Abstract
Background
This study aimed to assess the predictive factor of multifocality to identify patients at high risk of central lymph node metastasis (CLNM).
Patients and methods
Papillary thyroid microcarcinoma patients who underwent total or hemi-thyroidectomy with effective unilateral or bilateral central lymph node dissection were enrolled.
Results
Multifocality, age, sex, tumor size, extrathyroidal extension, and nodular goiter were significantly associated with CLNM. Multifocality was an independent predictor for CLNM in multivariate analysis. Compared with unifocal disease, the odds ratio for CLNM was 1.447 for patients with ≥2 tumor foci (P<0.001) and 2.978 for patients with ≥3 tumor foci (P<0.001). The significant association is at ≥3 foci diseases.
Conclusion
Multifocality with ≥3 tumor foci was an independent predictive factor for CLNM in papillary thyroid microcarcinoma. Multifocality should be assessed when selecting patients for prophylactic central neck lymph node dissection, and we speculate that patients with multifocality should undergo more radical treatment.
Introduction
The incidence of papillary thyroid cancer (PTC) has increased significantly worldwide in the past few decades.Citation1–Citation3 Papillary thyroid microcarcinoma (PTMC), a subset of PTCs, is defined as a thyroid carcinoma sized ≤1.0 cm along its greatest diameter.Citation4 PTMC accounts for about 35%–70% of all PTC cases,Citation5–Citation7 and its incidence has dramatically increased in both males and females.Citation8 PTMC in most cases is slow growing, and the majority of patients have a long survival time, although locoregional lymph node recurrence is common. The surgical treatment of PTMC remains controversial, especially in patients with multifocal disease.
Tumor multifocality is observed in approximately 20%–40% of patients with PTMCCitation5,Citation9–Citation12 and can be bilateral or unilateral. However, most staging systems do not include the tumor multifocality. While some studies fail to show a significant association between multifocality and recurrent disease,Citation5,Citation13 multifocality is generally regarded as a risk factor of lymph node metastasis and disease recurrence in PTMC.Citation9–Citation12,Citation14
Despite much attention having been paid to the increasing incidence of PTMC, there is still no consensus on the value of prophylactic central lymph node neck dissection (CLND) in PTMC. According to the 2015 ATA guidelines, thyroidectomy without CLND may be appropriate for small (T1 or T2), noninvasive, clinically node-negative PTC (cN0), but this does not address the issue of whether CLND is of value in PTMC.Citation15 Despite having small tumors, patients with PTMC have high incidence of lymph node metastasis and extrathyroidal extension (ETE); the incidence of central lymph node metastasis (CLNM) ranges from 30% to 60% in these patients.Citation5,Citation16–Citation21 Unfortunately, no preoperative clinical features can reliably identify CLNM, and preoperative sonography has an extremely low sensitivity for CLNM.Citation22 Therefore, it is imperative to develop a suitable approach to identify PTMC patients with a high risk for CLNM who may benefit from CLND.
The relationship between multifocality and CLNM in PTMC remains unclear. Few studies have assessed the association between the number of foci and the demographic characteristics of PTMC. This study aimed to assess the predictive factor of multifocality for CLNM in PTMC, which may improve our ability to identify patients who may benefit from CLND.
Patients and methods
Patient identification
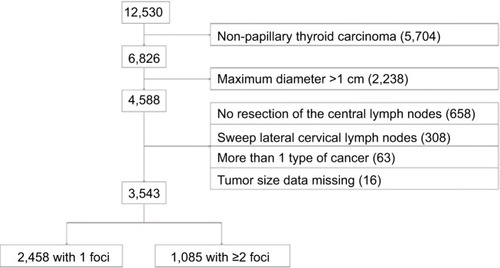
From January 2007 to December 2015, a total of 12,530 patients underwent thyroidectomy at Zhejiang Cancer Hospital. Of them, 6,826 patients were pathologically diagnosed with PTC (PTC >1 cm in diameter was noted in 2,238 patients) and 4,588 with PTMC. Patients who underwent thyroidectomy without effective central lymph node dissection (dissection of at least 1 lymph node) or lateral lymph node dissection with or without central lymph node dissection were excluded. Patients with PTMC and other types of thyroid cancer such as medullary carcinoma or anaplastic thyroid cancer were also excluded. Eventually, 3,543 patients with PTMC who underwent effective central lymph node dissection (dissection of ≥1 lymph nodes) were enrolled in this study. The 3,543 patients were divided into 2 groups: 2,458 patients with 1 lesion and 1,085 patients with ≥2 foci ().
All 3,543 patients in this cohort underwent total or hemi-thyroidectomy with effective unilateral or bilateral central lymph node dissection. If unilateral malignant lesion was present, ipsilateral central neck dissection of lymph nodes was employed; if bilateral malignant lesions were observed, bilateral central neck dissection of lymph nodes was done. The CLND was performed superior to the hyoid bone, inferior to the suprasternal notch, and lateral to the carotid sheaths. This study was approved by the Ethics Committee of Zhejiang Cancer Hospital. Written informed consent was obtained from all patients before surgery, and all operations were performed by experienced surgeons at the same cancer hospital. All patients gave a written informed consent to have their medical records reviewed for this study.
Data collection
Demographic characteristics, including age, sex, tumor size, multifocality, and tumor laterality (unilateral/bilateral), were collected from electronic medical records. According to the TNM staging system, age was dichotomized at 45 years. Tumor size was classified as <5 or ≥5 mm using the largest diameter. Multifocality was defined as ≥2 tumor foci in the same lobe or different lobes including the thyroid isthmus. The presence of ETE, lymphovascular invasion (LVI), lymphocytic thyroiditis, and nodular hyperplasia was recorded according to the pathological findings. In addition, the postoperative complications and prognosis were also evaluated in these patients. The postoperative complications’ information and prognosis were recorded through review of the medical records.
Statistical analysis
Descriptive statistics were employed to summarize study data. Continuous data are expressed as mean ± standard deviation. Statistical analyses were performed using SPSS version 23.0 for Mac (IBM Corporation, Armonk, NY, USA). χ2 test was used to assess the differences between groups in univariate analysis. Logistic regression analysis was applied in multivariate analysis to calculate the odds ratios (ORs) for the associations between CLNM and various clinicopathologic factors. A value of 2-tailed P<0.05 was considered statistically significant. Software Prism 6.0c (Graph Pad Software, Inc., La Jolla, CA, USA) was used to generate figures.
Results
Patients’ characteristics
A total of 3,543 patients with PTMC were enrolled (). There were 2,867 females (80.92%) and 676 males (19.08%) with an average age of 45.70±10.48 years (range, 12–81 years). The average size of the largest focus was 5.97±2.30 mm (range, 0.5–10 mm; median, 6 mm). Multifocality was suspected on ultrasonography in 806 patients, postoperative microscopy indicated multifocality in 1,085 patients, and the consistency rate of multifocality between ultrasonography and microscopy was 74.29% (806/1,085). Overall, from microscopic foci, 2,458/3,543 patients (69.38%) had unifocal PTMC and 1,085 (30.62%) had multifocal PTMC; 767/1,085 patients (70.69%) had 2 foci, 219 (20.18%) had 3 foci, and 99 (9.13%) had ≥4 foci.
The average number of central neck lymph nodes dissected was 4.29±3.23 (range, 1–29). CLNM was found in 1,057/3,543 patients (29.83%), of whom 781/1,057 (73.89%) had 1–2 CLNM, 238 (22.52%) had 3–5 CLNM, and 38 (3.59%) had ≥6 CLNM. ETE was present in 1,487/3,543 (41.97%) patients, LVI in 25/3,543 (0.71%) patients, lymphocytic thyroiditis in 337/3,543 (9.51%) patients, and nodular hyperplasia in 1,163/3,543 (32.83%) patients ().
Table 1 Demographic characteristics of the cohort of patients with PTMC (n=3,543)
Postoperative complications and prognosis
Hypocalcemia was found in 9.23% (327/3,543) of patients, hoarseness in 1.72% (61/3,543) of patients, and bleeding in 0.11% (4/3,543) of patients. Among these patients, 344 were lost to follow-up, recurrence was observed in 51 patients, and 5 patients died (1 died of cerebral hemorrhage).
Univariate analysis
The associations between CLNM and several demographic factors were further investigated. In univariate analysis, CLNM was significantly associated with patients younger than 45 years, males, and patients with a largest focus of ≥5 mm, multifocality, ETE, or nodular hyperplasia (all P<0.001). There were no significant associations of CLNM with bilateral tumors (P=0.813), LVI (P=0.120), and lymphocytic thyroiditis (P=0.187; ).
Table 2 Factors associated with CLNM in PTMC (n=3,543) in univariate analysis using the χ2 test
Multivariate analysis
Binary logistic regression was employed for multivariate analysis to calculate the ORs for CLNM ( and ). Age, sex, size of largest focus, ETE, nodular hyperplasia, and multifocality (≥2 foci) were controlled in 3,543 patients. Multifocality (≥2 foci) was significantly associated with CLNM (OR =1.447, 95% confidence interval [CI] =1.236–1.693; P<0.001). Five other factors were also independent predictors for CLNM (): age (OR =1.462; P<0.001), female sex (OR =0.682; P<0.001), the largest focus ≥5 mm (OR =1.854; P<0.001), ETE (OR =1.552; P<0.001), and nodular hyperplasia (OR =0.826; P=0.023).
Table 3 Factors associated with CLNM in PTMC (n=3,543) in multivariate logistic regression analysis
Table 4 Factors associated with CLNM in multifocal PTMC (n=1,085) in multivariate logistic regression analysis
In subsequent analysis, the association between CLNM and multifocality was evaluated with the same model; patients with ≥3 foci had a significantly higher OR for CLNM than patients with ≤2 foci (multifocal; OR =2.978, 95% CI =2.256–3.932; P<0.001; n=1,085). Similarly, among patients with multifocality, having the largest focus of ≥5 mm (OR =2.432; P<0.001) and ETE (OR =1.474; P=0.005) were significantly associated with CLNM. Being female was also a risk factor for CLNM in multifocal PTMC (OR =1.500; P=0.020). Age (P=0.897) and nodular hyperplasia (P=0.678) were not associated with CLNM in multifocal PTMC (; data not shown).
The association between the number of the foci and CLNM was also assessed. The incidence of CLNM was 26.77% in unifocal disease patients, 29.07% in patients with 2 foci, 47.95% in patients with 3 foci, and 71.72% in patients with ≥4 foci. A significant association existed between multifocality and CLNM in PTMC (P<0.001; ).
Figure 2 Association between CLNM and the number of foci in PTMC (n=3,543).
Abbreviations: CLNM, central lymph node metastasis; PTMC, papillary thyroid microcarcinoma.
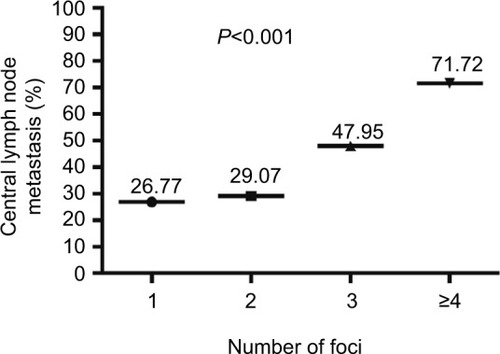
Multifocality was observed in 30.62% of patients with PTMC, and the incidence of CLNM was 36.77% (399/1,085) in multifocal PTMC compared to 26.77% (658/2,458) in patients with unifocal disease. The incidence of CLNM increased with the number of PTMC foci. There was no significant difference in CLNM between unifocal PTMC (26.77%) and PTMC with 2 foci (29.07%). However, the incidence of CLNM was 1.79-fold higher for PTMC with 3 foci (47.95%) and 2.68-fold higher for PTMC with ≥4 foci (71.72%), compared to unifocal disease. Multivariate analysis confirmed that multifocality (≥2 foci) was significantly associated with CLNM (OR =1.447). Furthermore, patients with ≥3 foci had a higher risk of CLNM than patients with 2 foci (OR =2.978; P<0.001).
Discussion
This study investigated the relationship between multifocality and CLNM in PTMC. This study reveals that multifocality is significantly associated with CLNM in PTMC. The high incidence of CLNM for patients with multifocal PTMC may indicate higher tumor aggressiveness and a higher risk of regional recurrence.
Consensus regarding the association between multifocality and CLNM has not yet been reached. Similar to our study, Afif et alCitation23 reported an association between the number of foci in PTC and CLNM (P<0.001; OR =2.355 for ≥3 tumor foci; P=0.026), although PTMC was not analyzed as a separate subgroup. A meta-analysis by Qu et alCitation24 revealed that CLNM was associated with multifocality in PTMC (relative risk =1.40, P=0.001). Guo et alCitation17 reported the presence of 2, 3, or ≥4 foci was associated with a significantly higher risk of CLNM in PTMC (OR =1.675, 2.360, and 2.703, respectively) compared to unifocal PTMC. Similar findings have been reported by other studies.Citation19,Citation20 In contrast, multifocality was significantly related to CLNM in univariate analysis (P=0.020) but not in multivariate analysis (P=0.138) in the study by Zhou et al,Citation21 and Lee et alCitation18 suggested multifocality was not associated with CLNM and did not significantly influence the predictive factor of CLNM. It is possible that the differences in sample size and selection criteria among studies may explain the discrepancies. Our study indicates that multifocality is significantly associated with CLNM in PTMC.
Prophylactic CLND in the management of PTC remains controversial, especially in PTMC.Citation25 Chang et alCitation16 reported 39% (239/613) of patients with PTMC had CLNM, and they recommended prophylactic CLND for all patients. In contrast, Wada et alCitation26 stated only patients with palpable lymph nodes should undergo prophylactic CLND. In the last decade, patients with PTMC in our hospital have routinely undergone prophylactic CLND, regardless of the presence of palpable lymph nodes. However, it would be desirable to identify subgroups of patients with less aggressive PTMC at low risk of CLNM who could avoid prophylactic CLND.
The reported incidence of CLNM in PTMC ranges from 30% to 60%.Citation5,Citation16–Citation21 In this study, 29.83% of patients had CLNM, which is lower than previously reported. This could be attributed to the fact that most patients had earlier stage disease and because patients who underwent lateral lymph node dissection were excluded from this study. Several studies have identified lymph node metastasis, including lateral lymph node metastasis, as a prognostic factor for poor outcome in PTMC.Citation9–Citation12,Citation14,Citation27 Mercante et alCitation14 found that lymph node metastasis was an independent risk factor for recurrence or distant metastasis (P<0.05). Usluogullari et alCitation12 reported that lymph node metastasis was an independent predictor of recurrence in multivariate analysis (OR =51.4; P=0.003). Agarwal et alCitation27 stated that lymph node metastasis was associated with more aggressive disease, recurrence, and poorer prognosis. Multifocality has also been associated with aggressive disease and poor prognosis in PTMC. Chow et alCitation9 found the risk of cervical lymph node recurrence was 6.2-fold (P<0.01) and 5.6-fold (P<0.02) higher if lymph node metastasis and multi-focal disease were present at diagnosis. Qu et alCitation11 reported that the presence of >3 tumor foci was an independent predictor of recurrence in multivariate analysis (HR =2.60, P=0.001). However, Mehanna et alCitation13 failed to find that tumor multifocality and lymph node involvement were significantly associated with recurrence in PTMC. Kim et alCitation5 found that multifocality was an independent risk factor for disease recurrence in PTC but not in PTMC. Further studies are required to define the prognostic value of CLNM in PTMC in the absence of lateral lymph node metastasis as a confounding factor. Moreover, large, multicentered studies with longer follow-up period are warranted to draw definitive conclusions.
In this study, CLNM was significantly associated with age, sex, tumor size, multifocality, ETE, and nodular hyperplasia in univariate analysis. Multivariate analysis confirmed that all these factors are independent predictive factors for CLNM in PTMC, in agreement with previous findings.Citation20,Citation28–Citation30 Moreover, bilateral tumors, LVI, and lymphocytic thyroiditis were not significantly associated with CLNM in this study, consistent with previously reported studies.Citation31,Citation32 Otherwise, coexistence of thyroiditis was previously identified as a negative independent predictor for CLNM.Citation33,Citation34 However, in the analysis of patients with multifocal PTMC, age and nodular hyperplasia were not found to be independent predictors for CLNM. It is difficult to explain the role of sex as a predictor for CLNM. In the entire cohort, being female (OR =0.682, P<0.001) was associated with reduced risk of CLNM, whereas being female was a risk factor for CLNM in multifocal PTMC (OR =1.500, P=0.020). Similar reports have not been published on the effect of sex on the risk of CLNM, and the predictive factor of sex for CLNM in multifocal PTMC still needs to be further elucidated in future studies.
Although a large number of patients were included, there were still limitations in this study. First, the retrospective nature precluded the identification of the mechanistic relationships between prognosis and multifocality or CLNM. Second, all patients were treated at a single hospital. Third, a number of patients did not undergo CLND due to the low rate of ultrasonography-guided preoperative fine-needle aspiration in PTMC; these patients were diagnosed using the final pathology as having incidental PTMC and excluded from this study. In general, incidental PTMC has features suggestive of less aggressive disease and a lower incidence of CLNM. Although this bias does not affect the association between multifocality and CLNM, the incidence of CLNM reported in this study may be lower than that in other studies of PTMC. Moreover, the relationship between lateral lymph node metastasis and multifocality was not assessed in this study. Lastly, the impact of molecular marker such as a determinant of sensitivity to proteasome inhibitors (BRAFV600E) on multifocality and CLNM requires further research.
Conclusion
This study indicates that multifocality is associated with an increased risk of CLNM in PTMC. Multifocality with ≥3 tumor foci was an independent predictor for CLNM. In addition to the size of the largest focus and ETE, multifocality should be considered when selecting subgroups of patients with PTMC for prophylactic CLND. We recommend that patients with multifocality should receive more radical treatment.
Acknowledgments
We thank all the patients who participated in this study. This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LQ14H160002, LY15H160008).
Author contributions
WHZ and JBS conceived and designed the experiments; WHZ performed the experiments; WHZ analyzed the data; KJW, JZW, WDW, and JBS contributed reagents, materials, and analysis tools; and WHZ wrote the paper. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- McNallyRJBlakeyKJamesPWGomez PozoBBastaNOHaleJIncreasing incidence of thyroid cancer in Great Britain, 1976–2005: age-period-cohort analysisEur J Epidemiol201227861562222760704
- MorrisLGSikoraAGTostesonTDDaviesLThe increasing incidence of thyroid cancer: the influence of access to careThyroid201323788589123517343
- WangYWangWIncreasing incidence of thyroid cancer in Shanghai, China, 1983–2007Asia Pac J Public Health2015272NP223NP22922345304
- SobinLHHistological typing of thyroid tumoursHistopathology1990165513
- KimKJKimSMLeeYSChungWYChangHSParkCSPrognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patientsAnn Surg Oncol201522112513125092159
- KutlerDICrummeyADKuhelWIRoutine central compartment lymph node dissection for patients with papillary thyroid carcinomaHead Neck201234226026321416550
- LeboulleuxSTuttleRMPaciniFSchlumbergerMPapillary thyroid microcarcinoma: time to shift from surgery to active surveillance?Lancet Diabetes Endocrinol201641193394227550849
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- ChowSMLawSCChanJKAuSKYauSLauWHPapillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocalityCancer2003981314012833452
- LombardiCPBellantoneRDe CreaCPapillary thyroid micro-carcinoma: extrathyroidal extension, lymph node metastases, and risk factors for recurrence in a high prevalence of goiter areaWorld J Surg20103461214122120052467
- QuNZhangLJiQHNumber of tumor foci predicts prognosis in papillary thyroid cancerBMC Cancer20141491425471041
- UsluogullariCAOnalEDOzdemirEA retrospective analysis of prognostic factors predictive of lymph-node metastasis and recurrence in thyroid papillary microcarcinomaMinerva Endocrinol2015401152224699706
- MehannaHAl-MaqbiliTCarterBDifferences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-upJ Clin Endocrinol Metab20149982834284324828487
- MercanteGFrasoldatiAPedroniCPrognostic factors affecting neck lymph node recurrence and distant metastasis in papillary micro-carcinoma of the thyroid: results of a study in 445 patientsThyroid200919770771619348581
- HaugenBRAlexanderEKBibleKC2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancerThyroid2016261113326462967
- ChangYWKimHSKimHYLeeJBBaeJWSonGSShould central lymph node dissection be considered for all papillary thyroid microcarcinoma?Asian J Surg201639419720125913730
- GuoYLiuZYuPUsing foci number to predict central lymph node metastases of papillary thyroid microcarcinomas with multifocalityInt J Clin Exp Med2015869925993026309677
- LeeSHLeeSSJinSMKimJHRhoYSPredictive factors for central compartment lymph node metastasis in thyroid papillary microcarcinomaLaryngoscope2008118465966218176339
- LimYCChoiECYoonYHKimEHKooBSCentral lymph node metastases in unilateral papillary thyroid microcarcinomaBr J Surg200996325325719224514
- WangWHXuSYZhanWWClinicopathologic factors and thyroid nodule sonographic features for predicting central lymph node metastasis in papillary thyroid microcarcinoma: a retrospective study of 1,204 patientsJ Ultrasound Med201635112475248127794131
- ZhouYLGaoELZhangWFactors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective studyWorld J Surg Oncol2012106722540396
- KhokharMTDayKMSangalRBPreoperative high-resolution ultrasound for the assessment of malignant central compartment lymph nodes in papillary thyroid cancerThyroid201525121351135426431908
- Al AfifAWilliamsBARigbyMHMultifocal papillary thyroid cancer increases the risk of central lymph node metastasisThyroid20152591008101226161997
- QuNZhangLJiQHRisk factors for central compartment lymph node metastasis in papillary thyroid microcarcinoma: a meta-analysisWorld J Surg201539102459247026099728
- WongKPLangBHThe role of prophylactic central neck dissection in differentiated thyroid carcinoma: issues and controversiesJ Oncol2011201112792921977029
- WadaNDuhQYSuginoKLymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissectionAnn Surg2003237339940712616125
- AgarwalSAgarwalAChandGIncidental papillary microcarcinoma of the thyroid – further evidence of a very low malignant potential: a retrospective clinicopathologic study with up to 30 years of follow-upAnn Surg Oncol201118Suppl 3S30621710325
- AhnBHKimJRJeongHCLeeJSChangESKimYHPredictive factors of central lymph node metastasis in papillary thyroid carcinomaAnn Surg Treat Res2015882636825692116
- SiddiquiSWhiteMGAnticTClinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinomaThyroid201626680781527117842
- SoYKSonYIHongSDSubclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resectionsSurgery2010148352653120189620
- QuNZhangLLinDZJiQHZhuYXWangYThe impact of coexistent Hashimoto’s thyroiditis on lymph node metastasis and prognosis in papillary thyroid microcarcinomaTumour Biol20163767685769226692097
- WreesmannVBNixonIJRiveraMPrognostic value of vascular invasion in well-differentiated papillary thyroid carcinomaThyroid201525550350825748079
- KimSSLeeBJLeeJCCoexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma: the influence of lymph node metastasisHead Neck20113391272127721837696
- PaulsonLMShindoMLSchuffKGRole of chronic lymphocytic thyroiditis in central node metastasis of papillary thyroid carcinomaOtolaryngol Head Neck Surg2012147344444922547555