainSUrbiZSuleARahmanHAndrographis paniculata (Burm. f.) wall. ex nees: a review of ethnobotany, phytochemistry, and pharmacologyScientific World J20142014274905
- RobatiMHoltzDDuntonCJA review of topotecan in combination chemotherapy for advanced cervical cancerTher Clin Risk Manag20084121321818728710
- HormannVKumi-DiakaJDurityMRathinaveluAAnticancer activities of genistein-topotecan combination in prostate cancer cellsJ Cell Mol Med201216112631263622452992
- AdamsDJSandvoldMLMyhrenFJacobsenTFGilesFRizzieriDAAnti proliferative activity of ELACY (CP-4055) in combination with cloretazine (VNP40101M), idarubicin, gemcitabine, irinotecan and topotecan in human leukemia and lymphoma cellsLeuk Lymphoma200849478679718398748
- YangTYaoSZhangXGuoYAndrographolide inhibits growth of human T-cell acute lymphoblastic leukemia Jurkat cells by downregulation of PI3K/AKT and upregulation of p38 MAPK pathwaysDrug Des Devel Ther20161013891397
- KumarDDasBSenRAndrographolide analogue induces apoptosis and autophagy mediated cell death in U937 cells by inhibition of PI3K/Akt/mTOR pathwayPLoS One20151010e013965726436418
- WuCCHuangKFYangTYThe topoisomerase 1 inhibitor austrobailignan-1 isolated from koelreuteria henryi induces a G2/M-phase arrest and cell death independently of p53 in non-small cell lung cancer cellsPLoS One2015107e013205226147394
- MauryaDKAyuzawaRDoiCTroyerDTamuraMTopoisomerase I inhibitor SN-38 effectively attenuates growth of human non-small cell lung cancer cell lines in vitro and in vivoJ Environ Pathol Toxicol Oncol201130111021609311
- LiJCheungHYZhangZChanGKFongWFAndrographolide induces cell cycle arrest at G2/M phase and cell death in HepG2 cells via alteration of reactive oxygen speciesEur J Pharmacol20075681–3314417512926
- TomicicMTChristmannMKainaBTopotecan triggers apoptosis in p53-deficient cells by forcing degradation of XIAP and survivin thereby activating caspase-3-mediated Bid cleavageJ Pharmacol Exp Ther2010332131632519812371
- SchmidtFRiegerJWischhusenJNaumannUWellerMGlioma cell sensitivity to topotecan: the role of p53 and topotecan-induced DNA damageEur J Pharmacol20014121212511166732
- PratheeshkumarPSheejaKKuttanGAndrographolide induces apoptosis in B16F-10 melanoma cells by inhibiting NF-κB-mediated bcl-2 activation and modulating p53-induced caspase-3 gene expressionImmunopharmacol Immunotoxicol201234114315121682651
- YanJChenYHeCYangZZLuCChenXSAndrographolide induces cell cycle arrest and apoptosis in human rheumatoid arthritis fibroblast-like synoviocytesCell Biol Toxicol2012281475622012578
- YangSEvensAMPrachandSMitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculataClin Cancer Res201016194755476820798229
- YunosNMMutalipSSJauriMHYuJQHuqFAnti-proliferative and pro-apoptotic effects from sequenced combinations of andrographolide and cisplatin on ovarian cancer cell linesAnticancer Res201333104365437124123004
- UckunFMStewartCFReamanGIn vitro and in vivo activity of topotecan against human B-lineage acute lymphoblastic leukemia cellsBlood19958510281728287742543
