Abstract
Background
Giant cell tumor (GCT) of bone is an intermittent and locally aggressive tumor with increasing pulmonary metastatic potential. In this study, we evaluated the interim clinical outcome of denosumab in patients with pulmonary metastatic GCT.
Materials and methods
We retrospectively reviewed seven patients with pulmonary metastatic GCT who received denosumab treatment after local tumor surgery during January 2014 and July 2016. Denosumab treatment for all patients lasted for at least 12 months. Serial chest computerized tomography scan was used to monitor the drug response and RECIST 1.1 standard was used to evaluate the therapeutic efficacy.
Results
All patients experienced chest pain relief in the first month of treatment. Three patients showed partial response. Four patients got stable disease after denosumab treatment. Adverse events included one patient with hypocalcemia and two patients with fever. No treatment-related deaths were reported. No patient with metastatic disease progression was found during an average of 28.6 months follow-up period.
Conclusion
We presented a promising interim clinical outcome using denosumab to treat patients with pulmonary metastatic GCT. Denosumab might be considered as the first-line treatment for patients with inoperable metastatic pulmonary GCT. However, Phase II clinical study with larger number of patients and longer follow-up period is needed to detect the further efficacy and safety of this drug for lung metastatic GCT.
Introduction
Giant cell tumor (GCT) of bone is an intermittent tumor that is responsible for ~6% of all primary bone tumors. Reported annual incidence of this tumor ranges between 1 and 6 per 10 million persons and shows a relatively high incidence in Chinese populations.Citation1 It typically affects adults aged between 20 and 40 years, with a slightly higher incidence among females.Citation2 The tumor is locally aggressive but with low metastatic potential despite maintaining a benign histology.Citation3 The most common site of distant metastasis is lung, occurring at a frequency of 1%–9% in all GCT patients.Citation4,Citation5 Because of the unpredictable behavior, no standard treatment for GCT lung metastasis exists, and treatment options vary from metastasectomy, chemotherapy, radiation, or simple observation.Citation6,Citation7 The use of systemic antineoplastic chemotherapeutic agents has been confined to these few patients albeit with limited success.Citation8 There are a lot of reports about bisphosphonates treatment for primary or recurrent GCT which have shown a variable but generally beneficial effect on tumor size. It may reduce the local recurrence rate after surgery.Citation9 However, there are no exact reports about efficacy of bisphosphonate treatment for patients with pulmonary metastatic GCT. Surgery resection might be beneficial for patients with single metastatic lesion or other resectable pulmonary metastatic lesions. In order to avoid rapid increase of metastasis in volume and number, early detection and optimal follow-up observation periods are essential.
Treatment for GCT may considerably change with the advent of denosumab, which is a nuclear factor kappa-B ligand (RANKL) inhibitor. Giant cells in GCT have been confirmed to express RANKL, which causes the local aggressive nature of the tumor. In June 2013, FDA approved the application of denosumab in adults and skeletally mature adolescents with GCT deemed unresectable or requiring morbid surgery.Citation10 But the efficacy of denosumab in pulmonary metastasis is currently unknown. Here, we followed up seven patients with pulmonary metastatic GCT treated in our hospital. These patients received aggressive curettage, bone cement filling, internal fixation for local tumor, and denosumab subcutaneously after surgery. Safety and efficacy of denosumab for these patients are evaluated.
Materials and methods
We retrospectively reviewed the charts of seven patients who underwent denosumab treatment during January 2014 and July 2016. The diagnoses of primary tumor of all patients were histologically confirmed. Specific tumor- and therapy-related data were extracted from medical records, histologic sections, and radiographs for each patient after obtaining institutional review board approval for the study ().
Table 1 Summary of the clinical features of the series
Lung metastasis of GCT was diagnosed when histological examination of the metastatic lesions was confirmed or when radiological images met the following criteria: 1) development of abnormal lesions as single or multiple pulmonary nodules on chest radiography or nodular, rounded, well-defined opacities on chest computerized tomography (CT), and 2) growth either in number or size of the lesions during follow-up.Citation11
Primary tumor locations are three at proximal tibias, two at distal femurs, one at pelvic, and one at distal radius. Local tumor of all seven patients was managed with aggressive curettage and bone graft, or following internal fixation with plate and screws, if it was necessary. Five patients were found to have lung metastasis disease within 2 years after local tumor surgery. Two patients were with pulmonary metastases at the initial diagnosis. Five patients received bisphosphonates treatment prior to denosumab.
Chest CT scan was used to assess lung metastasis lesions in all seven patients. Two patients received thoracoscopic surgery so that metastases of pulmonary lesions were histologically confirmed. After screening for contraindications and informed consent acquisition, patients were treated with denosumab with a dosage of 120 mg subcutaneously on days 1, 8, 15, 28, and every 4 weeks thereafter. All patients took daily supplements of calcium (≥500 mg) and vitamin D (≥400 IU). Responses and toxicity of this drug were assessed every 3 months based on physical examination, patients’ reporting symptoms, and radiological imaging assessments. Adverse events were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Therapeutic efficacy of all patients was evaluated according to their chest CT scans every 3 months. New Response Evaluation Criteria in Solid Tumors (RECIST 1.1) was used to evaluate the therapeutic efficacy. The revised RECIST guideline (version 1.1) was published by the RECIST Working Group in January 2009, based in part on the investigations using the database consisting of >6,500 patients with >18,000 target lesions.Citation12
Ethics approval and informed consent
This study was approved by the Medical Ethics Committee of the West China Hospital. Written informed consent with regard to publication of this article and accompanying images was obtained from all the patients.
Results
There were four male patients and three female patients, with an average age of 33.3 years ranging from 23 to 44 years. The average follow-up period was 22.9 months (ranging from 15 to 36 months; ).
During the first month of treatment, all patients reported significant decrease in chest pain. Four patients reported free of chest pain after 6 months of treatment. The other three patients had significant reduction in chest pain after 6 months. All patients continually responded to treatment radiologically in 3 months. After 1 year, chest CT scans of three patients showed reduction in size and number of lung metastases, indicating partial response according to RECIST1.1. The other four patients got a stable disease and showed no disease progression. During the treatment, three patients (42.9%) reported grade I or II adverse events (fever, pain). No severe adverse events were reported. Up to now, all the seven patients are still under denosumab therapy.
Case report
The third patient with distal radius GCT in was a 29-year-old female. In March 2010, she received curettage, bone graft, and internal fixation with plate and screws for local tumor. Lung metastases were found through chest CT scans 2 years after surgery. Then, she was given bisphosphonates every month for 1 year. This patient started to use denosumab treatment in February 2014, as she experienced disease progression after the bisphosphonates treatment. The dosage of denosumab for this patient was 120 mg subcutaneously on days 1, 8, 15, 28, and every 4 weeks thereafter ().
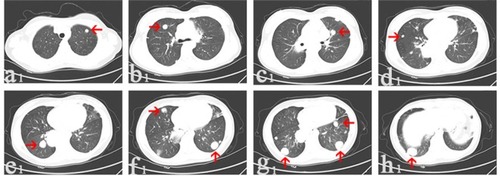
Figure 1 In February 2014, CT scans of the third patient before denosumab treatment showed multi-lung metastatic nodules (arrows).
Notes: The nodule was located on the left lung and grew to 1 cm in diameter (a1); the nodule was 1.2 cm in diameter (b1); the nodule was 1.2 cm in diameter (c1); the nodule was 0.6 cm in diameter (d1); the nodule was 2.2 cm in diameter (e1); the nodules were 1.2 and 2.2 cm in diameter (f1); two nodules were 2.2 cm and the other one was 1.2 cm in diameter (g1); the nodule was 2.4 cm in diameter (h1).
Abbreviation: CT, computerized tomography.
The multi-lung metastatic nodules reduced in size after denosumab treatment. In August 2014, CT scans of this patient indicated a partial response according to RECIST 1.1 (). In March 2015, there was no new measurable nodule and a total of three lesions disappeared on chest CT scans (). In July 2016, that is, over 2 years after denosumab treatment, CT scans showed no new measurable nodules arisen. Compared to the CT scans in March 2015, the number and size of these nodules maintained stability (). For this patient, there were no severe adverse events during denosumab treatment.
Figure 2 In August 2014, CT images collected after denosumab treatment for 6 months showed multi-lung metastatic nodules (arrows) reduced in size and number.
Notes: The nodule reduced to 0.4 cm in diameter (a2); the nodule reduced to 0.6 cm in diameter (b2); the nodule was with lower density than before and reduced to 1.0 cm in diameter (c2); the nodule reduced to 0.4 cm in diameter (d2); the nodule reduced to 1.2 cm in diameter (e2); the nodule on the right side reduced to 1.2 cm in diameter and the one on the left side disappeared (f2); one nodule disappeared and two nodules reduced to 1.0 and 0.8 cm in diameter (g2); the nodule reduced to 1.4 cm in diameter (h2).
Abbreviation: CT, computerized tomography.
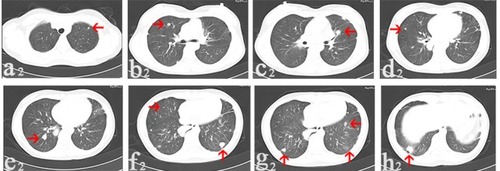
Figure 3 In March 2015, CT images collected after denosumab treatment for 13 months.
Notes: The nodule remained stable in size (a3); the nodule remained stable with size 0.6 cm in diameter (b3); the diameter of the nodule decreased to 0.8 cm (c3); one nodule disappeared on the right side (d3); there was no change in the size of the nodule (e3); the one on the left side reduced to 1.0 cm in diameter (f3); the two nodules reduced to 0.8 and 0.6 cm in diameter, respectively (g3); the nodule reduced to 1.2 cm in diameter (h3). Arrows point to the nodules.
Abbreviation: CT, computerized tomography.
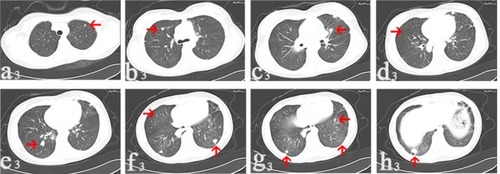
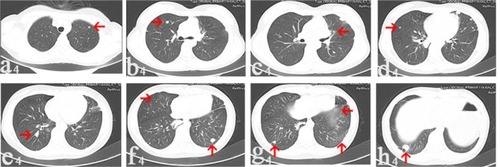
Figure 4 In July 2016, there was no new measurable nodule (arrows) on CT images collected after denosumab treatment for 29 months.
Notes: The nodule on the left side disappeared (g4). Number and size of these metastatic nodules maintain stability on all these eight images (a4, b4, c4, d4, e4, f4, h4), when compared with images in March 2015.
Abbreviation: CT, computerized tomography.
Discussion
GCT patients who are younger, presenting with Enneking grade 3 disease, developing local recurrence, or presenting with axial disease have an increased risk of pulmonary metastasis. The mean interval between primary diagnosis and the onset of lung metastases was 2.8 years in our study, which is similar to those of larger series with a mean interval of 2.0–4.1 years.Citation7,Citation13,Citation14 The mode of treatment was not found to be associated with the development of pulmonary metastasis.Citation6
The previous general consensus is that patients with persistent untreated pulmonary metastases of GCT of bone have a good long-term prognosis and excellent survival rate.Citation13,Citation15,Citation16 However, as GCT have an increasing tendency of pulmonary metastasis in China, early detection of metastasis in GCT with regular long-term follow-up is recommend.Citation4 Treatment for these patients with pulmonary disease is debatable. If possible, after the evaluation by thoracic surgeon, appropriate surgical resection including metastasectomy, wedge resection, or lobectomy might be performed to prevent progressive pulmonary dysfunction.
However, these metastatic pulmonary diseases are sometimes inoperative or surgery resection is aggressive and intolerable for patients. Besides, literature showed that pulmonary metastasectomy and chemotherapy might fail to produce a cure.Citation17 In our study, five patients received bisphosphonates treatment prior to denosumab. Bisphosphonates have demonstrated therapeutic efficacy in osteolytic cancers and bone metastases, and combining with postoperative radiotherapy treatment might lead to a good local tumor control.Citation18 It may be useful in controlling disease progression in GCT, and these agents directly inhibit GCT-derived osteoclast resorption.Citation19 Clinical use of bisphosphonates as adjuvants in treating patients with extremity GCT reduced the local recurrence rates to 4.2% (9% in stage III GCT).Citation20 The drug with proper dose can promote apoptosis of the stromal cell component in GCT and can reduce RANK-ligand expression in GCT stromal cell.Citation21
Denosumab works by binding to RANKL and thus blocking binding to RANK on osteoclasts and osteoclast precursors, therefore inhibiting differentiation of osteoclasts and osteoclast-mediated bone reabsorption. It has been used to treat osteoporosis, bone metastases from solid tumors, and hypercalcemia of malignancy. As the giant cells in GCT also express RANK, denosumab is an attractive target-specific therapy for this tumor.Citation22,Citation23 It is already an effective and useful drug for managing GCT, especially when morbid surgery will be used to get good local tumor control. In addition, the drug has been used successfully for control of metastatic lung disease and may make resection of previously unresectable metastases possible according to a single case report.Citation24 It might be considered as the gold standard for first-line treatment of patients with inoperable or metastatic GCT and can be used to downstage those with metastatic disease requiring aggressive surgery resection, but the timing of the use of neoadjuvant therapy in pulmonary tumors is debatable.Citation25 Our study demonstrates a sustained clinical benefit, including chest pain reduction and radiological disease control, similar to a previous study.Citation26 Approximately 42.9% of patients got partial response according to RECIST 1.1, and the remaining 57.1% patients belonged to stable disease in this study.
Common adverse events after denosumab treatment include fatigue, nausea, dyspnea, and hypocalcemia.Citation27 In our study, denosumab was associated with a decrease in skeletal-related events, possibly reflecting superior inhibition of osteoclasts compared with zoledronic acid because of a different mechanism of action. Tsukamoto et al reported development of high-grade osteosarcoma in a patient with recurrent GCT of the ischium while receiving treatment with denosumab. This finding suggests that the scientific community should be aware of the possible malignant transformation of giant cell tumor of bone during denosumab treatment.Citation28 George reported a case of rapid recurrent following cessation of denosumab therapy. Thus, he concluded that patients need to maintain regular life-long denosumab therapy or definitive surgical treatment should be performed prior to cessation of therapy.Citation29 So, there is a major concern that denosumab withdrawal is associated with a high rate of subsequent progression.Citation29,Citation30
Overall, our study presents a promising interim clinical outcome using denosumab in patients with pulmonary metastatic GCT. Denosumab treatment might produce a surgery chance for those who are with unresectable pulmonary metastatic disease. However, there is still much to do in considering its clinical use for different stages of GCT. The optimal treatment schedule in long-term maintenance therapy with less frequent dosage is not known and should be the subject of ongoing research. Full dataset of large Phase II study is essential to confirm the safety for long-term use. Due to the challenges of treating this metastatic disease and the unanswered questions regarding optimal use of denosumab, referral and follow-up of complicated cases of GCT requiring denosumab should be within expert bone cancer centers.
Acknowledgments
We wish to acknowledge funding in support of Yi Luo by the Support Program for Science and Technology of Sichuan Province, China (2017SZ0106).
Disclosure
The authors report no conflicts of interest in this work.
References
- Beebe-DimmerJLCetinKFryzekJPSchuetzeSMSchwartzKThe epidemiology of malignant giant cell tumors of bone: an analysis of data from the Surveillance, Epidemiology and End Results Program (1975–2004)Rare Tumors200912e5221139931
- MendenhallWMZloteckiRAScarboroughMTGibbsCPMendenhallNPGiant cell tumor of boneAm J Clin Oncol2006291969916462511
- TandraVSKothaKMSatyanarayanaMGVadlamaniKVYerravalliVSynchronous multicentric giant cell tumour of distal radius and sacrum with pulmonary metastasesCase Rep Oncol Med2015201535415826106496
- NiuXZhangQHaoLGiant cell tumor of the extremity: retrospective analysis of 621 Chinese patients from one institutionJ Bone Joint Surg Am201294546146722398741
- BertoniFPresentDSudaneseABaldiniNBacchiniPCampanacciMGiant-cell tumor of bone with pulmonary metastases. Six case reports and a review of the literatureClin Orthop Relat Res1988237275285
- ChanCMAdlerZReithJDGibbsCPJrRisk factors for pulmonary metastases from giant cell tumor of boneJ Bone Joint Surg Am201597542042825740033
- DominkusMRuggieriPBertoniFHistologically verified lung metastases in benign giant cell tumours-14 cases from a single institutionInt Orthop200630649950416909252
- OsakaSToriyamaMTairaKSanoSSaotomeKAnalysis of giant cell tumor of bone with pulmonary metastasesClin Orthop Relat Res1997335253261
- ArpornchayanonOLeerapunTEffectiveness of intravenous bisphosphonate in treatment of giant cell tumor: a case report and review of the literatureJ Med Assoc Thai200891101609161218972907
- GoldenbergMMPharmaceutical approval updatePT2013388443445
- RosarioMKimHSYunJYHanISurveillance for lung metastasis from giant cell tumor of boneJ Surg Oncol2017116790791328650536
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- SiebenrockKAUnniKKRockMGGiant-cell tumour of bone metastasising to the lungs. A long-term follow-upJ Bone Joint Surg Br199880143479460951
- TubbsWSBrownLRBeaboutJWRockMGUnniKKBenign giant-cell tumor of bone with pulmonary metastases: clinical findings and radiologic appearance of metastases in 13 casesAJR Am J Roentgenol199215823313341729794
- ChengJCJohnstonJOGiant cell tumor of bone. Prognosis and treatment of pulmonary metastasesClin Orthop Relat Res1997338205214
- GuptaRSeethalakshmiVJambhekarNAClinicopathologic profile of 470 giant cell tumors of bone from a cancer hospital in western IndiaAnn Diagn Pathol200812423924818620989
- JacopinSViehwegerEGlardYFatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old childOrthop Traumatol Surg Res201096331031320488151
- WolanczykMJFakhrianKAdamietzIARadiotherapy, bisphosphonates and surgical stabilization of complete or impending pathologic fractures in patients with metastatic bone diseaseJ Cancer20167112112426722368
- BalkeMCampanacciLGebertCBisphosphonate treatment of aggressive primary, recurrent and metastatic Giant Cell Tumour of BoneBMC Cancer20101046220799989
- TseLFWongKCKumtaSMHuangLChowTCGriffithJFBisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case-control studyBone2008421687317962092
- ChengYYHuangLLeeKMXuJKZhengMHKumtaSMBisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of boneCalcif Tissue Int2004751717715037971
- HanleyDAAdachiJDBellABrownVDenosumab: mechanism of action and clinical outcomesInt J Clin Pract201266121139114622967310
- XuSFAdamsBYuXCXuMDenosumab and giant cell tumour of bone-a review and future management considerationsCurr Oncol2013205e442e44724155640
- KarrasNAPolgreenLEOgilvieCManivelJCSkubitzKMLipsitzEDenosumab treatment of metastatic giant-cell tumor of bone in a 10-year-old girlJ Clin Oncol20133112e200e20223509309
- GastonCLGrimerRJParryMCurrent status and unanswered questions on the use of Denosumab in giant cell tumor of boneClin Sarcoma Res2016611527651889
- TraubFSinghJDicksonBCEfficacy of denosumab in joint preservation for patients with giant cell tumour of the boneEur J Cancer20165911226990281
- LiptonAStopeckAMoosRVA meta-analysis of results from two randomized, double-blind studies of denosumab versus zoledronic acid (ZA) for treatment of bone metastasesJ Clin Oncol20102815 Suppl9015
- TsukamotoSRighiAVanelDHonokiKDonatiDMErraniCDevelopment of high-grade osteosarcoma in a patient with recurrent giant cell tumor of the ischium while receiving treatment with denosumabJpn J Clin Oncol201747111090109629048579
- MatcukGRJrPatelDBScheinAJWhiteEAMenendezLRGiant cell tumor: rapid recurrence after cessation of long-term denosumab therapySkeletal Radiol20154471027103125712768
- RosarioMTakeuchiAYamamotoNPathogenesis of osteosclerotic change following treatment with an antibody against RANKL for giant cell tumour of the boneAnticancer Res201737274975428179326
