Abstract
Extramedullary hematopoiesis (EMH) usually occurs in hematological disease, but more rarely develops in cases of malignant solid tumors. Due to its features on computed tomography (CT) and magnetic resonance imaging (MRI) that are atypical, EMH in tumor patients might easily be misdiagnosed as metastasis leading to the improper TNM staging and inappropriate therapy. Here, we reported the first case of pleural EMH occurring in a patient with esophageal carcinoma whose pleural lesion was first diagnosed as metastasis and confirmed EMH after the needle biopsy. In addition, a retrospective review was conducted by analyzing patients presented with EMH with malignant solid tumors from PubMed and Medline databases. A total of 42 solid tumor patients with EMH were enrolled, and breast cancer was the most common (n=13, 31.0%), followed by renal carcinoma (n=7, 16.7%) and lung cancer (n=6, 14.3%). A wide variety of body sites may be affected by EMH in malignant solid tumor patients, of which the lymph nodes (n=8, 19.0%) and liver (n=7, 16.7%) were the most common, followed by the kidney (n=6, 14.3%). All patients were diagnosed with EMH by excision, biopsy, or autopsy. Treatment strategies for EMH included surgery (n=25, 59.5%), hydroxyurea (n=1, 2.4%), and blood transfusions (n=2, 4.8%); a further 14 patients (33.3%) were subjected to clinical observation without intervention. Of the patients for whom outcome was reported, 10 patients maintained a good performance status (23.8%) and a further six patients died from the malignant tumor. This was the first study to summarize the presentations of EMH in malignant solid tumors, and our findings might provide some useful guidance for clinical practice, especially for treating patients harboring nonresponse lesions during the antitumor treatment.
Introduction
Extramedullary hematopoiesis (EMH) is defined as the production of normal blood cells outside of the bone marrow.Citation1,Citation2 It is a compensatory mechanism that is closely related to inadequate functioning of medullary hematopoiesis, especially myeloproliferative disorders and hemolytic anemia.Citation1,Citation3 However, there have also been reports of EMH in cases of malignant solid tumors, including breast cancer,Citation4 lung cancer,Citation5 and Kaposi’s sarcoma.Citation6 The majority of patients are generally asymptomatic, but EMH may also manifest as a mass or organomegaly, which can be detected by imaging techniques. There is little information in the literature to guide the management of EMH in cases of malignant solid tumors due to the low incidence of this condition. Furthermore, in the absence of typical imaging characteristics of EMH within cases of malignant tumors, radiologists may misdiagnose EMH as malignancy, affecting clinical decision making.
In the present study, we report a new case of pleural EMH occurring in a patient with esophageal cancer. In addition, we conduct a systematic review of case reports on EMH within malignant tumors in order to improve diagnosis, staging, and treatment of this disorder and better understand its prognosis.
Case report
A 48-year-old Asian man sought medical assessment on October 25, 2016 due to a 1-month history of black stool and progressive difficulty in swallowing. He had no medical history of hematological system disease. Contrast-enhanced computed tomography (CT) scans of the chest revealed a significantly thickened esophageal wall, which was considered to be an esophageal neoplasm (red arrows in ); the pleural soft tissue at the eighth right posterior rib was perceived to be metastasis (yellow arrows in and D). Pathological analysis of a gastroscopic biopsy revealed squamous cell carcinoma (). Physical examination indicated no significant abnormalities. Laboratory studies included a red blood cell count of 3.56×1012/L and a hemoglobin density of 105 g/L. The fecal occult blood test was positive. Moreover, biochemical tests and tumor markers in the patient’s serum were negative.
Figure 1 Changes in CT in the ESCC.
Notes: (A and D) the well-circumscribed mass in right pleura (yellow arrows) and thickened esophageal wall (red arrow) prior to treatment, respectively. (B and E) Reduction in the lesions for the esophageal wall (red arrow) and stabilization of the lesion for the right pleura (yellow arrows) after two cycles of TP chemotherapy. (C and F) Esophageal wall (red arrow) thickness decrease and pleural lesion (yellow arrows) size stability after four cycles of TP chemotherapy.
Abbreviations: CT, computed tomography; ESCC, esophageal squamous cell carcinoma; TP, cisplatin–docetaxel.
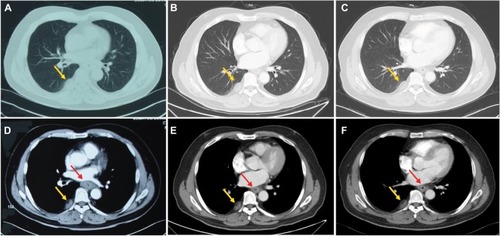
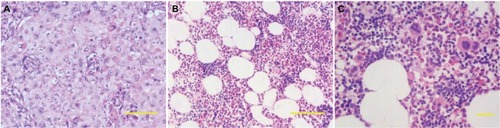
Figure 2 (A) Pathocytology of esophageal tissue revealed squamous cell carcinoma. (B and C) Pathocytology of pleural soft tissue showed a few of lymphocytes, erythropoiesis, megakaryocytes, and degraded cells.
Notes: (A and B) H&E staining ×100. Scale bar: 100 µm and (C) H&E staining ×200. Scale bar: 100 µm.
Abbreviation: H&E, hematoxylin and eosin.
The patient was diagnosed with esophageal squamous cell carcinoma (ESCC) with pleural metastasis based on radiographic examination combined with the biopsy pathology, and the clinical stage was classified as IV (T4bN0M1) according to the 7th edition of the American Joint Committee on Cancer staging guidelines. The patient subsequently underwent a total of four cycles of triweekly chemotherapy, consisting of docetaxel (70 mg/m2 body surface area on days 1 and 8) combined with cisplatin (70 mg/m2 body surface area on days 1–3). According to response evaluation criteria in solid tumors criteria, the patient achieved partial remission and stable disease (SD) of the original esophageal mass after two and four cycles of chemotherapy, respectively (, C, E, and F). However, the right pleural nodule was not observed to change after two therapeutic assessments.
A CT-guided percutaneous needle biopsy of the pleura was performed. Pathological analysis revealed a few lymphocytes, erythropoiesis, megakaryocytes, and degraded cells ( and C). Immunohistochemical findings revealed positive staining for CD68, lysozyme, CD15, CD7, CD20, CD30, CD38, and CD34, while CK7 and CK5 staining was absent, and the Ki-67 index was 80–90%, supporting a diagnosis of EMH (). As the diagnosis of pleural metastasis from esophageal cancer was excluded, the TNM classification of the esophageal tumor was updated to T4bN0M0, stage IIIC. In combination with enhanced CT, intensity-modulated radiation therapy was administered only to the esophageal lesion with the use of X-ray generated at 6 MV. The post-treatment CT and magnetic resonance imaging (MRI) scans, performed 1 month after the completion of the treatments, revealed a decrease in the thickness of the esophageal wall, which was considered to represent a partial response. During the whole course of chemotherapy and radiotherapy, the pleural EMH did not alter in size and the patient had no chest pain or shortness of breath ( and B and 5).
Figure 3 Immunohistochemical of pleural mass revealed EMH.
Notes: Immunohistochemical staining ×200. Scale bar: 200 µm. (A) CD15+; (B) CD20+; (C) CD34+; (D) CD30+; (E) CD38+; (F) CD68+; (G) CD7+; (H) Ki67+ (80–90%); (I) lysozyme+; (J) CK5−; and (K) CK7−.
Abbreviation: EMH, extramedullary hematopoiesis.
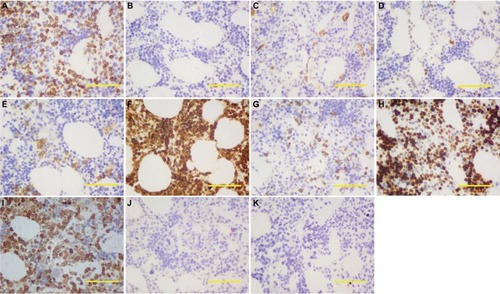
Figure 4 Variations in CT in the ESCC 1 month after finishing all the treatments.
Note: (A and B) The stabilization of the right pleural mass (yellow arrows) and decrease of the thickened esophageal wall (red arrow) after the treatment, respectively.
Abbreviations: CT, computed tomography; ESCC, esophageal squamous cell carcinoma.
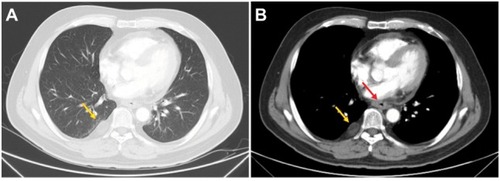
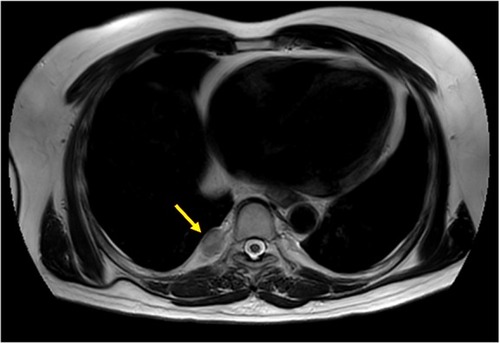
Figure 5 T2W/TSE sagittal MRI image 1 month after the completion of all the treatments, showing no considerable change in the size of the right pleural lesion (yellow arrow).
Abbreviations: MRI, magnetic resonance imaging; T2W/TSE, T2 weighted/turbo spin echo.
This case report was approved by the Institutional Review Board of Shandong Cancer Hospital Affiliated to Shandong University, and the patient has provided written informed consent for permitting the case details and accompanying pictures to be published publicly.
Methods
A retrospective chart review was conducted on all identified case reports. We comprehensively searched the PubMed and Medline databases for articles published between 1980 and 2017, using the search terms “extramedullary haematopoiesis”, “cancer”, and “sarcoma”. Searches were limited to studies on human subjects. All case reports were included according to the eligibility criteria and are presented in . A manual search of references was implemented in relevant articles. The following data were collected from each case report: patient age and gender, type of malignant solid tumor, location of EMH, presence of hematopathy, type of therapy, and reported outcome (Table S1).
Table 1 Eligibility criteria for the literature review
Pure malignant solid tumors were defined as those with no reported hematopathy. Malignant solid tumors complicated by hematopathy included cases of thalassemia, hemolytic anemia, sickle cell anemia, thrombotic thrombocytopenic purpura, and primary myelofibrosis. Pernicious anemia was defined as anemia occurring in malignant tumor patients secondary to nonhematological disease. Diagnosis patterns included excision (defined as surgical removal of the entirety of the mass), biopsy, and autopsy. Treatment strategies included observation (defined as no reported intervention), surgery (defined as complete excision), hydroxyurea, and blood transfusion. If imaging studies reported no change on follow-up or no explicit statement of symptomatology, the outcome was assumed to be SD.
Results
A total of 35 unique articles comprising 42 cases of EMH occurring with a malignant solid tumor were identified in the literature,Citation1,Citation4–Citation37 as shown in Table S1. These patients included 13 males and 28 females, ranging in age from 1 to 84 years (mean, 52.4 years). The most common malignancies were breast cancer (n=13, 31.0%), renal cancer (n=7, 16.7%), and lung cancer (n=6, 14.3%) (). EMH could develop in a variety of tissues and organs, including the lymph nodes (n=8, 19.0%), liver (n=7, 16.7%), and kidney (n=6, 14.3%) ().
Table 2 Summary of the malignant solid tumors
Table 3 Summary of the extramedullary hematopoiesis sites
The majority of patients were asymptomatic, but physical and imaging findings of 13 patients (31.0%) revealed hepatomegaly or splenomegaly (). In 12 of the 42 patients, malignant solid tumors were reported to be combined with hematopathy, including thalassemia (n=1), hemolytic anemia (n=2), sickle cell anemia (n=1), primary myelofibrosis (n=6), thrombotic thrombocytopenic purpura (n=1), and unknown disease (n=1). A further three patients suffered from pernicious anemia. Of the 12 patients with hematopathy, four patients exhibited malignant solid tumors secondary to hematological disease, six patients developed hematological disease secondary to a malignant solid tumor, and two patients were diagnosed with hematological disease and malignant solid tumor simultaneously. However, the mutual mechanisms of EMH, tumor, and hematological disease remain unclear.
Table 4 Summary of data collected from case reports included in the literature review
The majority of the patients (n=27, 64.3%) underwent imaging examinations, including X-ray imaging (n=9, 21.4%), ultrasound (n=11, 26.2%), CT (n=20, 47.6%), MRI (n=5, 11.9%), and 18F-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) (n=3, 7.1%) (). All cases were diagnosed as EMH by pathological examination, including 25 patients through surgery (59.5%), 14 patients through biopsy (33.3%), and three patients through autopsy (7.1%). On pathological examination, tissue composition included pure EMH (n=29, 69.0%) and mixed EMH (n=13, 31.0%), in which EMH was present in the tumor tissues. A total of 10 patients (23.8%) were recorded to receive bone marrow biopsy assay, six of whom were in accordance with the diagnosis of hematological diseases and the remaining patients showed normal marrow ().
Antitumor treatments were administered to 69.4% of patients, and EMH was treated with surgical excision (n=25, 59.5%), hydroxyurea (n=1, 2.4%), blood transfusion (n=2, 4.8%), or clinical observation (n=14, 33.3%). Notably, 25 patients underwent surgery in an attempt to remove the cancer and the subsequent postoperative pathological examination revealed the presence of EMH. Of the patients for whom outcome was reported, 10 patients maintained a good performance status (23.8%) and a further six patients died from the malignant tumor ().
Discussion
EMH refers to the presence of hematopoietic elements outside the bone marrow, causing reduced erythrocyte production or accelerated destruction.Citation1 Although this process may be physiological during the fetal stage, its occurrence after birth is usually considered abnormal.Citation20,Citation38 EMH is typically associated with hematological disease, such as myelofibrosis and hemolytic anemia.Citation1,Citation3 Reports of EMH in cases of malignant solid tumor are less common.
Most patients are asymptomatic; however, any symptoms exhibited correspond to the location of EMH. For example, lung EMH may cause a pneumonia-like process and patients may present with a cough and sputum production;Citation38 serosal EMH may lead to pleural effusion and ascites, with patients presenting with chest congestion, labored breathing, and abdominal distension or pain;Citation38 and paravertebral EMH occupying the epidural space of the spinal canal may lead to the compression of nerve roots, with patients complaining of lumbago.Citation39 In the present study, we identified two cases of pleural effusion and one patient with osphyalgia due to nerve root compression. However, the statistics indicated that symptoms are likely to be atypical in the malignant solid tumors.
The etiology of EMH in patients with solid tumors remains unclear. Granulocyte colony-stimulating factor (G-CSF) may be an inducing factor of EMH.Citation4,Citation9,Citation14,Citation17,Citation21,Citation40 In the present review, it was identified that five patients had been administered G-CSF during chemotherapy and radiotherapy due to chemotherapy-related bone marrow suppression. As a growth factor, G-CSF can stimulate the bone marrow to produce granulocytes, thereby increasing the release of granulocytes into the blood, by promoting stem cell production in the bone marrow.Citation21 Additionally, it has been reported that doxorubicin, which is widely used as a neoadjuvant chemotherapy for breast cancer, is potentially involved in the etiopathogenesis of EMH in animal models without the administration of G-CSF.Citation4,Citation41 Thus, chemotherapeutic agents may also play roles in the pathogenesis of EMH. Furthermore, some abnormal cytokine or paracrine growth factors may be secreted by tumors, which may evoke the differentiation of stem cells into hematopoietic cells and stimulate regional hyperplasia of circulating hematopoietic progenitors.Citation3 Moreover, three cases of pernicious anemia were identified in the present study; the shortage of erythrocytes in these patients could evoke a natural homeostatic response to increase the production of red blood cells by a compensatory mechanism of the bone marrow.Citation3 Thus, the pathogenesis of EMH in cases of malignant solid tumors is very complicated and requires further study.
Among the reviewed cases, the majority of patients (n=27, 64.3%) received imaging examinations. X-ray and ultrasound imaging revealed abnormal masses. CT imaging was performed for 20 patients (47.6%); on this imaging modality, EMH appeared as a well-circumscribed, inhomogeneous, hypovascular mass, often interspersed with areas of fat attenuation and without calcification or bone destruction. EMH can also appear with heterogeneous enhancement on contrast-enhanced CT.5,7,13,15,16,19,27,42–45 Among the five patients who underwent MRI (11.9%), EMH appeared with a higher signal intensity than that of the adjacent normal tissue on T1- and T2-weighted images, with intermediate to high signal intensity particularly noted on T2-weighted images.Citation42–Citation45 EMH also contained lipid components, in which the enhancement was variable.Citation23,Citation24,Citation42–Citation45 The aforementioned imaging features of EMH were atypical and similar to those of tumors, and therefore, EMH can be easily misdiagnosed. Aspiration cytology or biopsy is amenable for the accurate diagnosis of EMH, which had hematopoietic elements, including erythroid, myeloid precursors, and megakaryo-cytes.Citation35,Citation36,Citation38 In this study, 14 of the 27 patients who underwent imaging examination were not initially diagnosed with EMH. This is exemplified by our reported case of esophageal cancer, where the contrast-enhanced CT findings of pleural soft tissue were originally misdiagnosed as pleural metastases. Subsequently, a CT-guided pleural aspiration biopsy led to the correct diagnosis of EMH.
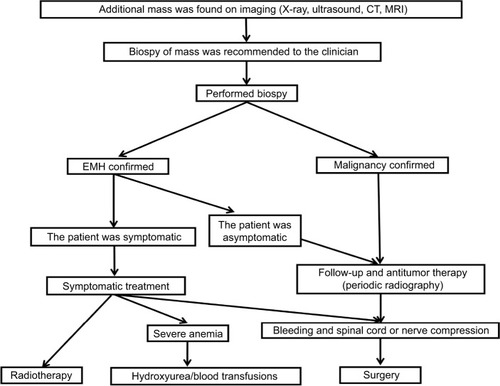
It is usually unnecessary to treat asymptomatic patients with EMH, whereas there are several options for the treatment of symptomatic patients, including hydroxyurea, transfusion, radiotherapy, and surgery.Citation43 In the reviewed cases, 25 (59.5%) patients underwent surgery in an attempt to remove the cancer, and resultant postoperative pathological examination revealed the presence of EMH. All three patients who received hydroxyurea and blood transfusions eventually died from the malignant solid tumor. illustrates the diagnosis and treatment of EMH in cases of malignant solid tumor in a simplified sequence flow diagram.
Figure 6 Process for the diagnosis and treatment of EMH in malignant solid tumors.
Abbreviations: CT, computed tomography; EMH, extramedullary hematopoiesis; MRI, magnetic resonance imaging.
Research indicates that antitumor therapy is crucial in cases of malignant tumor with EMH in order to prevent the development of the cancer and improve the survival of patients. Radiotherapy may result in the remission of symptoms and reduction in the volume of the EMH mass, representing an effective treatment strategy, since the hematopoietic tissue is extremely radiosensitive.Citation1,Citation38 Numerous studies have indicated that EMH does not affect the prognosis of patients, and the follow-up outcomes of EMH are fairly satisfactory in the context of hematological diseases.Citation38 EMH did not seem to affect the outcomes of malignant solid tumor cases according to our retrospective analysis, since all six of the mortalities were due to the malignant solid tumor rather than EMH.
There were several limitations inherent to our study. First, as it is a review of case reports, complete information regarding demographics and follow-up data are lacking. The imaging examinations, diagnostic approaches, and subsequent treatments were often based on the emphasis point of the journal in which the report was published. Finally, the retrospective manner of data collection may have introduced bias. Despite these limitations, this is, to the best of our knowledge, the first study to summarize the presentations of EMH in malignant solid tumors and provide some guidance for clinical practice.
Conclusion
We present a novel case of a patient with esophageal cancer and pleural EMH. Based on the literature review on EMH in malignant solid tumors, various mechanisms for the formation of EMH have been proposed, including induction by G-CSF, stimulation by chemotherapeutic agents, secretion of cytokines, and pernicious anemia. EMH is a benign lesion but can be challenging to diagnose on the basis of imaging findings alone. Biopsy was the most common method for accurately diagnosing the benign and malignant masses. Antitumor therapy should be the main focus for asymptomatic patients. For patients with symptomatic EMH, treatments such as radiotherapy and excision may be considered if symptoms are unremitting.
Acknowledgments
We are grateful to Spandidos Language Editing Service for their contribution in polishing language and thank the patient and his family members who agreed to publish this case.
Supplementary material
Table S1 Summary of the basic characteristics of patients
References
- MeyklerSObstfeldAJhalaNVergaraNGuptaPKPleural mass forming extramedullary hematopoiesis masquerading as a malignant neoplasmDiagn Cytopathol2015431299699926303071
- BowenJMPerryAMQuistEAkhtariMExtramedullary hematopoiesis in a sentinel lymph node as an early sign of chronic myelomonocytic leukemiaCase Rep Pathol2015201559497025960906
- PantanowitzLKupermanMGoulartRAClinical history of HIV infection may be misleading in cytopathologyCytojournal20107720607096
- TakharASNeyAPatelMSharmaAExtramedullary haematopoiesis in axillary lymph nodes following neoadjuvant chemotherapy for locally advanced breast cancerBMJ Case Rep201320138943
- ArdakaniNMKumarasingheMPSpagnoloDVStewartCJExtramed-ullary hematopoiesis associated with organizing peritoneal hemorrhage: a report of 5 cases in patients presenting with primary gynecologic disordersInt J Gynecol Pathol201433331732224681745
- WangJDarvishianFExtramedullary hematopoiesis in breast after neoadjuvant chemotherapy for breast carcinomaAnn Clin Lab Sci200636447547817127738
- TokumitsuSTokumitsuKKohnoeKTakeyaMTakeuchiTExtramedullary hematopoiesis presenting as mediastinal tumorActa Pathol Jpn19803023153227386205
- Yablonski-PeretzTSulkesAPolliackAWeshlerZOkonECataneRSecondary myelofibrosis with metastatic breast cancer simulating agnogenic myeloid metaplasia: report of a case and review of the literatureMed and Pediatr Oncol198513292963982370
- LemosLBBaligaMBenghuzziHACasonZNodular hematopoiesis of the liver diagnosed by fine-needle aspiration cytologyDiagn Cytopathol199716151549034738
- Prieto-GranadaCSetiaNOtisCNLymph node extramedullary hematopoiesis in breast cancer patients receiving neoadjuvant therapy: a potential diagnostic pitfallInt J Surg Pathol201321326426623493877
- CriderSKroszer-HamatiAKrishnanKIsolated pancreatic extramedullary hematopoiesisActa Haematol199899138409490565
- TamiolakisDVenizelosJPrassopoulosPIntrahepatic extramedullary hematopoietic tumor mimicking metastatic carcinoma from a colonic primaryOnkologie2004271656715007251
- DuEOverstreetKZhouWFine needle aspiration of splenic extramedullary hematopoiesis presenting as a solitary mass. A case reportActa Cytol20024661138114212462096
- HsuFIFilippaDACastro-MalaspinaHDowneyRJExtramedullary hematopoiesis mimicking metastatic lung carcinomaAnn Thorac Surg1998664141114139800847
- MillarEKInderSLynchJExtramedullary haematopoiesis in axillary lymph nodes following neoadjuvant chemotherapy for locally advanced breast cancer--a potential diagnostic pitfallHistopathology200954562262319413641
- YangXBhuiyaTEspositoMSclerosing extramedullary hematopoietic tumorAnn Diagn Pathol20026318318712089730
- TalmonGAPure erythropoiesis in clear cell renal cell carcinomaInt J Surg Pathol201018654454620667923
- CelikBBulutTSedeleMSezerCKarakusVExtramedullary hematopoiesis within cystic renal cell carcinoma with oncocytic and chromophobe cell types: A case reportOncol Lett20147390991324520308
- Orphanidou-VlachouETziakouri-ShiakalliCGeorgiadesCSExtramedullary hemopoiesisSemin Ultrasound CT MR201435325526224929265
- TammEPRabushkaLSFishmanEKHrubanRHDiehlAMKleinAIntrahepatic, extramedullary hematopoiesis mimicking hemangioma on technetium-99m red blood cell SPECT examinationClin Imaging199519288917773882
- ArkadopoulosNKyriaziMYiallourouAIA rare coexistence of adrenal cavernous hemangioma with extramedullar hemopoietic tissue: a case report and brief review of the literatureWorld J Surg Oncol200971319193247
- GroismanGMLobular Carcinoma of the Breast Metastatic to the Spleen and Accessory Spleen: Report of a CaseCase Rep Pathol20162016516018027672468
- DekmezianRSneigeNPopokSOrdonezNGFine-needle aspiration cytology of pediatric patients with primary hepatic tumors: a comparative study of two hepatoblastomas and a liver-cell carcinomaDiagn Cytopathol1988421621682854046
- PaydasSSarginOGonlusenGPET CT imaging in extramedullary hematopoiesis and lung cancer surprise in a case with thalassemia intermediaTurk J Haematol2011281606227263943
- ChouSSubramanianVLauHMAchanARenal Anastomosing Hemangiomas With a Diverse Morphologic Spectrum: Report of Two Cases and Review of LiteratureInt J Surg Pathol201422436937323816823
- LewisDJMoulJWWilliamsSCSesterhennIAColonEPerirenal liposarcoma containing extramedullary hematopoiesis associated with renal cell carcinomaUrology19944311061098284868
- VarrasMStylianidoAAkrivisCGalanisPStefanakiSAntoniouNExtramedullary hematopoiesis in the uterine isthmus: a case report and review of the literatureEur J Gynaecol Oncol200223322723012094960
- LaraJFRosenPPExtramedullary hematopoiesis in a bronchial carcinoid tumor. An unusual complication of agnogenic myeloid metaplasiaArch Pathol Lab Med199011412128312852252428
- Policarpio-NicolasMLBregmanSGIhsanMAtkinsKAMass-forming extramedullary hematopoiesis diagnosed by fine-needle aspiration cytologyDiagn Cytopathol2006341280781117115434
- ZornKCOrvietoMAMikhailAASolitary ureteral metastases of renal cell carcinomaUrology2006682428e425427
- BoscoMCarucciPPacchioniDEndoscopic ultrasound-guided fine needle aspiration diagnosis of extramedullary hematopoiesis in mediastinumEndoscopy200941Suppl 2E6719177294
- WrightPKThiryayiSARanaDNFine needle aspiration cytology diagnosis of extramedullary haematopoiesis presenting as a pre-sacral mass: a pitfall avoidedCytopathology201223213313421955299
- VassiliouVPapamichaelDLutzSPresacral Extramedullary Hematopoiesis in a Patient with Rectal Adenocarcinoma: Report of a Case and Literature ReviewJ Gastrointest Cancer201243Suppl 1S13113522318765
- WilliamsonSRMastKJChengLIdreesMTClear cell renal cell carcinoma with intratumoral and nodal extramedullary megakaryopoiesis: a potential diagnostic pitfallHum Pathol20144561306130924704159
- MakoniSNLaberDAClinical spectrum of myelophthisis in cancer patientsAm J Hematol2004761929315114608
Disclosure
The authors report no conflicts of interest in this work.
References
- VassiliouVPapamichaelDLutzSPresacral extramedullary hematopoiesis in a patient with rectal adenocarcinoma: report of a case and literature reviewJ Gastrointest Cancer201243suppl 1S131S13522318765
- MunnRKKramerCAArnoldSMSpinal cord compression due to extramedullary hematopoiesis in beta-thalassemia intermediaInt J Radiat Oncol Biol Phys19984236076099806521
- O’MalleyDPBenign extramedullary myeloid proliferationsMod Pathol200720440541517334344
- WangJDarvishianFExtramedullary hematopoiesis in breast after neoadjuvant chemotherapy for breast carcinomaAnn Clin Lab Sci200636447547817127738
- HsuFIFilippaDACastro-MalaspinaHDowneyRJExtramedullary hematopoiesis mimicking metastatic lung carcinomaAnn Thorac Surg1998664141114139800847
- PantanowitzLKupermanMGoulartRAClinical history of HIV infection may be misleading in cytopathologyCytojournal20107720607096
- MeyklerSObstfeldAJhalaNVergaraNGuptaPKPleural mass forming extramedullary hematopoiesis masquerading as a malignant neoplasmDiagn Cytopathol2015431299699926303071
- BowenJMPerryAMQuistEAkhtariMExtramedullary hematopoiesis in a sentinel lymph node as an early sign of chronic myelomonocytic leukemiaCase Rep Pathol2015201559497025960906
- TakharASNeyAPatelMSharmaAExtramedullary haematopoiesis in axillary lymph nodes following neoadjuvant chemotherapy for locally advanced breast cancerBMJ Case Rep201320138943
- ArdakaniNMKumarasingheMPSpagnoloDVStewartCJExtramedullary hematopoiesis associated with organizing peritoneal hemorrhage: a report of 5 cases in patients presenting with primary gynecologic disordersInt J Gynecol Pathol201433331732224681745
- TokumitsuSTokumitsuKKohnoeKTakeyaMTakeuchiTExtra-medullary hematopoiesis presenting as mediastinal tumorActa Pathol Jpn19803023153227386205
- Yablonski-PeretzTSulkesAPolliackAWeshlerZOkonECataneRSecondary myelofibrosis with metastatic breast cancer simulating agnogenic myeloid metaplasia: report of a case and review of the literatureMed Pediatr Oncol198513292963982370
- LemosLBBaligaMBenghuzziHACasonZNodular hematopoi-esis of the liver diagnosed by fine-needle aspiration cytologyDiagn Cytopathol199716151549034738
- Prieto-GranadaCSetiaNOtisCNLymph node extramedullary hematopoiesis in breast cancer patients receiving neoadjuvant therapy: a potential diagnostic pitfallInt J Surg Pathol201321326426623493877
- CriderSKroszer-HamatiAKrishnanKIsolated pancreatic extramedullary hematopoiesisActa Haematol199899138409490565
- TamiolakisDVenizelosJPrassopoulosPIntrahepatic extramed-ullary hematopoietic tumor mimicking metastatic carcinoma from a colonic primaryOnkologie2004271656715007251
- DuEOverstreetKZhouWFine needle aspiration of splenic extramedullary hematopoiesis presenting as a solitary mass. A case reportActa Cytol20024661138114212462096
- MillarEKInderSLynchJExtramedullary haematopoiesis in axillary lymph nodes following neoadjuvant chemotherapy for locally advanced breast cancer – a potential diagnostic pitfallHistopathology200954562262319413641
- YangXBhuiyaTEspositoMSclerosing extramedullary hematopoietic tumorAnn Diagn Pathol20026318318712089730
- TalmonGAPure erythropoiesis in clear cell renal cell carcinomaInt J Surg Pathol201018654454620667923
- CelikBBulutTSedeleMSezerCKarakusVExtramedullary hematopoiesis within cystic renal cell carcinoma with oncocytic and chromophobe cell types: a case reportOncol Lett20147390991324520308
- Orphanidou-VlachouETziakouri-ShiakalliCGeorgiadesCSExtra-medullary hemopoiesisSemin Ultrasound CT MR201435325526224929265
- TammEPRabushkaLSFishmanEKHrubanRHDiehlAMKleinAIntrahepatic, extramedullary hematopoiesis mimicking hemangioma on technetium-99m red blood cell SPECT examinationClin Imaging199519288917773882
- ArkadopoulosNKyriaziMYiallourouAIA rare coexistence of adrenal cavernous hemangioma with extramedullar hemopoietic tissue: a case report and brief review of the literatureWorld J Surg Oncol200971319193247
- GroismanGMLobular carcinoma of the breast metastatic to the spleen and accessory spleen: report of a caseCase Rep Pathol20162016516018027672468
- DekmezianRSneigeNPopokSOrdonezNGFine-needle aspiration cytology of pediatric patients with primary hepatic tumors: a comparative study of two hepatoblastomas and a liver-cell carcinomaDiagn Cytopathol1988421621682854046
- PaydasSSarginOGonlusenGPET CT imaging in extramedullary hematopoiesis and lung cancer surprise in a case with thalassemia intermediaTurk J Haematol2011281606227263943
- ChouSSubramanianVLauHMAchanARenal anastomosing hemangiomas with a diverse morphologic spectrum: report of two cases and review of literatureInt J Surg Pathol201422436937323816823
- LewisDJMoulJWWilliamsSCSesterhennIAColonEPerirenal liposarcoma containing extramedullary hematopoiesis associated with renal cell carcinomaUrology19944311061098284868
- VarrasMStylianidoAAkrivisCGalanisPStefanakiSAntoniouNExtramedullary hematopoiesis in the uterine isthmus: a case report and review of the literatureEur J Gynaecol Oncol200223322723012094960
- LaraJFRosenPPExtramedullary hematopoiesis in a bronchial carcinoid tumor. An unusual complication of agnogenic myeloid metaplasiaArch Pathol Lab Med199011412128312852252428
- Policarpio-NicolasMLBregmanSGIhsanMAtkinsKAMass-forming extramedullary hematopoiesis diagnosed by fine-needle aspiration cytologyDiagn Cytopathol2006341280781117115434
- ZornKCOrvietoMAMikhailAASolitary ureteral metastases of renal cell carcinomaUrology2006682428e425427
- BoscoMCarucciPPacchioniDEndoscopic ultrasound-guided fine needle aspiration diagnosis of extramedullary hematopoiesis in mediastinumEndoscopy200941suppl 2E6E719177294
- WrightPKThiryayiSARanaDNFine needle aspiration cytology diagnosis of extramedullary haematopoiesis presenting as a pre-sacral mass: a pitfall avoidedCytopathology201223213313421955299
- WilliamsonSRMastKJChengLIdreesMTClear cell renal cell carcinoma with intratumoral and nodal extramedullary megakaryopoiesis: a potential diagnostic pitfallHum Pathol20144561306130924704159
- MakoniSNLaberDAClinical spectrum of myelophthisis in cancer patientsAm J Hematol2004761929315114608
- KochCALiC-YMesaRATefferiANonhepatosplenic extramedullary hematopoiesis: associated diseases, pathology, clinical course, and treatmentMayo Clin Proc200378101223123314531481
- SauerBBuyXGangiARoyCExceptional localization of extramedullary hematopoiesis: presacral and periureteral massesActa Radiol200748224624817354150
- RedmondJ3rdKantorRSAuerbachHESpiritosMDMooreJTExtramedullary hematopoiesis during therapy with granulocyte colony-stimulating factorArch Pathol Lab Med199411810101410157524465
- ComereskiCRPedenWMDavidsonTJWarnerGLHirthRSFrantzJDBR96-doxorubicin conjugate (BMS-182248) versus doxorubicin: a comparative toxicity assessment in ratsToxicol Pathol19942254734887899776
- SohawonDLauKKLauTBowdenDKExtramedullary haematopoiesis: a pictorial review of its typical and atypical locationsJ Med Imaging Radiat Oncol201256553854423043573
- ZhouPPClarkEKapadiaMRA systematic review of presacral extramedullary haematopoiesis: a diagnosis to be considered for presacral massesColorectal Dis201618111033104027329993
- GeorgiadesCSNeymanEGFrancisIRSneiderMBFishmanEKTypical and atypical presentations of extramedullary hemopoiesisAJR Am J Roentgenol200217951239124312388506
- GinzelAWKransdorfMJPetersonJJGarnerHWMurpheyMDMass-like extramedullary hematopoiesis: imaging featuresSkeletal Radiol201241891191622101909
