Abstract
Introduction
Breast cancers develop different patterns of sialylation to modulate their tumor-infiltrating lymphocyte (TIL) environment. We studied the relationship between α-2,6 sialyltransferases and the TIL in different breast cancer molecular subgroups.
Materials and methods
Immunohistochemical preparations were made from 39 luminal (LUM), 13 human epidermal growth factor receptor 2-overexpressing (HER2) and 47 triple-negative (TN) breast carcinomas. Targeted proteins included ST6Gal-I, ST6Gal-II, ST6GalNac-I, CD8, CD4 and granzyme-B in both cytotoxic T lymphocytes and NK lymphocytes (CTL/NK).
Results
CTL/NK populations were significantly more frequent in TN than LUM (P <0.001). TN showed a lower level of ST6Gal-I expression than LUM or HER2 (both P > 0.001). ST6GalNac-I expression was lower in LUM than in TN or HER2 (P = 0.002 and P = 0.02, respectively). In HER2, a significant association was found between a low level of ST6Gal-I expression and a high TIL level. In TN, a significant association was observed between a high level of ST6Gal-II expression and a high TIL level.
Conclusion
An increase in infiltrating lymphocytes could be influenced by low expression of ST6Gal-I in HER2 and by high expression of ST6Gal-II in TN breast cancers. Thus, targeting these sialylation pathways could modulate the levels of TIL.
Introduction
To date, the molecular classification of breast cancers (BCs) proposed by Perou et al has been categorized according to the targeted therapy against these tumors. These authors have described three main molecular subtypes: luminal (LUM) BCs that express hormonal estrogen and progesterone receptors (ER+ and PR+) but no human epidermal growth factor receptor 2 (HER2−), overexpressed HER2 BC (HER2; phenotype: ER±PR±HER2+) and triple-negative (TN; phenotype: ER−/PR−/HER2−).Citation1
It is now well established that tumor-infiltrating lymphocytes (TILs) play an important role in chemotherapy and that their stimulation or inhibition can influence the tumor therapeutic response and patient prognosis.Citation2,Citation3 However, cell interactions between cancer cells and the TIL are complex, and our present knowledge represents only the tip of the iceberg. One of these intercellular communication mechanisms likely consists of changes to the cytoplasmic membrane, particularly in glycoproteins. In cancer cells, the most important glycoprotein alteration is sialylation.Citation4
Sialylation consists of adding sialic acids (Sia) on an N- or O-linked glycoprotein. Sialylation is regulated by a balance of two enzyme families: the sialyltransferases, which add Sia, and the sialidases (or the neuraminidases, Neu), which remove Sia. Sialyltransferases are a family of ~20 different enzymes linking Sia to a galactose (Gal) in an α-2,3 position (ST3Gal)or an α-2,6 position (ST6Gal) or to an N-acetylgalactosamine (ST6GalNac). The third sialyltransferase family (ST8) promotes α-2,8 linkage. Four sialidases remove Sia residues: Neu1, localized in lysosomes and the cell surface; Neu2 or Neu4, located in the cytosol and Neu3, located in the cell membrane.Citation5
Alpha-2,6 sialylation is the most commonly observed sialylation mechanism in tumor cells and is associated with an upregulation of both ST6Gal-I and ST6GalNac-I sialyltransferases.Citation6 Two of the most well-characterized substrates of these sialyltransferases are MUC1 and EGFR. Moreover, Wreschner et al demonstrated an association between MUC1 and EGFR.Citation7 Interestingly, Lillehoj et al suggested that both glycoproteins could be associated with Neu, one of the desialylation enzymes.Citation8 These authors suggested that Neu1 could regulate EGFR and MUC1 signaling.Citation7,Citation8 We recently demonstrated that Neu1 and MUC1 have reduced expression in TN compared to LUM tumors, and that EGFR is more expressed in TN than LUM tumors, suggesting that the EGFR-MUC1-Neu1 molecular pathway is complex.Citation9 Intriguingly, in breast cancer, EGFR has been observed to be highly sialylated, and these changes have been shown to influence its metabolic activities and induce chemoresistance.Citation10
Here, we investigated whether TILs and malignant cell sialylation were associated, and we discussed the possibility that this plays a role in the development of resistance to chemotherapy or targeted therapy.
To understand the α-2,6-sialylation pathway and its relationship with TIL, we determined the expression of three α-2,6-sialylidases (ST6Gal-I, ST6Gal-II and ST6GalNac-I), as well as their relationship with different TIL subsets, including CD4, CD8 and CTL/NK cells (granzyme B-positive cells; cytotoxic T/natural killer lymphocytes, respectively), in different molecular breast cancer groups.
Materials and methods
Patient population
Archival paraffin-embedded surgical material and clinical data from 99 women presenting with breast cancer were available for this study. All cases were classified according to immunohistochemical classification by means of a preliminary immunohistochemical study and subsequent confirmation by tissue microarray (TMA). Our data set included 39 luminal carcinomas (LUM, age = 59.3 ± 12.9 years), 13 HER2 over-expressing carcinomas (HER2, age = 58.1 ± 13.1 years) and 47 TN carcinomas (age = 61.7 ± 14.5 years, P = ns). No neo-adjuvant chemotherapy was performed.
This study was performed with the approval of the local ethics committee (Centre de Resources Biologiques de l’Intitut Jean Godinot) and conformed to the Declaration of Helsinki. Written informed consent was provided by all participants and all patients were informed and agreed to contribute to this study.
Histological procedures and Tissue Micro Array (TMA) construction
All surgical specimens were initially fixed in 4% buffered formaldehyde solution for 8–48 h, then embedded in paraffin and cut into 4-µm-thick sections. From these archival formaldehyde/paraffin blocks, we built a TMA to receive a paraffin block to perform all immunohistochemical studies. We used an automated TMA device, Minicore2 (Excilone, Elancourt, France) associated with a needle core 0.6 mm in diameter. We chose 3 distant core needle samples of each donor tumor paraffin block.
Immunohistochemical methods
Immunohistological staining was performed with a Dako Autostainer Link 48® immunostaining system (Dako Denmark A/S, Glostrup, Denmark). After dewaxing, antigenic retrieval was performed using citrate-buffered (pH 6) or EDTA-buffered (pH 9) antigenic retrieval solution at 99°C in a warm bath (EnVision Flex Target Retrieval solutions, high and low pH, Dako). Endogenous peroxidase was inhibited with a hydrogen peroxide phosphate-buffered solution (EnVision Flex Peroxidase Blocking Reagent, Dako). After incubation with the primary antibodies (ST6Gal-I – rabbit polyclonal, dilution 1:400, pH 6, Cliniscience®; ST6Gal-II – rabbit polyclonal, dilution 1:300, pH 6, Cliniscience®; ST6GalNac-I – rabbit polyclonal, dilution 1:500, pH 6, Novus®; CD4 – 4B12 clone, RTU, pH 9, Dako®; CD8 – C8/144B clone, RTU, pH 9, Dako®, and Granzyme B – GrB-7 clone, dilution 1:20, pH 9, Dako®), the immunological reaction was detected with a polymer dextran coupled with secondary antibody and peroxidase for 15 min (EnVision Flex HRP, Dako) and diaminobenzidine for 10 min (EnVision DAB + chromogen, Dako). Counterstaining was performed with hematoxylin for 10 min (EnVision Flex hematoxylin, Dako). Negative controls were obtained using mouse or rabbit IgG1 (Universal Negative Control Mouse or Universal Negative Control Rabbit, Dako) diluted 1:100, in place of the primary antibodies. MDA-MB231 and MCF7 breast cancer culture cells were known to express ST6Gal-I, ST6Gal-II and ST6GalNac-I.Citation11 From MDA-MB231 and MCF7 cells, we performed cell blocks as positive controls. Methods were previously described.Citation12
Immunostaining quantification
The staining results were evaluated by CG and CM based on the intensity and percentage of staining tumor cells, and agreement was reached.
The parametric results were edited as a score by the addition of intensity (0 = none, 1 = weak, 2 = intermediated, 3 = strong) and the percentage of tumor cells (0 = none, 1 = 1%, 2 = 2% to 10%, 3 = 11% to 33%, 4 = 34% to 66% and 5 > 66% to 100%). The range of scores was 0 to 8. Nonparametric results were calculated as positive for a score >5.
Lymphocytes were counted in one hot spot of lymphocytes close to the tumor cells in a high-power field (HPF) and expressed as the number per HPF (400× magnification).
Statistics
The results were expressed as means and standard error. Mann–Whitney and Spearman’s correlation rank tests were performed. A P-value <0.05 was considered significant. WinSTAT® version 2012 (Fitch Software, Bad Krozinger, Germany) and Excel 2013 (Microsoft Corporation, Redmond, WA, USA) programs were used for statistical analysis.
Results
TIL subsets in BCs
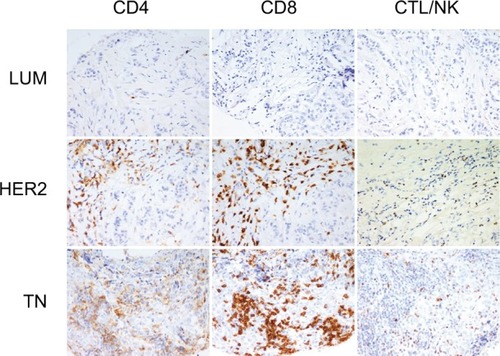
We first determined the molecular subtypes of BC TIL subsets, including CD4, CD8 and CTL/NK (granzyme B-positive) (). The results reveal that cytotoxic lymphocytes were significantly increased in TN compared to LUM (LUM = 1.5 ± 2.2; HER2 = 4.8 ± 7.5 and TN = 9.6 ± 9.9 lymphocytes/HPF; P <0.0001 only for LUM versus TN). Although no statistically significant increase in CD4 (LUM = 18.4 ± 9.5; HER2 = 11.1 ± 15.0 and TN = 14.4 ± 16.6 lymphocytes/HPF) or CD8 (LUM = 22.5 ± 23.1; HER2 = 19.5 ± 27.6 and TN = 28.7 ± 28.5 lymphocytes/HPF) was observed in all molecular BC subgroups, we noted that CD8 was the predominant T-cell population.
Figure 1 Tumor-infiltrating lymphocyte (TIL) subsets in different breast cancer groups.
Notes: CD4, CD8 and granzyme-B peroxidase-positive immunohistochemical staining in LUM, HER2 and TN breast cancers: LUM shows a low TIL level in comparison with HER2 or TN. CD8 is the predominant T-cell population. LUM, HER2 and TN are the same case of . (200× magnification).
Abbreviations: LUM, luminal; HER2, human epidermal growth factor receptor 2; TN, triple negative; CTL/NK; cytotoxic T lymphocytes/natural killer lymphocytes.
Correlation between TIL and ST6Gal-I
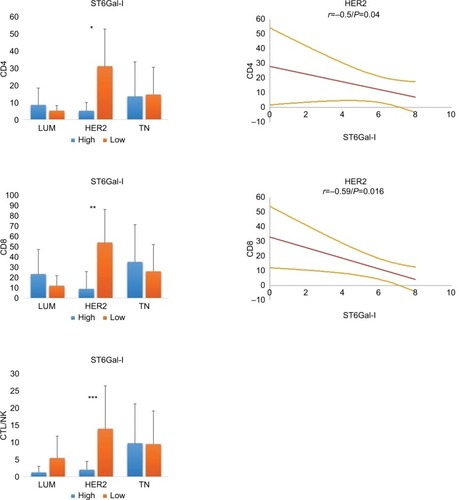
We next evaluated whether ST6Gal-I influenced the TILs in different BCs ( and ). We found that ST6Gal-I expression was significantly lower in TN than in LUM or HER2 (TN: 2.1 ± 3.0 versus LUM: 7.2 ± 0.99, P <0.0001 and TN versus HER2: 6.4 ± 2.3, P <0.0001). Concerning TILs, low levels of ST6Gal-I were correlated with an increase in all lymphocytes subsets only for HER2, with no changes observed in LUM or TN. Only in HER2, Spearman’s correlations were statistically positive and inverse between ST6Gal-I and CD4 (r = –0.5; P = 0.04) or CD8 (r = 0.59; P = 0.016). We conclude that ST6Gal-I plays an important role in HER2 and negatively influences the TIL. Consequently, overexpression of ST6Gal-I in HER2 led to reduced TILs.
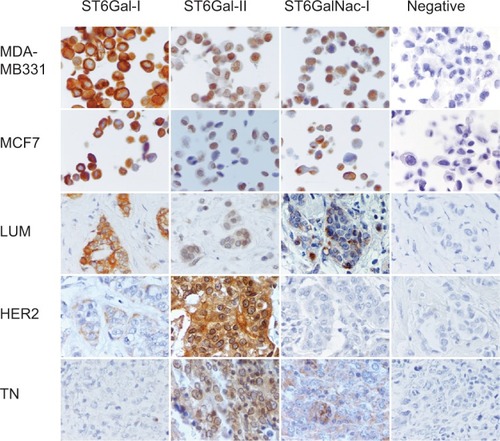
Figure 2 Immunohistochemical expression of ST6Gal-I, ST6Gal-II and ST6GalNac-I.
Figure 3 Relationship between ST6Gal-I and TILs in different breast cancer subtypes.
Notes: In HER2 tumors, low levels of ST6Gal-I are correlated with an increase in all lymphocyte subsets (*CD4: P = 0.01; **CD8: P = 0.02; ***CTL/NK: P = ns). On the right, statistical negative Spearmen’s correlations are documented for CD4 and CD8 positive T lymphocytes in HER2 breast carcinoma (regression line and 95% CI). For LUM and TN, we found no significant correlation. We conclude that high expression of ST6Gal-I in HER2 leads to reduced TILs.
Abbreviations: TILs, tumor-infiltrating lymphocytes; HER2, human epidermal growth factor receptor 2; CTL/NK; cytotoxic T lymphocytes/natural killer lymphocytes; LUM, luminal; TN, triple negative; ns, not significant.
Notes: Peroxidase-positive immunohistochemical staining in breast carcinomas: MDA-MB231 and MCF7 controls are positive for ST6Gal-I, ST6Gal-II and ST6GalNac-I. ST6Gal-I presents a high expression level in LUM. ST6Gal-II and shows a high expression in TN and HER2. ST6GalNac-I is expressed more in TN. ST6Gal-I shows diffuse cytoplasmic expression, ST6Gal-II shows diffuse cytoplasmic and perinuclear expression and ST6GalNac-I shows granular cytoplasmic positivity. LUM, HER2 and TN are the same case of . Negative controls were performed with IgG1 negative control mouse or rabbit (Dako®) (400× magnification).
Abbreviations: LUM, luminal; HER2, human epidermal growth factor receptor 2; TN, triple negative; IgG1, immunoglobulin G1.

Correlation between TILs and ST6Gal-II
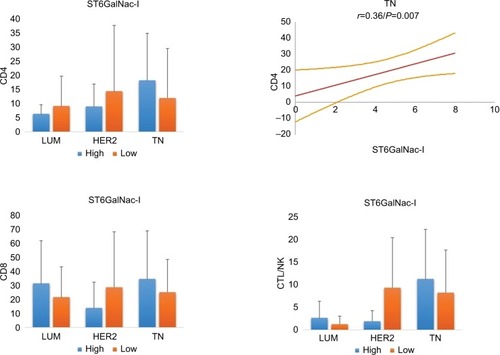
We next characterized ST6Gal-II and found that its expression was similar in all 3 BC groups (LUM: 5.9 ± 1.5; HER2: 6.6 ± 0.6; TN: 5.8 ± 1.8; P = ns) ( and ). Concerning the amount of TILs, we noted that a low expression level of ST6Gal-II was significantly correlated with a decrease in TILs, mainly in the TN group. Only in TN, Spearman’s correlations were statistically positive between ST6Gal-II and CD4 (r = 0.36; P = 0.006) or CD8 (r = 0.41; P = 0.0026) or CTL (r = 0.28; P = 0.02).
Figure 4 Relationship between ST6Gal-II and TILs in different breast cancer subtypes.
Notes: In TN, low levels of ST6Gal-II are significantly correlated with a decrease in lymphocyte subsets (*CD4: P = 0.01; **CD8: P = 0.004; ***CTL/NK: P = ns). On the right, statistical positive Spearmen’s correlations are documented for CD4 and CD8 positive T lymphocytes in TN breast carcinoma (regression line and 95% CI). For LUM and HER2, we found no significant correlation. We conclude that a loss of expression of ST6Gal-II in TN leads to reduced TILs.
Abbreviations: TILs, tumor-infiltrating lymphocytes; TN, triple negative; CTL/NK; cytotoxic T lymphocytes/natural killer lymphocytes; LUM, luminal; HER2, human epidermal growth factor receptor 2; ns, not significant.
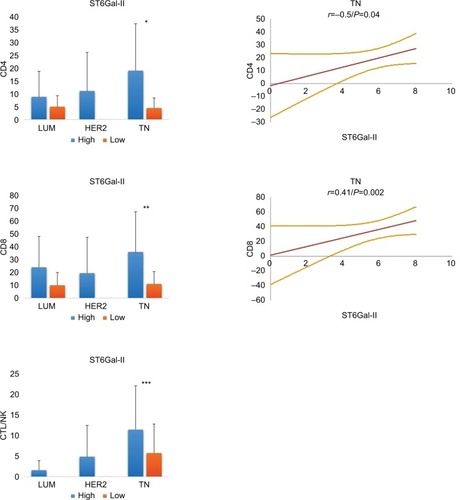
To our knowledge, this is the first time that ST6Gal-II has been described, in situ, in BC.
Correlation between TILs and ST6GalNac-I
ST6GalNac-I is the enzyme associated with the sialylation of Thomsen-nouvelle antigen (Tn), which is localized on the external glycosylated region of MUC1-VNTR ( and ).Citation13 In our previous study, we demonstrated increased expression of MUC1 in LUM compared to TN.Citation9 Here, we observed a difference in expression between LUM and HER2 BCs (2.8 ± 2.7 versus 4.6 ± 2.9, respectively; P = 0.02) and TN (4.6 ± 2.5 respectively; P = 0.002). However, we found only a Spear-man’s correlation between ST6GalNac-I and CD4 for TN (r = 0.36; P = 0.007), suggesting that modulation of this sialylation pathway of MUC1 is not sufficient to have influence on TILs.
Figure 5 Relationship between ST6GalNac-I and TILs in different breast cancer subtypes.
Notes: On the right, statistical positive Spearmen’s correlation is only documented for CD4 positive T lymphocytes in TN breast carcinoma (regression line and 95% CI). For ST6GalNac-I, no other significant correlation was observed, suggesting that ST6GalNac-I does not have a major effect on TILs.
Abbreviations: TN, triple negative; TILs, tumor-infiltrating lymphocytes; CTL/NK; cytotoxic T lymphocytes/natural killer lymphocytes.
Discussion
Sialylation of tumor cells is complex, involving numerous enzymes that play a major role in oncogenesis and associated processes, such as adhesion, migration, apoptosis and receptor regulation. However, this mechanism of sialylation is not well studied, likely due to the absence of sufficient laboratory tools or complicated investigation techniques. To date, immunohistochemistry, which is used in the present study, is still the primary investigational method of choice.Citation5
Here, we evaluated the relationship between α-2,6 sialylation and intratumoral T-cell lymphocytes. We found that sialylation could modulate the TILs and that this cell signaling pattern was altered depending on the molecular signature of the BC. Our observations appear particularly relevant in TN for ST6Gal-II and in HER2 for ST6Gal-I.
Moreover, clinical practice has previously described the importance of TILs in the prognosis or therapeutic response of breast cancers. TILs are heterogeneous lymphocyte subsets that are highly variable in different BC subgroups. Previously, TN and HER2 were found to exhibit increased TILs, which were correlated with better overall survival and better chemotherapeutic responses.Citation2,Citation14,Citation15. Interestingly, we also observed that ST6Gal-I and ST6Gal-II influenced TILs only in HER2 and TN tumors.
Therefore, we propose that the sialyl charge of the membrane glycoproteins could regulate the immune response. First, ST6Gal-I and ST6Gal-II are involved in the α-2,6 sialylation of numerous cell membrane glycoproteins, including EGFR, CD45, β1 integrin, PECAM, Fas and immunoglobulin.Citation16 Second, it has been shown in B16 murine melanoma cell cultures that hypersialylation influences tumor growth and immune escape by alteration of T-cell activity, particularly NK cells, by inhibition of DC maturation.Citation17 Sialylation has also been associated with other immune mechanisms, such as the sialic acid-binding immunoglobulin-like lectins (siglecs), which are a family of receptors linking cancers cells to immune cells. Moreover, Hudak et alCitation16 demonstrated that increasing global sialylated glycans on cancer cells inhibit human NK activation through the recruitment of siglec.Citation6,Citation18
Finally, ST6GalNac-I is an enzyme associated with the sialylation of Tn antigen localized on the external glycosylated region of MUC1-VNTR.Citation13 Moreover, sialyl-Tn is known to play a role in the cellular immunologic regulation of tumor cells.Citation18 It has also been reported that aberrant glycosylation of MUC1 modulates the immunologic environment through the sialic acid and sialic acid-binding immunoglobulin-like lectin (siglec) pathway.Citation19,Citation20 In our study, the relationship between ST6GalNac-I and TIL was not obvious, but the low levels of CTL/NKs in LUM or HER2, which broadly express MUC1 compared to TN (no significant difference between LUM and HER2, data not shown), suggest the loss of ST6GalNac-I sialylation activity due to the absence of MUC1 as a substrate. Similarly, EGFR, which is a substrate of ST6Gal-I and ST6Gal-II, is less expressed in LUM or HER2 tumors than in TN tumors (no significant difference between LUM and HER2, data not shown), which likely explains the different influences of these enzymes on TILs following molecular BC classification.Citation9,Citation12 Other sialylated membrane glycoproteins or others sialylation enzymes could also be involved, such as ST3Gal or ST6GalNac-II, which also use MUC1 as a substrate.Citation21,Citation22
Concerning the relationship between therapeutic resistance or prognosis and sialyltransferases, the most studied enzyme in the literature is ST6Gal-I. Interestingly, in our series, we observed that the loss of ST6Gal-I was associated with a poor prognosis in TN (5-year disease-free survival: 91% for high expression versus 76% for low expression, P = 0.04; data no shown). Park et al demonstrated that loss of ST6Gal-I enhanced EGFR phosphorylation, which was also correlated with the sialylation of EGFR. These authors also showed that the overexpression of ST6Gal-I decreased the effects of gefitinib (an EGFR inhibitor).Citation10 Additionally, several authors have demonstrated the colocalization of MUC1 and EGFR at both the cell membrane and in the nucleus, involving internalization of EGFR and its activation.Citation23,Citation24 Moreover, it is known that both MUC1 and EGFR show severe alterations of their glycosylation patterns, which are correlated with the tumor’s capacity for metastasis.Citation25 It has been demonstrated that ST6Gal-I is associated with chemoresistance to docetaxel in hepatocarcinoma and to gemcitabine in pancreatic ductal adenocarcinoma.Citation26,Citation27 Curiously, EGFR is also described as highly glycosylated, and its sialylation confers resistance to tyrosine kinase inhibitors in lung carcinoma cell lines.Citation28,Citation29 To our knowledge, we report, for the first time, an inverse relationship between TILs and ST6Gal-I in HER2 tumors. As discussed, the high levels of TILs in HER2 tumors are associated with a good prognosis. However, MUC4, a highly glycosylated glycoprotein that can also be sialylated, is also associated with trastuzumab (an HER2 inhibitor) resistance.Citation30,Citation31 Considering these observations, we hypothesize that the sialylation patterns of MUC1, EGFR and likely other membrane glycoproteins could serve as a regulatory signal between TILs and the tumor cells and could be one of the pathways underlying therapeutic resistance.
To date, this is the first study to describe ST6Gal-II expression in human BC in situ. We demonstrated that ST6Gal-II is correlated with TILs in TN tumors. Only one additional report, in bovine breast epithelial cell cultures, has found that ST6Gal-II stimulates pro-inflammatory cytokines, particularly IL-6, known to be active in BCs, therefore suggesting that sialyltransferases could play an important role in the tumor immune microenvironnment.Citation32 In human bronchial mucosa and pancreatic cancer cell lines, IL-6 has been shown to increase the expression of ST6Gal-I and ST6Gal-II respectively, suggesting regulation by the immune system.Citation33
Conclusion
Sialylation, mediated by different sialyltransferases, is one of the most important and complex mechanisms involving cell surface receptors and the relationship between the immune microenvironment and the tumor cells. It is not surprising that sialyltransferases are associated with therapeutic resistance, likely by inhibition of the immune response.
Disclosure
The authors report no conflicts of interest in this work.
References
- PerouCMMolecular stratification of triple-negative breast cancersOncologist201116Suppl 16170
- MiyanMSchmidt-MendeJKiesslingRPoschkeIde BonifaceJDifferential tumor infiltration by T-cells characterizes intrinsic molecular subtypes in breast cancerJ Transl Med20161422727473163
- MaoYQuQChenXHuangOWuJShenKThe prognostic value of tumor-infiltrating lymphocytes in breast cancer: A systematic review and meta-analysisPLoS One2016114e015250027073890
- VajariaBNPatelPSGlycosylation: A hallmark of cancer?Glycoconj J201734214715627975160
- SchultzMJSwindallAFBellisSLRegulation of the metastatic cell phenotype by sialylated glycansCancer Metastasis Rev2012313–450151822699311
- LuJGuJSignificance of β-galactoside α2,6 sialyltranferase 1 in cancersMolecules20152057509752725919275
- WreschnerDHMcGuckinMAWilliamsSJGeneration of ligand-receptor alliances by “SEA” module-mediated cleavage of membrane-associated mucin proteinsProtein Sci200211369870611847293
- LillehojEPHyunSWFengCNEU1 sialidase expressed in human airway epithelia regulates epidermal growth factor receptor (EGFR) and MUC1 protein signalingJ Biol Chem2012287118214823122247545
- GarbarCMascauxCGiustinianiJAutophagy is decreased in triple-negative breast carcinoma involving likely the MUC1-EGFR-NEU1 signalling pathwayInt J Clin Exp Pathol2015854344435526191126
- ParkJJYiJYJinYBSialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cellsBiochem Pharmacol201283784985722266356
- MaXDongWSuZFunctional roles of sialylation in breast cancer progression through miR-26a/26b targeting ST8SIA4Cell Death Dis2016712e256128032858
- GarbarCMascauxCGiustinianiJMerroucheYBensussanAChemotherapy treatment induces an increase of autophagy in the luminal breast cancer cell MCF7, but not in the triple-negative MDA-MB231Sci Rep201771720128775276
- MadsenCBLavrsenKSteentoftCVester-ChristensenMBClausenHWandallHHPedersenAEGlycan elongation beyond the mucin associated Tn antigen protects tumor cells from immune-mediated killingPLoS One201389e7241324039759
- LoiSSirtaineNPietteFPrognostic and predictive value of tumor-infiltrating lymphocytes in a Phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98J Clin Oncol201331786086723341518
- LuenSJSalgadoRFoxSTumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: A retrospective analysis of the CLEOPATRA studyLancet Oncol2017181526227964843
- HudakJECanhamSMBertozziCRGlycocalyx engineering reveals a siglec-based mechanism for NK cell immunoevasionNat Chem Biol2014101697524292068
- PerdicchioMCornelissenLAStreng-OuwehandITumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cellsOncotarget2016788771878226741508
- OgataSMaimonisPJItzkowitzSHMucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicityCancer Res19925217474147461511439
- BeatsonRTajadura-OrtegaVAchkovaDThe mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9Nat Immunol201617111273128127595232
- TanidaSAkitaKIshidaABinding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growthJ Biol Chem201328844318423185224045940
- MarcosNTPinhoSGrandelaCRole of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigenCancer Res200464197050705715466199
- SolatyckaAOwczarekTPillerFMUC1 in human and murine mammary carcinoma cells decreases the expression of core 2 β1,6-N-acetylglucosaminyltransferase and β-galactoside α2,3-sialyltransferaseGlycobiology20122281042105422534569
- BitlerBGGoverdhanASchroederJAMUC1 regulates nuclear localization and function of the epidermal growth factor receptorJ Cell Sci2010123Pt 101716172320406885
- DharmarajNeerajaEngelBJCarsonDDActivated EGFR stimulates MUC1 expression in human uterine and pancreatic cancer cell linesJ Cell Biochem2013114102314232223686469
- SenapatiSDasSBatraSKMucin-interacting proteins: from function to therapeuticsTrends Biochem Sci201043542364519913432
- ChenXWangLZhaoYST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathwayOncotarget2016732519555196427340870
- ChakrabortyADorsettKATrummellHQST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damageJ Biol Chem2018293398499429191829
- YenHYLiuYCChenNYEffect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibitionProc Natl Acad Sci USA2015112226955696025971727
- PeirisDSpectorAFLomax-BrowneHCellular glycosylation affects Herceptin binding and sensitivity of breast cancer cells to doxorubicin and growth factorsSci Rep201774300628223691
- WimanaZGebhartGGuiotTN-acetylcysteine breaks resistance to trastuzumab caused by MUC4 overexpression in human HER2 positive BC-bearing nude mice monitored by 89Zr-Trastuzumab and 18F-FDG PET imagingOncotarget2017834561855619828915583
- LaporteBGonzalez-HilarionSMaftahAPetitJMThe second bovine β-galactoside-α2,6-sialyltransferase (ST6Gal II): Genomic organization and stimulation of its in vitro expression by IL-6 in bovine mammary epithelial cellsGlycobiology200919101082109319617256
- Groux-DegrooteSKrzewinski-RecchiMACazetAIL-6 and IL-8 increase the expression of glycosyltransferases and sulfotransferases involved in the biosynthesis of sialylated and/or sulfated LewisX epitopes in the human bronchial mucosaBiochem J2008410121322317944600
- BassagañasSAllendeHCoblerLOrtizMRLlopEde BolósCPeracaulaRInflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell linesCytokine201575119720625934648
