Abstract
Pioglitazone has been reported to increase the risk of bladder cancer but the conclusions of published clinical studies are confusing. We conducted a systematic review and meta-analysis of all eligible randomized controlled trial (RCT) studies and observational studies, in order to identify a more precise relationship between pioglitazone and risk of bladder cancer. We searched for publications up to January 24, 2018, in PubMed, EMBASE, Scopus, Web of Science, Cochrane register, and Chinese National Knowledge Infrastructure databases, and the references of the retrieved articles and relevant reviews were also checked. Relative risk and 95% confidence interval (CI) were used to assess this correlation. A dose-related meta-analysis was performed as well. Data on RCT studies showed a null association between pioglitazone and bladder cancer. The pooled RR estimates of the 12 included studies illustrated that pioglitazone is associated with a 14% increased risk of bladder cancer (95% CI 1.03–1.26). No evidence of publication bias was detected. In the dose effect analysis, patients who used a higher dose of pioglitazone had an increased risk of bladder cancer. In conclusion, this meta-analysis indicated that pioglitazone is associated with an increased risk of bladder cancer. Further research should be conducted to confirm our findings and reveal the potential biological mechanisms.
Introduction
Bladder cancer has become a common cancer worldwide and ranks as the ninth most frequently diagnosed cancer. In 2012, approximately 430000 new cases of bladder cancer were diagnosed.Citation1 Cigarette smoking, specific exposure to arylamine, and chronic schistosoma infection are the most associated factors with increased risk for bladder cancer.Citation2 In recent years, diabetes mellitus (DM) has been reported to increase the risk of bladder cancer.Citation3,Citation4 Meanwhile, pioglitazone, an antidiabetic agent of the thiazolidinedione (TZD) class, which is broadly used for glycemic control in patients with type 2 diabetes mellitus (T2DM), has been reported to increase the risk of bladder cancer as well. At first, the risk was reported in male rats,Citation5 then, a series of clinical studies was performed to identify this relationship. However, the conclusions of published observational clinical studies remain confusing.Citation6–Citation25 This may result from problems associated with study design, limited sample size, and a lack of attention to potential biases. In 2016, one randomized controlled trial (RCT) (PROactive) ended its 10-year observational follow-up drawing a conclusion that the imbalance in bladder cancer cases observed during the double-blind period did not persist, and there was no overall increase in malignancies.Citation26 But only one RCT result could not end the argument. In 2017, another RCT (TOSCA.IT) also reported null association between pioglitazone and bladder cancer, but the number of bladder cancer cases in this RCT was only 16, which may not be convincing.Citation27 A large cohort study including 193099 people by Lewis et al also reported no statistically significant increased risk of bladder cancer associated with pioglitazone use.Citation8 However, the updated US Food and Drug Administration review in 2016 concluded that use of T2DM medicine pioglitazone may be linked to an increased risk of bladder cancer,Citation28 which conflicted with the newly updated RCT results by Erdmann et al,Citation26 Vaccaro et al,Citation27 and cohort study by Lewis et al.Citation8 In the most recently updated meta-analysis conducted by Davidson and Pan,Citation29 they suggested the resurrection of the use of pioglitazone, but failed to perform sensitivity analysis and simply pooled observational studies together without excluding the duplicate population and low-quality studies ().Citation16,Citation17,Citation20–Citation22 In another recent meta-analysis by Filipova et al,Citation30 they failed to give any details about quality assessment and simply pooled RCT and observational studies together without excluding the duplicate population and low-quality studies. They did not perform subgroup analysis either, though his result showed significant heterogeneity. Other meta-analyses are not convincing mainly because inadequate evidence acquisition, poor methodology, and lack of appropriate quality assessment. Consequently, despite a number of observational studies and previous reviews,Citation29,Citation31–Citation33 whether pioglitazone increases the risk of bladder cancer or not still remains an enigma. We conducted a systematic review and meta-analysis of all eligible RCTs, cohort studies, and nested case–control studies about pioglitazone use in patients with diabetes and bladder cancer in order to identify a more precise and reliable relationship between pioglitazone and risk of bladder cancer. Furthermore, we also examined whether the association between them converts according to sensitivity analysis.
Materials and methods
Publication search
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.Citation34 We carried out a search in PubMed, EMBASE, Scopus, Web of Science, Cochrane register, and Chinese National Knowledge Infrastructure databases, covering all the eligible papers published up to January 24, 2018. The search strategy included terms for outcome (bladder neoplasm* or bladder cancer* or bladder tumor* or bladder tumour*) and exposure (pioglitazone* or TZD* or thiazolidinedione*). We carefully evaluated every potentially relevant publication by examining their titles and abstracts. All the studies matching the inclusion criteria were retrieved. The references from retrieved articles and reviews were also thoroughly checked to identify any additional relevant studies. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Inclusion criteria
Studies included in this meta-analysis had to meet all the following criteria: a) an RCT design or a cohort design or a nested case–control design; b) the population included in studies were patients with diabetes; c) one of the exposures of interest was pioglitazone; d) one of the outcomes of interest was incidence of bladder cancer and the result is recorded in a reliable database or using ICD codes of cancer; e) studies provided rate ratio, hazard ratio (HR) or standardized incidence ratio (SIR) with their 95% confidence intervals (CIs), or sufficient data to calculate them; and f) they were of high quality according to the Newcastle–Ottawa scale (NOS) (more than 6)Citation35 or were assessed to have low risk of bias, using the recommended tool in the Cochrane Handbook for Systematic Reviews of Interventions.Citation36 Research on mortality rates of bladder cancer was not included because it could be unpredictably confounded by survival-related factors. We also did not consider studies in which the population included healthy people because diabetes was also considered to increase the risk of bladder cancer. If multiple publications from the same study population were available, the most recent and detailed study was entitled to be included in this meta-analysis.
Data extraction
Two authors extracted data independently by using a predefined data collection form, with disagreements being resolved by consensus. For each study, the following characteristics were collected: first author’s name, year of publication, the country in which the study was carried out, participant characteristics (age and sex), study design, study population, range for follow-up, number of events and non-event subjects exposed to pioglitazone and comparison, medication use in comparison group, estimate effects with their 95% CIs and covariates adjusted for in the analysis. From each of the studies, we optionally excerpted the relative risk estimate that was adjusted for the greatest number of potential confounders. In dose effect analysis, we defined low dose as ≤8268 mg or ≤10500 mg or ≤14000 mg; moderate dose as 10501–28000 mg or 14001–40000 mg; high dose as >28000 mg or >40000 mg.
Quality assessment of searched papers was also undertaken independently by two authors according to the NOS for observational studies, and Cochrane Tool Review Manager 5.3 for RCTs. The disagreements between authors were resolved by discussion and consensus.
Statistical methods
Studies that reported various measures of RR were included in this meta-analysis: rate ratio, HR, and SIR. In practice, these three measures of effect yield similar estimates of RR because the absolute risk of bladder cancer in patients with diabetes is low.
Overall RR estimates with their corresponding 95% CIs were calculated with the DerSimonian and LairdCitation37 random effects models, which consider both within-study and between-study variation. Heterogeneity among studies was measured by Q test and quantified by I2 with higher value indicating a greater degree of heterogeneity. Random effects model was chosen to analyze the data.Citation38 Publication bias was assessed using Begg’s testCitation39 (rank correlation method) and Egger’s test (linear regression method).Citation40 P<0.05 was considered to be representative of a significant statistical publication bias. Dose effect analysis of pioglitazone was conducted if data were sufficient. Sensitivity analyses were also performed to assess the stability of obtained results with a single study deleted each time to manifest the influence of the individual dataset to the pooled RR. All of the statistical analyses were performed with STATA 14.0 (StataCorp LP, College Station, TX, USA), using two-sided P-values.
Results
Literature search
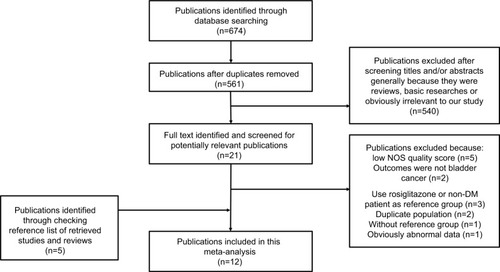
outlines our study selection process. Briefly, after removing duplicates, the search strategy generated 561 articles. Of these, the majority was excluded after the first screening based on abstracts or titles, mainly because they were reviews, basic research, case–control studies, or obviously not relevant to our analysis. After full-text review of 21 papers, 14 studies were excluded for the following reasons: low NOS quality score;Citation17,Citation18,Citation20,Citation41,Citation42 outcomes were not bladder cancer;Citation43,Citation44 duplicate population;Citation16,Citation22 used rosiglitazone as reference group;Citation45 used non-DM people as reference group; Citation46,Citation47 without reference;Citation11 obviously abnormal data (details of exclusion criteria in ).Citation23 We also identified five publications through checking reference lists of retrieved studies and reviews. Thus, a total of 12 cohort studies or nested case–control studies,Citation7–Citation10,Citation12–Citation15,Citation19,Citation23–Citation25 which met the inclusion criteria, were included in the meta-analysis. Two RCT studiesCitation26,Citation27 were also identified in this process and were systematically reviewed.
Data obtained from RCTs
Two RCT studies, PROactiveCitation26 and TOSCA.IT,Citation27 which reported bladder cancer cases in patients with diabetes, were eligible for this study. As it is meaningless to perform a meta-analysis for two studies, we systematically reviewed them. Characteristics including population, interventions, years of follow-up, and number of cases are shown in . Risk of bias assessment of the studies is demonstrated in Figure S1.
The PROactive study was performed in patients with T2DM who were aged 35–75 years. Among a total of 5238 patients who were enrolled in the PROactive study, 2605 patients were randomized to the pioglitazone group and 27 bladder cancer cases were identified finally after double-blind period +10-year observational follow-up. Meanwhile, 26 cases were reported in the placebo group which consisted of 2633 T2DM patients who took placebo. The authors found no significant association between pioglitazone and bladder cancer with RR and 95% CI 1.05 (0.61–1.79). The other study, TOSCA.IT, was a multicenter prospective, randomized, open label, blinded end point designed. The authors reported eight cases in metformin + pioglitazone (n=1535) and metformin + sulfonylurea group (n=1493). Similar to the PROactive study, no significant relationship was detected. Both studies were assessed to have a low risk of bias (Figure S1).
Data obtained from observational studies
Study characteristics
The characteristics of the 12 observational studies are presented in . Of these, eightCitation6,Citation8,Citation10,Citation12,Citation14,Citation15,Citation19,Citation25 were cohort studies and fourCitation7,Citation9,Citation13,Citation24 were nested case–control studies. The studies were conducted in the following regions: Europe (n=5),Citation10,Citation13–Citation15,Citation19 Asia (n=4),Citation6,Citation7,Citation9,Citation24 and USA (n=3).Citation8,Citation12,Citation25 The study population in 12 studies consisted of both sexes. All included studies were published between 2012 and 2018. The cohort ranged in size from 878Citation24 to 1.01 million.Citation19 Medication use in comparison group was either “never used any TZD”Citation13,Citation24 or “never used pioglitazone”Citation6–Citation10,Citation12,Citation14,Citation15,Citation19 or “use of sulfonylureas”.Citation25 Adjustments were made for potential confounders of more than two factors in all studies.
Table 1 Characteristics of observational studies of pioglitazone and bladder cancer
Pioglitazone use and risk of bladder cancer
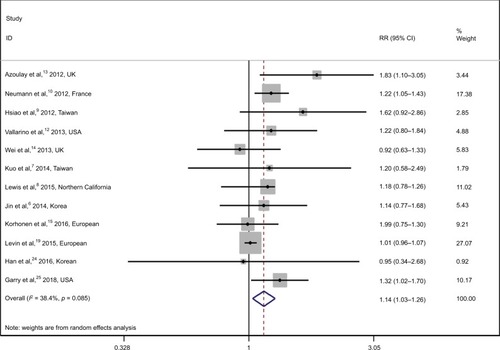
The overall RR with its 95% CI showed a statistically slight but significant association between pioglitazone use and risk of bladder cancer (, RR 1.14, 95% CI 1.03–1.26). There is no significant heterogeneity among these studies with Q=17.86, P=0.085, and I2=38.4%.
Figure 2 Relative risks for the association between pioglitazone use and risk of bladder cancer.
Notes: Diamonds represent study-specific relative risks (RRs) or summary relative risks with 95% confidence intervals (CIs). Horizontal lines represent 95% CIs. Test for heterogeneity among studies: P=0.085, ICitation2=38.4%.
We also performed dose effect analysis of pioglitazone (). We defined low dose as ≤8268 mg or ≤10500 mg or ≤14000 mg; moderate dose as 10501–28000 mg or 14001–40000 mg; high dose as >28000 mg or >40000 mg. Although there were not statistically significant results, the risk of bladder cancer showed a rising trend when the dose of pioglitazone increased (low dose, RR 1.12, 95% CI 0.95–1.33; moderate dose, RR 1.20, 95% CI 0.99–1.46; high dose, RR 1.29, 95% CI 0.75–2.22).
Table 2 Dose effect analysis of relative risks for the association between pioglitazone and bladder cancer
Sensitivity analysis
Sensitivity analyses of the pooled RRs were performed to test the stability of the pooled results. All of the integrated RRs were once again calculated by means of the random effects model. When omitting each study in the meta-analysis, the pooled RRs () always remained stable.
Publication bias
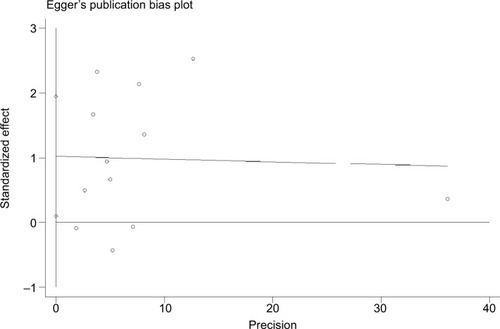
There was no evidence indicating significant publication bias either with the Begg’s test (P=1.0) or with the Egger’s test (=0.90).
Discussion
The finding of this meta-analysis of 12 studies indicates that pioglitazone is associated with a 14% increased risk of bladder cancer (95% CI 1.04–1.26). Quantified Q test and I2 test were carried out to assess the extent of heterogeneity among the included studies. No statistically significant heterogeneity was found among the included studies (I2=38.4%, P=0.085). Our conclusion was convincing because a fixed model showed a similar result (RR 1.05, 95% CI 1.01–1.10) and our heterogeneity was not significant. In sensitivity analysis we found that heterogeneity largely decreased (I2=0, P=0.591) when excluding Levin et al’sCitation19 study. This result may be due to the difference in methodology of Levin et al’s paper. Unlike other cohort studies, Levin used a multi-population pooled cohort to assess the risk of bladder cancer and use of pioglitazone in patients with type 2 diabetes. This might be the origin of the heterogeneity. Although the result of two RCT studies providing high-level evidence showed a null association between pioglitazone and bladder cancer, it is still not convincing because a total of only 35 bladder cancer cases were reported in the intervention group. During our search process, another high-quality RCT study assessing risk of bladder cancer and pioglitazone in patients without diabetes was identified.Citation47 Similar to two other RCTs, 12 bladder cancer cases in the pioglitazone group were reported and no significant relationship was detected. In spite of its high quality, this study was excluded from the systematic review because the non-diabetic population did not fit our study goal.
One important molecular signaling pathway involved in carcinogenesis concerns of pioglitazone intake and risk of bladder cancer. Pioglitazone is a member of TZDs, which are agonists of PPARγ.Citation48 The effects of PPARγ agonists on human bladder cell lines have continually demonstrated significantly decreased proliferation, increased differentiation and apoptosis, suggesting that PPARγ is involved in antitumor action.Citation49–Citation51 However, recent research reported by Yang et al indicates that higher expression of PPARγ or its activation by agonist TZD can promote bladder cancer cell migration and invasion,Citation52 suggesting that different expression levels of PPARγ may induce different outcomes. In light of this point, pioglitazone, the PPARγ agonist, might cause higher expression of PPARγ which promotes the process of carcinogenesis. In the future, more basic experimental research should be conducted to further elucidate the possible molecular mechanism of pioglitazone intake and risk of bladder cancer.
Our results of dose effect support this molecular theory. Patients who used a higher dose of pioglitazone had an increased risk of bladder cancer, indicating that larger amounts of pioglitazone may cause higher expression of PPARγ which promotes the process of carcinogenesis.
Additionally, no evidence of significant publication bias was observed in the analyses either with the Begg’s test or Egger’s test. These results greatly improved the predictability and reliability of our meta-analysis.
Compared to other related meta-analyses, our meta-analysis evaluating the correlation between pioglitazone and risk of bladder cancer is more convincing because: a) a transparent and robust approach was taken to examine the evidence base, including adherence to PRISMA guidelines; b) we included the newest and the broadest studies, to the best of our knowledge, with rigid inclusion criteria restriction in which only RCTs and high-quality (NOS quality score >6) cohort studies or nested case–control studies were included. We did not include the other high-quality RCT by Kernan et alCitation47 because the study population excluded patients with type 2 diabetes, which did not fit our aim. One RCT had another quality assessment methodCitation36 and was systematically reviewed rather than pooling with observational studies. A previous meta-analysisCitation33 simply pooled results of RCTs and observational studies which makes the conclusions unconvincing. c) Patients’ outcomes were only recorded in reliable databases or using ICD codes. This approach ensured methodological rigor. d) We did not include case–control studies to avoid latent high selection and recall bias; e) previous meta-analysesCitation29,Citation30 did not exclude studies with duplicate populations.Citation9,Citation16,Citation22
Nevertheless, some limitations should be mentioned: a) we only pooled cohort studies and nested case–control studies without RCTs. Two RCTs,Citation26,Citation27 which provided convincing level 1 evidence, reported null association between pioglitazone use and bladder cancer, but there was only a total of 35 bladder cancer cases in the intervention group. More future RCT studies should be performed to provide more convincing evidence. b) Although no publication bias was found in our meta-analysis by either Egger’s or Begg’s test, the selection strategy of published studies and language limitation could bring about potential publication bias which may have affected our ultimate findings; c) the uncontrolled or unmeasured latent risk factors could have produced biases. We cannot rule out the possibility that remaining confounding factors could have affected the results. Recently, Lewis et al54 reported that proteinuria testing could potentially cause unmeasured confounding in studies of pioglitazone and bladder cancer but most of the studies included in this meta-analysisCitation6,Citation7,Citation9,Citation10,Citation12–Citation14 did not adjust for proteinuria testing. d) Although our heterogeneity was not significant, the results of our meta-analysis should be interpreted with caution because heterogeneity of our study was not unapparent (Q=17.86, P=0.085 and I2=38.4%).
In conclusion, this meta-analysis indicated that pioglitazone is associated with an increased risk of bladder cancer. Despite some study limitations, we still suggest that doctors should carefully assess the overall risks and benefits of pioglitazone. Further research should be conducted to confirm our findings and clarify the potential biological mechanisms.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81472375 and No. 81702500) and Zhejiang Province Medical Technology Project (2016KYA173).
Supplementary materials
Figure S1 Risk of bias assessment of two RCT studies.
Abbreviation: RCT, randomized controlled trial.
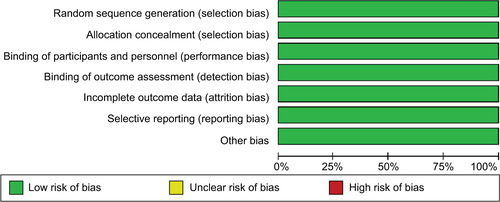
Table S1 Detailed reasons of excluded studies
Table S2 Characters of included RCT studies
Table S3 Sensitivity analysis of relative risks for the association between pioglitazone and bladder cancer
References
- JinSMSongSOJungCHRisk of bladder cancer among patients with diabetes treated with a 15 mg pioglitazone dose in Korea: a multi-center retrospective cohort studyJ Korean Med Sci201429223824224550651
- KuoHWTiaoMMHoSCYangCYPioglitazone use and the risk of bladder cancerKaohsiung J Med Sci2014302949724444539
- LewisJDHabelLAQuesenberryCPPioglitazone use and risk of bladder cancer and other common cancers in persons with diabetesJAMA2015314326527726197187
- HsiaoFYHsiehPHHuangWFTsaiYWGauCSRisk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case-control studyDrug Saf201336864364923797604
- NeumannAWeillARicordeauPPioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort studyDiabetologia20125571953196222460763
- MackenzieTAZahaRSmithJKaragasMRMordenNEDiabetes pharmacotherapies and bladder cancer: a medicare epidemiologic studyDiabetes Ther201671617326894755
- VallarinoCPerezAFuscoGComparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weightsClin Drug Investig2013339621631
- AzoulayLYinHFilionKBThe use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control studyBMJ2012344e364522653981
- WeiLMacDonaldTMMackenzieISPioglitazone and bladder cancer: a propensity score matched cohort studyBr J Clin Pharmacol201375125425922574756
- KorhonenPHeintjesEMWilliamsRPioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countriesBMJ2016354i390327530399
- LeeMYHsiaoPJYangYHLinKDShinSJThe association of pioglitazone and urinary tract disease in type 2 diabetic Taiwanese: bladder cancer and chronic kidney diseasePloS One201491e8547924427312
- BalajiVSeshiahVAshtalakshmiGRamananSGJanarthinakaniMA retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian populationIndian J Endocrinol Metab201418342542724944944
- GuptaSGuptaKRaviRPioglitazone and the risk of bladder cancer: an Indian retrospective cohort studyIndian J Endocrinol Metab201519563964326425474
- LevinDBellSSundRPioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysisDiabetologia201558349350425481707
- FujimotoKHamamotoYHonjoSPossible link of pioglitazone with bladder cancer in Japanese patients with type 2 diabetesDiabetes Res Clin Pract2013992e212323228390
- TsengCHPioglitazone and bladder cancer: a population-based study of TaiwaneseDiabetes Care201235227828022210574
- TuccoriMFilionKBYinHPioglitazone use and risk of bladder cancer: population based cohort studyBMJ2016352i154127029385
- HanEJangSYKimGRosiglitazone use and the risk of bladder cancer in patients with type 2 diabetesMedicine (Baltimore)2016956e278626871835
- GarryEMBuseJBLundJLPateVSturmerTComparative safety of pioglitazone versus clinically meaningful treatment alternatives concerning the risk of bladder cancer in older US adults with type 2 diabetesDiabetes Obes Metab201820112914028661561
- ErdmannEHardingSLamHPerezATen-year observational followup of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetesDiabetes Obes Metab201618326627326592506
- VaccaroOMasulliMNicolucciAEffects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trialLancet Diabetes Endocrinol201751188789728917544
- HsuYHYangYTChenPNAssociation between pioglitazone and bladder cancer among patients with type II diabetes: a propensity score matched cohort studyValue in Health2014173A73
- PiccinniCMotolaDMarchesiniGPoluzziEAssessing the association of pioglitazone use and bladder cancer through drug adverse event reportingDiabetes Care20113461369137121515844
- LinHCHsuYTKachingweBHDose effect of thiazolidinedione on cancer risk in type 2 diabetes mellitus patients: a six-year populationbased cohort studyJ Clin Pharm Ther201439435436024661226
- KaoCHSunLMChenPCA population-based cohort study in Taiwan–use of insulin sensitizers can decrease cancer risk in diabetic patients?Ann Oncol201324252353023110810
- MamtaniRHaynesKBilkerWBAssociation between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort studyJ Natl Cancer Inst2012104181411142122878886
- BazelierMTde VriesFVestergaardPLeufkensHGDe BruinMLUse of thiazolidinediones and risk of bladder cancer: disease or drugs?Curr Drug Saf20138536437024215315
- KernanWNViscoliCMFurieKLPioglitazone after ischemic stroke or transient ischemic attackN Engl J Med2016374141321133126886418
Disclosure
The authors report no conflicts of interest in this work.
References
- AntoniSFerlayJSoerjomataramIBladder cancer incidence and mortality: a global overview and recent trendsEur Urol20177119610827370177
- Murta-NascimentoCSchmitz-DragerBJZeegersMPEpidemiology of urinary bladder cancer: from tumor development to patient’s deathWorld J Urol200725328529517530260
- XuXWuJMaoYDiabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studiesPloS One201383e5807923472134
- McConkeyDJChoiWOchoaADinneyCPIntrinsic subtypes and bladder cancer metastasisAsian J Urol20163426026729264194
- SuzukiSArnoldLLPenningtonKLEffects of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on the urine and urothelium of the ratToxicol Sci2010113234935719858066
- JinSMSongSOJungCHRisk of bladder cancer among patients with diabetes treated with a 15 mg pioglitazone dose in Korea: a multi-center retrospective cohort studyJ Korean Med Sci201429223824224550651
- KuoHWTiaoMMHoSCYangCYPioglitazone use and the risk of bladder cancerKaohsiung J Med Sci2014302949724444539
- LewisJDHabelLAQuesenberryCPPioglitazone use and risk of bladder cancer and other common cancers in persons with diabetesJAMA2015314326527726197187
- HsiaoFYHsiehPHHuangWFTsaiYWGauCSRisk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case-control studyDrug Saf201336864364923797604
- NeumannAWeillARicordeauPPioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort studyDiabetologia20125571953196222460763
- MackenzieTAZahaRSmithJKaragasMRMordenNEDiabetes pharmacotherapies and bladder cancer: a medicare epidemiologic studyDiabetes Ther201671617326894755
- VallarinoCPerezAFuscoGComparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weightsClin Drug Investig2013339621631
- AzoulayLYinHFilionKBThe use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control studyBMJ2012344e364522653981
- WeiLMacDonaldTMMackenzieISPioglitazone and bladder cancer: a propensity score matched cohort studyBr J Clin Pharmacol201375125425922574756
- KorhonenPHeintjesEMWilliamsRPioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countriesBMJ2016354i390327530399
- LeeMYHsiaoPJYangYHLinKDShinSJThe association of pioglitazone and urinary tract disease in type 2 diabetic Taiwanese: bladder cancer and chronic kidney diseasePloS One201491e8547924427312
- BalajiVSeshiahVAshtalakshmiGRamananSGJanarthinakaniMA retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian populationIndian J Endocrinol Metab201418342542724944944
- GuptaSGuptaKRaviRPioglitazone and the risk of bladder cancer: an Indian retrospective cohort studyIndian J Endocrinol Metab201519563964326425474
- LevinDBellSSundRPioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysisDiabetologia201558349350425481707
- FujimotoKHamamotoYHonjoSPossible link of pioglitazone with bladder cancer in Japanese patients with type 2 diabetesDiabetes Res Clin Pract2013992e212323228390
- SongSOKimKJLeeBWThe risk of bladder cancer in korean diabetic subjects treated with pioglitazoneDiabetes Metab J201236537137823130322
- TsengCHPioglitazone and bladder cancer: a population-based study of TaiwaneseDiabetes Care201235227828022210574
- TuccoriMFilionKBYinHPioglitazone use and risk of bladder cancer: population based cohort studyBMJ2016352i154127029385
- HanEJangSYKimGRosiglitazone use and the risk of bladder cancer in patients with type 2 diabetesMedicine (Baltimore)2016956e278626871835
- GarryEMBuseJBLundJLPateVSturmerTComparative safety of pioglitazone versus clinically meaningful treatment alternatives concerning the risk of bladder cancer in older US adults with type 2 diabetesDiabetes Obes Metab201820112914028661561
- ErdmannEHardingSLamHPerezATen-year observational followup of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetesDiabetes Obes Metab201618326627326592506
- VaccaroOMasulliMNicolucciAEffects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trialLancet Diabetes Endocrinol201751188789728917544
- fda.gov [homepage on the Internet]FDA Drug Safety Communication: Updated FDA review concludes that use of type 2 diabetes medicine pioglitazone may be linked to an increased risk of bladder cancerUS Food and Drug Administration2016 Available from: https://www.fda.gov/Drugs/DrugSafety/ucm519616Accessed May 2, 2018
- DavidsonMBPanDAn updated meta-analysis of pioglitazone exposure and bladder cancer and comparison to the drug’s effect on cardiovascular disease and non-alcoholic steatohepatitisDiabetes Res Clin Pract201713510211029146119
- FilipovaEUzunovaKKalinovKVekovTPioglitazone and the risk of bladder cancer: a meta-analysisDiabetes Ther20178470572628623552
- PaiSAKshirsagarNAPioglitazone utilization, efficacy & safety in Indian type 2 diabetic patients: a systematic review & comparison with European Medicines Agency Assessment ReportIndian J Med Res20161445671680
- TurnerRMKwokCSChen-TurnerCThiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysisBr J Clin Pharmacol201478225827324325197
- HeSTangYHZhaoGPioglitazone prescription increases risk of bladder cancer in patients with type 2 diabetes: an updated meta-analysisTumor Biol201435320952102
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementAnn Intern Med2009151426426919622511
- Ottawa Hospital Research Institute [homepage on the Internet]WellsGASheaBO’ConnellDOThe Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed May 2, 2018
- HigginsJPGreenSCochrane Handbook for Systematic Reviews of Interventions Version 5.10 [updated32011The Cochrane Collaboration2011 Available from: http://handbookAccessed May 2, 2018
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- ZengDLinDYOn random-effects meta-analysisBiometrika2015102228129426688589
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- HsuYHYangYTChenPNAssociation between pioglitazone and bladder cancer among patients with type II diabetes: a propensity score matched cohort studyValue in Health2014173A73
- PiccinniCMotolaDMarchesiniGPoluzziEAssessing the association of pioglitazone use and bladder cancer through drug adverse event reportingDiabetes Care20113461369137121515844
- LinHCHsuYTKachingweBHDose effect of thiazolidinedione on cancer risk in type 2 diabetes mellitus patients: a six-year population-based cohort studyJ Clin Pharm Ther201439435436024661226
- KaoCHSunLMChenPCA population-based cohort study in Taiwan–use of insulin sensitizers can decrease cancer risk in diabetic patients?Ann Oncol201324252353023110810
- MamtaniRHaynesKBilkerWBAssociation between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort studyJ Natl Cancer Inst2012104181411142122878886
- BazelierMTde VriesFVestergaardPLeufkensHGDe BruinMLUse of thiazolidinediones and risk of bladder cancer: disease or drugs?Curr Drug Saf20138536437024215315
- KernanWNViscoliCMFurieKLPioglitazone after ischemic stroke or transient ischemic attackN Engl J Med2016374141321133126886418
- ChingJAmiridisSStylliSSThe peroxisome proliferator activated receptor gamma agonist pioglitazone increases functional expression of the glutamate transporter excitatory amino acid transporter 2 (EAAT2) in human glioblastoma cellsOncotarget2015625213012131426046374
- WangYTanHXuDThe combinatory effects of PPAR-gamma agonist and survivin inhibition on the cancer stem-like phenotype and cell proliferation in bladder cancer cellsInt J Mol Med201434126226824820432
- LangleYLodillinskyCBelgoroskyDSandesEOEijanAMRole of peroxisome proliferator activated receptor-gamma in bacillus Calmette-Guerin bladder cancer therapyJ Urol201218862384239023088980
- VarleyCLSouthgateJEffects of PPAR agonists on proliferation and differentiation in human urotheliumExp Toxicol Pathol200860643544118571911
- YangDRLinSJDingXFHigher expression of peroxisome proliferator-activated receptor gamma or its activation by agonist thiazolidinedione-rosiglitazone promotes bladder cancer cell migration and invasionUrology20138151109.e1623522297
- LewisJDHabelLQuesenberryCProteinuria testing among patients with diabetes mellitus is associated with bladder cancer diagnosis: potential for unmeasured confounding in studies of pioglitazone and bladder cancerPharmacoepidemiol Drug Saf201423663664524764283