Abstract
Modern radiotherapy (RT) is being enriched by big digital data and intensive technology. Multimodality image registration, intelligence-guided planning, real-time tracking, image-guided RT (IGRT), and automatic follow-up surveys are the products of the digital era. Enormous digital data are created in the process of treatment, including benefits and risks. Generally, decision making in RT tries to balance these two aspects, which is based on the archival and retrieving of data from various platforms. However, modern risk-based analysis shows that many errors that occur in radiation oncology are due to failures in workflow. These errors can lead to imbalance between benefits and risks. In addition, the exact mechanism and dose–response relationship for radiation-induced malignancy are not well understood. The cancer risk in modern RT workflow continues to be a problem. Therefore, in this review, we develop risk assessments based on our current knowledge of IGRT and provide strategies for cancer risk reduction. Artificial intelligence (AI) such as machine learning is also discussed because big data are transforming RT via AI.
Keywords:
Introduction
With the growth of cancer survivors, there was more than 15.5 million on January 1, 2016, in USA and is projected to reach more than 20 million by January 1, 2026.Citation1 Electronic health records for them are skyrocketing. The US Cancer Moonshot Initiative recommends large-scale genetic analysis of tumors and clinical trial networks to better harness the information gleaned from cancer patients and to optimize care, pushing forward the personalized medicine.Citation2
In radiotherapy (RT), cancer data are firmly in the realm of big data mainly due to ubiquitous images constituting one-third of total global storage demand. Medical imaging is crucial to RT. Its application in RT, referred to as image-guided RT (IGRT), encompasses tumor diagnosis, staging, prognosis, treatment planning, radiation targeting, and follow-up care.Citation3 Enormous digital data are created in the workflow, so IGRT can be redefined as information-guided RT. Large setup errors, ranges of organ motion, and changes in tumor position, size, and shape are most likely to be detected during the course of RT with frequent imaging, which has been becoming a requirement to attain the best tumor coverage improving local control and the most healthy tissue sparing, thereby improving quality of life.Citation4,Citation5
For decades, cancer patients have benefited from advances in IGRT. Survival times are likely to be longer for them, and the number of cancer survivors is increasing. Late sequelae of RT are becoming the next concern. Second malignant neoplasms (SMNs) are among the most serious and life-threatening sequelae for the growing number of cancer survivors, especially for younger patients who have a longer life expectancy. Numerous epidemiological cohort studies have demonstrated radiation-related risks of thyroid and breast cancers, leukemia, and other neoplasms.Citation6,Citation7
However, the exact mechanism and dose–response relationship for radiation-induced malignancy are not well understood. The cancer risk associated with RT is still an area of controversy in clinical radiation oncology with impact on treatment decision making and on patient management. Many debates on cancer risks have existed for a decade or more.Citation8–Citation10 In addition, modern risk-based analysis shows that many errors that occur in radiation oncology are due to failures in workflow and process.Citation11 These errors may further exacerbate the risk. In any case, research on cancer risk has substantially expanded our knowledge of clinical radiation oncology.
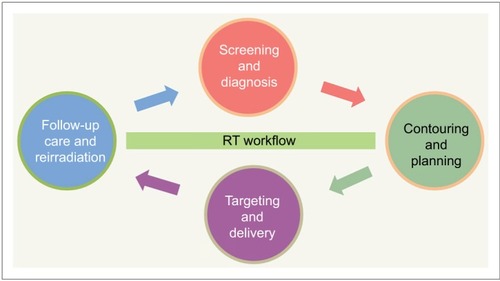
In this review, we highlight the latest research on IGRT workflow and discuss cancer risks in four key areas: screening and diagnosis, contouring and planning, targeting and delivery, and follow-up care and re-irradiation (). Ethics approval for this study was obtained from Ethics Committee of Chongqing Cancer Institute. All methods were performed according to the relevant guidelines and regulations. These insights will help clinicians better understand the technology and the IGRT process in general and have an effect on personal trade-off between the risks and benefits of treatment options, improving safe RT delivery and patient treatment outcomes. In addition, these insights have the potential to decrease health care costs with a more rational use of medical technology. To transform these data into knowledge, data-driven machine-learning approaches such as automatic diagnosis, automated RT planning, and medical image retrieval are being utilized in the modern RT, so they are also discussed in this review.
Screening and diagnosis
Technologies have improved, and cancers are being detected earlier. Cancer survival rates have improved over the past several decades.Citation12 The specific contribution of screening has been debated for lung cancer (low-dose computed tomography [LDCT] or chest radiography), breast cancer (mammography), colorectal cancer (computed tomography [CT] colonography, CTC), and other cancers.Citation13–Citation16 Several trials have demonstrated that screening can reduce death from both lung cancer and breast cancer by 20% with LDCT and mammography, respectively.Citation17,Citation18 Epidemiological analyses also show a steady decline in mortality rates of colorectal cancer over the past several decades, which are attributed to screening.Citation19
However, concerns have been raised about these routine screening and diagnostic evaluations because of the potential harms, including the radiation risks. The mean effective dose from an LDCT or a CTC is 1.6–2.1 mSv or 7.0–8.0 mSv, respectively.Citation20,Citation21 Mammography screening doses range from 2.0 to 5.0 mSv.Citation22 The total radiation dose for a diagnostic CT can vary widely ranging from 2.2 to 14.0 mSv.Citation23 The dose is so low that these risks are not precisely quantifiable. Most of the quantitative information comes from studies of survivors of the atomic bombs and cohorts of radiation workers but it is characterized by a great uncertainty. The theoretical risk is estimated as follows: doses below 20 mSv have a minimal risk of cancer of less than one in 1000 patients, doses of 20–100 mSv have a moderate risk greater than one in 1000 patients, and doses above 100 mSv have clear evidence of radiation-induced cancer.Citation24
Above 100 mSv: most scientific review groups consider that this dose is appropriate to use a linear dose–response model; below 100 mSv: it is difficult to get consensus on dose–response due to considerable uncertainties. Currently, the most commonly used model is a linear no-threshold (LNT) model, wherein dose–effect data at high dose are simply extrapolated linearly downward to zero without a threshold dose ().Citation25 The LNT model is recommended by many expert advisory bodies for setting radiation protection regulations. Lifespan study (LSS) of atomic bomb survivors also shows that dose–response is consistent with the LNT model over 0–2 Gy range for all solid cancers as a group.Citation26 However, the LNT model focuses only on molecular damage, while ignoring protective, organismal biological responses. There is a growing body of experimental and epidemiological evidence that does not support its usage for estimating cancer risks.Citation27 Moreover, LNT sometimes causes considerable stress-related casualties.Citation28 The French Academy of Sciences believes that the use of LNT for assessing carcinogenic risks induced by low doses below 100 mSv is unjustified and should be discouraged.Citation29
Figure 2 Dose–response curve for carcinogenesis and dose range of the IGRT workflow.
Abbreviations: IGRT, image-guided radiotherapy; LNT, linear no-threshold.
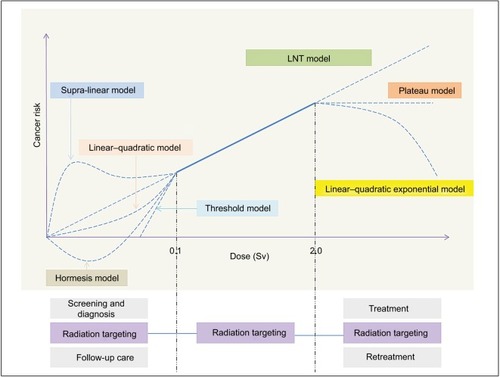
Apart from LNT model, there are various other contradicting models. A sub-linear model such as the linear–quadratic model based on “dual radiation action” theory which reflects the effect of dose and dose rate can explain current leukemia data from the LSS.Citation30 A hormesis model predicts protective effects below a threshold dose, even for noncancer deaths. It belongs to an adaptive response triggered by low-dose radiation, which is supported by multiple animal studies revealing the extension of lifespan of various mammalian models and many retrospective human studies exhibiting the reduced cancer rates or negative excess relative risk.Citation27,Citation31,Citation32 A threshold model predicts a negligible risk below a threshold dose such as immune suppression. This model is also compatible with leukemia data and sarcoma data, suggesting that thresholds cannot be larger than ~60 mSv for cancer or ~0.9 Sv for noncancer disease.Citation33 A supra-linear model fits well with the current cancer incidence data explained by hypersensitivity or bystander effect, and the low-dose hypersensitivity decreases with increasing dose and then disappears at doses of >0.5 Gy due to the biological defense system.Citation34
To better understand the nature of the dose–response in the low-dose zone, it is necessary to use biological models with low variability and high reproducibility. In addition, it is much more important to review the risks and benefits of screening and diagnostic tests. This may serve as an indication for the individual to make a more informed decision to undergo these procedures. For breast cancer risk associated with mammography, the estimated risk is 86 cancers and 11 deaths per 100,000 women screened annually from 40 to 55 years of age and biennially thereafter; the ratio of benefit to risk is 4.5:1 for lives saved and 9.5:1 for life-years saved.Citation35 The estimated number of radiation-related cancers from CTC every 5 years from age 50 to 80 years is 150 cases/100,000 individuals; the estimated number of colorectal cancer prevented by CTC ranges from 3580 to 5190/100,000, yielding a benefit–risk ratio that varied from 24:1 to 35:1.Citation21 The benefit-to-risk ratio of lung cancer screening with CT depends on several factors including efficacy of screening, smoking habits, sex of the screened subject, CT technology, and patient age at the commencement of screening. It can reach about 10:1 for special cohorts and screening efficacy, and it increases with advancing age.Citation36
The radiation-related cancer risk is not well understood at the levels of screening and diagnostic radiation, but there are clearly risks associated with not performing an examination, such as missing a diagnosis and/or initiating treatment too late to improve the medical outcome. Currently, there is rigorous evidence supporting the value of screening and diagnosis, and it is important to implement screening and diagnosis in a manner that is focused on maximizing benefits and minimizing harms. For example, the dose to the fetus resulting from most conventional radiography or nuclear medicine procedures is <0.01 Gy for pregnant women, and it has a risk of childhood cancer and leukemia with an incidence of about 3–4/1000;Citation37 if the dose exceeds 0.01 Gy, it can be reduced with proper tailoring of the examination or adopting another type of examination, such as ultrasonography or magnetic resonance imaging (MRI).
Recently, a new emerging field referred to as radiomics not only provides a quantitative way to assess tumor phenotype but also has shown promise in automatic detection of cancers, staging determinations, and predicting treatment response by applying a large number of quantitative features from screening or diagnosis images.Citation38 This new field will make CT, MRI, and positron emission tomography (PET) more standardized, reproducible, and quantitative. Its potential has been demonstrated in many cancers, in turn allowing for adapting and individualizing treatment at low cost.Citation39 In light of this, cancer risks may be more accurately predicted by radiomic changes, which are assessable prior to the development of observable changes on standard diagnostic imaging. Combining radiomics with genomic data, the so-called radiogenomics could provide the highest level of personalized risk stratification.
Contouring and planning
In IGRT, pretreatment images constitute the inputs to the treatment planning process, which is vulnerable to the GIGO effect (garbage in, garbage out). For this reason, a series of scientific articles on CT have been raising increasing concern about image quality.Citation40 However, the higher the dose contributing to the image, the less apparent is image noise and the easier it is to perceive low-contrast structures. In view of possible cancer risk, CT dose reduction strategies and scanning protocols have been suggested to match CT dose with the necessary image quality by many organizations.Citation41–Citation43 In contrast to therapeutic irradiation, the CT planning doses are too small to judge whether cancer patients benefit from the reduced dose strategies. Hence, it is not of great concern to these patients and their attending radiation oncologists. The acquisition of optimal CT data set fused with other imaging modalities, such as MRI and PET, to allow the radiation oncologist to accurately contour the tumor targets, is always the subject of the RT procedure.
Various studies indicate that inconsistencies in anatomy contouring may be larger than errors in the other steps of the treatment planning and delivery process.Citation44 They also could potentially lead to reduction in the dose to the tumor, increased locoregional recurrence and worse survival. Up to now, a large number of contouring consensus guidelines have been established, and structured training on image interpretation has been offered. In addition, semiautomatic and automatic contouring methods, such as probabilistic atlases and machine-learning technologies, have been proposed to minimize manual input and increase consistency in delineating target volume. Various margins are recommended to generate clinical target volume (CTV) or planning target volume (PTV). Frequent image guidance is suggested allowing margin reduction to several millimeters and dose escalation while maintaining sparing of healthy tissue. Standards for quality assurance (QA) of IGRT devices and reconstruction algorithms are implemented to quantify image quality in adaptive RT (ART) and online/offline planning process, improving positioning or registration accuracy.
However, traditional CT images show respiratory motion as artifacts, and target volumes based on these images may be distorted. Four-dimensional (4D) CT (4DCT) technology allows clinicians to view volumetric CT images changing over time for the observation of intra-fractional target motion and assessment of lung function in RT and to predict treatment response even better than static pretreatment images. Although a prospective 4DCT simulation can allow radiation exposure to be minimized to almost the same as that in helical CT, the measured effective dose was 28.7–33.2 mSv during a retrospective 4DCT simulation, approximately four times higher than that for helical CT.Citation45 From the radiological protection point of view, it is important to optimize the 4DCT scan protocol to minimize patient exposure.
To enhance target definition and RT planning, IGRT takes full advantage of these imaging advances. It markedly impacts the amount of radiation dose and volume of irradiated tissue as well as cancer risk. Large clinical studies have highlighted that patients who received RT had a higher risk of SMNs.Citation46 SMNs usually occur near volumes irradiated to intermediate doses (~2–50 Gy) or in volumes receiving full-dose radiation.Citation47 Although LSS shows that a linear relationship exists between cancer and dose from ~0.1 to ~2.0 Sv, there are great uncertainties at higher doses (). The bell-shaped Gray model (linear–quadratic–exponential model) hypothesizes that the relative increase in risk declines above a threshold dose (thyroid cancer, ~20 Gy) in terms of a balance between cell mutation and killing; a plateau model based on various biological parameters, such as cell sterilization, proliferation, and carcinogenic effects, speculates that there is a plateau beyond a threshold dose (breast cancer, ~10 Gy).Citation48 However, several epidemiological studies indicate that SMN risks increase significantly from 1 to 45 Gy for stomach and pancreas, but 1–60 Gy for bladder and rectum or 1–15 Gy for kidney.Citation46 It seems that the risk continues to rise as a linear function of dose.Citation47
In IGRT, especially for planning process, many factors such as fractionation schedule, linear accelerator (linac), RT techniques, beam energy, and dose/dose rate may affect SMN risks. Carcinoma and sarcoma risks are decreased bŷ10% and ~15% per gray with increasing fractionation dose.Citation49 The out-of-field dose is likely to be highly facility dependent due to leakage and scattering from head and accessories.Citation50 Intensity-modulated techniques increase the risks because of more monitor units (MUs), increasing leakage and scattering radiation; dynamic mode or cross-plane motion also increases SMN risks compared to segmental or in-plane mode; more fields are correlated with higher risks.Citation51–Citation53 Higher energies (>6 MV) produce larger scattered X-rays and small but significant neutrons due to photonuclear interactions with thresholds of 6–13 MeV for most materials.Citation54 Although the model-dependent SMN risks have been presenting in many literatures according to the Gy, plateau, or linear dose–response model, large discrepancies exist among them.Citation55
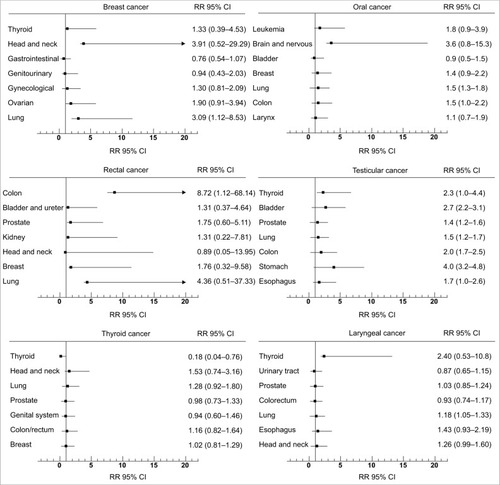
Clinically, choosing the least toxic radiation modality is of utmost importance. Previous studies have also demonstrated that increased risks for RT-related SMNs vary in different organs/tissues ();Citation56–Citation61 both size and shape of the PTV influence SMN.Citation62,Citation63 While providing equally good PTV coverage and following the specific organ-at-risk (OAR) constraints, the probability of SMN incidence should be carefully examined and weighed against the possibility of developing acute side effects for each patient individually as well as treatment efficacy. According to the target characteristics, a major decrease in the volume receiving a moderate radiation dose by any strategy (e.g., patient positioning, linac choice, and shielding strategies) is the appropriate way to substantially decrease the second cancer risk while designing the treatment plan. In addition, clinical trials have demonstrated that plan quality is associated with survival and local tumor control.Citation64 There have been intense research activities in planning quality evaluation using machine-learning approaches based on prior plans.Citation65–Citation67 Further, automatic treatment planning solutions such as knowledge-based planning and multi-criteria optimization have been proposed to improve the plan quality and workflow, including automatic learning-based beam angle selection and automatic optimized intensity for individual patients based on their unique anatomy.
Targeting and delivery
For decades, radiographic images have been the standard method of verification, providing localization, displacement, and deformation of tumors and/or OARs in two dimension (2D), three dimension (3D), or 4D to achieve the beam- and-target alignment, dose verification, and adaptation, which are dependent on both image quality and imaging frequency.Citation68 Nowadays, the emerging delta radiomics, a longitudinal fashion for radiomics, can be based on routine images to serve as a biomarker for treatment monitoring and optimization or active surveillance. An increase in the number of images will ensure accurate and precise RT delivery and yield smaller setup errors and CTV-to-PTV margins reducing SMN risks in distant tissues, but add more imaging radiation doses to target and adjacent healthy tissues. About 70% of SMNs occur in those regions.Citation69,Citation70 At some point, a cost/benefit balance needs to be reached, which is highly individualized to RT site and protocol. In fact, geometric precision is only one aspect of treatment, and its desire should be balanced with clinical gains and modest workloads on RT contouring, planning, and delivery.
Besides imaging frequency, the organ-absorbed doses also depend on imaged region, imaging parameters, and techniques provided by different vendors ().Citation71 Generally, 2D imaging dose is concentrated at the skin, while 3D/4D tomography dose is distributed nearly uniformly throughout the imaged volume. If imaging parameters are not optimized, out-of-field doses from imaging can be comparable to doses from the scatter and leakage radiation associated with therapeutic beam. There is an estimated probability of 0.08–3.59% of SMN.Citation72 Therefore, the imaging dose and its distribution need to be taken into account in the treatment planning, which results in about 4–5% reduction in MUs and control points,Citation73 or it is appropriate to optimize the image quality and minimize the imaging dose to ≤2% variation of the therapy dose, which is the internationally accepted beam output variation of RT linac, through varying the beam directions, field of view, number of projections per imaging, the tube voltage (kVp), current (mA), or mAs/frame. Especially for pediatric patients, it is necessary to perform personalized imaging according to patient characteristics considering the type of imaging, such as two orthogonal images or cone beam CT (CBCT), and acquisition modes, for example, CBCT head modes can be used to image the thorax, abdomen, or pelvis to selectively avoid irradiation of superficial organs.Citation74
Figure 4 The median dose of various imaging modalities.
Abbreviation: CBCT, cone beam computed tomography; FBCT, fan beam computed tomography; GE, general electrics; MV, megavoltage; kV, kilovoltage; TB, truebeam.
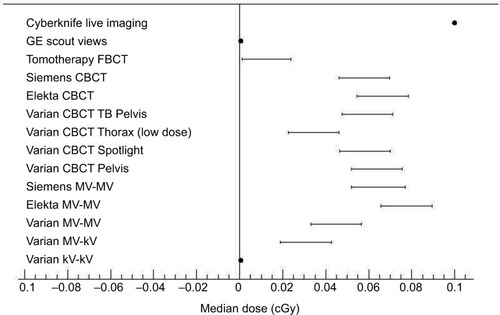
Unlike the diagnostic/planning imaging, radiation targeting adds the additional dose to an already high level of therapeutic radiation except for the first fraction (). The joint effect of targeting and delivery, which is additive or not, is not readily tested through randomized clinical trials. In theory, due to enhanced accuracy, the observed reduction in adverse effects of IGRT might in part be due to an adaptive response of normal tissues to the low doses of radiation from the imaging process before the delivery of high therapeutic doses. By coincidence, tumor cell radioresistance triggered by the imaging dose has been verified recently.Citation75 According to the generalized linear–quadratic model, the delay time between imaging and treatment also affects local tumor control while incorporating imaging dose into the therapeutic dose.Citation76 Other modalities such as optical imaging techniques also have an important role in IGRT without exposing the patient to additional radiation dose during RT delivery.Citation77 In conjunction with X-ray systems providing some information about internal anatomical structures such as bones, they can provide continuous monitoring of patients and detect these changes in the soft tissues, where the tumor is located.
Due to higher rates of medication errors among adult (7.1%) and pediatric (18.8%) patients with cancer, numerous programs for workflow-related QA are established and performed to detect errors to ensure that these personalized treatments are delivered safely.Citation78 These errors include unauthorized acts, operative errors, equipment failures, initiating events, accident precursors, near misses, and other mishaps.Citation79 Most of the errors are discovered in setup/treatment and during treatment follow-up phases. There are still errors that are not covered by regular QA checks, so individual clinics should perform a risk analysis of their unique practice, classifying and learning from incidents, and to determine appropriate testing frequencies to maximize physicist time efficiency and patient treatment quality, improving existing processes or implementing new workflows. But manually intensive procedures are more prone to errors. To further minimize human errors, all kinds of applications using state-of-the-art web technologies, data mining, and machine learning, are being designed to automate and model the clinical workflow and IGRT process.
As one of today’s most rapidly growing technical fields, machine learning also brings new treatment technologies via new learning algorithms, such as auto-adaptive margin generation for real-time tracking RT for motion management.Citation80 However, technological innovations themselves can lead to the development of new potential hazards, so it is important to carry out quality evaluation when they are implemented in the clinic. All aspects of the treatment workflow, from imaging to dose calculation and treatment delivery, should be carefully handled and recorded. A large amount of data will be produced. Although these data are available in one RT center or other centers separately, it is difficult to make comparisons of new treatment technologies on a large scale and explore treatment effects. Nowadays, information availability has become more elaborate and widespread. More and more national or international infrastructures are being developed to enable structured and automated data collection and secure sharing of RT data.Citation81 To reach the best patient care, most treatment decisions will be based on gathered relevant data, referred to as big data, while treatments can be further optimized with respect to increased survival, less SMN risk, and less burden on the health care sector.
Follow-up care and re-irradiation
The risk of second malignancies after RT is a subject not without controversy. Generally, all cancer survivors should follow applicable national guidelines for cancer screening. But it is more important than ever for clinicians and patients to have accurate risk estimates of secondary cancers after RT to permit the development of individualized follow-up guidelines and prevention and intervention strategies.
To date, there are many investigations of interactions between RT and potential confounding factors such as age, sex, race, tobacco and alcohol use, dietary intake, energy balance, and other cofactors, as well as genetic susceptibility. One of the most important factors correlated with an SMN is the age of the patient at the time of RT. For the same dose, it shows that children are considered to be 3–15 times more sensitive to radiation-induced SMN than adults, and the cancer risk decreases from about 15%/Sv of whole body uniform irradiation for children under 10 years of age to about 1%/Sv for adults exposed at over 60 years of age.Citation82 Greater systematic checkup should be implemented after RT for this higher risk population. Compared to the therapeutic benefit, SMN might not be as significant and should not factor into treatment decisions for the older population.
It has been known that there is a significant dependence of tissue and organ to SMN risk. According to the National Council on Radiation Protection and Measurements (NCRP) report No. 116, stomach, lung, and colon are the most common sites for developing a fatal second cancer after radiation exposure. In addition, the thyroid gland is known to have a low threshold for radiation-induced cancer, especially in children and young adults (a mean organ dose as low as 0.05 Gy).Citation83 For a given dose, females have a higher SMN incidence compared to men due to the increased risk of breast and thyroid cancers in females.Citation84 Therefore, follow-up care should be managed on a case-by-case basis, but the cost to both the health care system and patients need to be evaluated.
The greatest challenge in determining risk is that second cancers after RT have a latency of onset of 5–10 years for leukemia and about 10–60 years for solid tumors after the initial treatment.Citation85 Only longer follow-up will allow a true assessment of the SMN risk. Taking into account what is known with regard to first primary cancers and adding evidence on SMN risks, a detailed survivorship care plan should be made to record the patient’s treatment and anticipated long-term effects.Citation86 In addition, a risk-adapted strategy can be made to optimize the routine follow-up policy such as screening frequency and follow-up duration and to minimize the probability of second cancers according to the follow-up care guidelines.
Indeed, new SMNs now are representing about 16% of all cancers reported to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program.Citation87 Further, SMNs particularly solid tumors are a major cause of mortality among cancer survivors. Fortunately, technological advances in RT and imaging have made treatment of patients with re-irradiation possible. However, re-irradiation with overlapping volumes of previously irradiated tissues is not without risks because of severe toxicity. Due to some disease- and patient-related factors, such as previous treatment, second cancer site, and performance status, the identification of who might derive the most benefit from which technology with what kind of dose and fractionation schedules is of utmost importance.
Nowadays, although the selection criteria for re-irradiation remain poorly defined and vary across centers, a careful second course of irradiation might provide a symptomatic and survival benefit in special patients. A single institutional experience shows that thoracic re-irradiation with conventional RT appears to deliver a meaningful survival benefit in new primary or recurrent lung cancer with low target volume (PTV <300cc).Citation88 Re-irradiation also may be considered an option for recurrent or new primary cancer of the head and neck, rectum, breast, cervix, or other sites in carefully selected patients using a variety of techniques and fractionation schedules, providing good local control rates while toxicity remains acceptable.Citation89–Citation94 Despite a paucity of large randomized studies, re-irradiation has been adopted in different clinical scenarios by many institutions, and the role of contemporary methods, such as IGRT, remains an area of active investigation in re-irradiation. Regardless of these aspects, careful attention to RT planning and delivery is critical to optimize the outcomes, so the corresponding guidelines are beginning to emerge for certain indications.
In recent years, enormous advances in RT have been achieved, for instance, introducing particle therapy into clinical routine, or the development of MRI-guided radiotherapy.Citation95,Citation96 These high-energy particle beams can often achieve excellent disease control while delivering minimal radiation dose to healthy tissue near cancer targets, offering a significantly lower second cancer incidence rates than photons. In a ROCOCO in silico clinical trial, a reduction in mean dose to OARs is also demonstrated using particle therapy compared to photons in the re-irradiation of patients with squamous cell carcinoma of the head and neck.Citation97 However, due to the high cost of the particle therapy facility, the cost/benefit ratio is being debated. MRI provides the gold standard for defining soft tissue structures during RT planning, and the use of MRI-guided treatment delivery is providing a further argument for an MRI-only workflow, which will eliminate setup and registration error while also reducing workload and strain on the patient, especially additional radiation in the RT workflow. But the dosimetric errors in an MRI-only RT workflow need to be considered due to the specific geometric distortion from MRI.Citation98
Conclusion
This review describes the cancer risks in numerous processes of IGRT, including screening and diagnosis, contouring and planning, targeting and delivery, and follow-up care and re-irradiation. Although we do not know the exact mechanism and dose–response relationship for radiation-induced malignancy, enormous advances in IGRT will help clinicians better understand the technology and the process in general and have an effect on individualized RT guidelines and strategies for cancer risk reduction, improving safe RT delivery and patient treatment outcomes. This review only describes external beam radiation therapy, and it is conceivable that brachytherapy faces a similar challenge. In the future, we believe that utilizing artificial intelligence (AI) to translate and combine all data sources into knowledge will enable health care to move to individualized, high-quality, and safe cancer treatments.
Acknowledgments
This study was supported by the National Natural Science Foundation of China under grant number 11575038.
Disclosure
The authors report no conflicts of interest in this work.
References
- MillerKDSiegelRLLinCCCancer treatment and survivorship statistics, 2016CA Cancer J Clin201666427128927253694
- EditorialsPrevention is as good as a cureNature2016539467
- VerellenDDe RidderMLinthoutNTournelKSoeteGStormeGInnovations in image-guided radiotherapyNat Rev Cancer200771294996018034185
- DawsonLASharpeMBImage-guided radiotherapy: rationale, benefits, and limitationsLancet Oncol200671084885817012047
- DawsonLAJaffrayDAAdvances in image-guided radiation therapyJ Clin Oncol200725893894617350942
- SwerdlowAJHigginsCDSmithPSecond cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort studyJ Clin Oncol201129314096410421969511
- Berrington de GonzalezACurtisREKrySFProportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registriesLancet Oncol201112435336021454129
- BrennerDJHallEJComputed tomography-an increasing source of radiation exposureN Engl J Med20073572277228418046031
- BrennerDJDollRGoodheadDTCancer risks attributable to low doses of ionizing radiation: assessing what we really knowProc Natl Acad Sci U S A200310024137611376614610281
- MoulJWRadiotherapy: secondary malignancies after prostate cancer treatmentNat Rev Clin Oncol2010724925020428221
- HuqMSFraassBADunscombePBThe report of Task Group 100 of the AAPM: Application of risk analysis methods to radiation therapy quality managementMed Phys2016437420927370140
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- National Lung Screening Trial Research TeamChurchTRBlackWCResults of initial low-dose computed tomographic screening for lung cancerN Engl J Med2013368211980199123697514
- EvansWPBreast cancer screening: successes and challengesCA Cancer J Clin2012625922252587
- ShaukatAMonginSJGeisserMSLong-term mortality after screening for colorectal cancerN Engl J Med2013369121106111424047060
- SmithRAAndrewsKBrooksDCancer screening in the United States, 2016: A review of current American Cancer Society guidelines and current issues in cancer screeningCA Cancer J Clin20166629611426797525
- HumphreyLLDeffebachMPappasMScreening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendationAnn Intern Med2013159641142023897166
- MyersERMoormanPGierischJMBenefits and harms of breast cancer screening: a systematic reviewJAMA2015314151615163426501537
- SiegelRLWardEMJemalATrends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992–2008Cancer Epidemiol Biomark Prev2012213411416
- AberleDRAbtinFBrownKComputed tomography screening for lung cancer: has it finally arrived? Implications of the national lung screening trialJ Clin Oncol20133181002100823401434
- Berrington de GonzálezAKimKPKnudsenABRadiation-related cancer risks from CT colonography screening: a risk-benefit analysisAJR Am J Roentgenol2011196481682321427330
- MullendersLAtkinsonMParetzkeHSabatierLBoufflerSAssessing cancer risks of low-dose radiationNat Rev Cancer20099859660419629073
- AshaSCurtisKAGrantNComparison of radiation exposure of trauma patients from diagnostic radiology procedures before and after the introduction of a panscan protocolEmerg Med Australas2012241435122313559
- KritsaneepaiboonSJutiyonAKrisanachindaACumulative radiation exposure and estimated lifetime cancer risk in multiple-injury adult patients undergoing repeated or multiple CTsEur J Trauma Emerg Surg2018441192727084545
- CalabreseEJOrigin of the linearity no threshold (LNT) dose-response conceptArch Toxicol20138791621163323887208
- OzasaKShimizuYSuyamaAStudies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseasesRadiat Res2012177322924322171960
- CalabreseEJO’ConnorMKEstimating risk of low radiation doses - a critical review of the BEIR VII report and its use of the linear no-threshold (LNT) hypothesisRadiat Res2014182546347425329961
- DossMAdoption of linear no-threshold model violated basic scientific principles and was harmful: Letter from Mohan Doss regarding Edward Calabrese’s paper “How the US National Academy of Sciences misled the world community on cancer risk assessment: new findings challenge historical foundations of the linear dose response”Arch Toxicol2013872063208123912675
- CiceroneRalph JArch Toxicol20148817117224311193
- Arch Toxicol20148884985224504165
- TubianaMDose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: the joint report of the Académie des Sciences (Paris) and of the Académie Nationale de MédecineInt J Radiat Oncol Biol Phys20056331731916168825
- PrestonDLPierceDAShimizuYEffect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimatesRadiat Res2014162377389
- DossMLinear no-threshold model vs. radiation hormesisDose Response20131148049724298226
- SiegelJAPenningtonCWSacksBWelshJSThe birth of the illegitimate linear no-threshold model: an invalid paradigm for estimating risk following low-dose radiation exposureAm J Clin Oncol201841217317726535990
- DossMLittleMPOrtonCGPoint/counterpoint: low-dose radiation is beneficial, not harmfulMed Phys20144107060124989368
- JacobPMeckbachRKaiserJCSokolnikovMPossible expressions of radiation-induced genomic instability, bystander effects or low-dose hypersensitivity in cancer epidemiologyMutat Res20106871–2343920096708
- WarnerEClinical practice. breast-cancer screeningN Engl J Med20113651025103221916640
- MascalchiMBelliGZappaMRisk-benefit analysis of X-ray exposure associated with lung cancer screening in the Italung-CT trialAJR Am J Roentgenol2006187242142916861547
- KalHBStruikmansHRadiotherapy during pregnancy: fact and fictionLancet Oncol20056532833315863381
- YipSSAertsHJApplications and limitations of radiomicsPhys Med Biol20166113R150R16627269645
- AertsHJVelazquezERLeijenaarRTDecoding tumour phenotype by noninvasive imaging using a quantitative radiomics approachNat Commun20145400624892406
- LiHDollySChenHCA comparative study based on image quality and clinical task performance for CT reconstruction algorithms in radiotherapyJ Appl Clin Med Phys2016174377390
- PearceMSSalottiJALittleMPRadiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort studyLancet2012380984049950522681860
- MathewsJDForsytheAVBradyZCancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million AustraliansBMJ2013346f236023694687
- HeveziJMMaheshMOptimizing CT dose and image quality for radiotherapy patientsJ Am Coll Radiol2012915222305705
- SegedinBPetricPUncertainties in target volume delineation in radiotherapy - are they relevant and what can we do about them?Radiol Oncol201650325426227679540
- MatsuzakiYFujiiKKumagaiMTsuruokaIMoriSEffective and organ doses using helical 4DCT for thoracic and abdominal therapiesJ Radiat Res201354596297023603303
- SuitHGoldbergSNiemierkoASecondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjectsRadiat Res20071671124217214511
- Berrington de GonzalezAGilbertECurtisRSecond solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationshipInt J Radiat Oncol Biol Phys201386222423323102695
- HallEJIntensity-modulated radiation therapy, protons, and the risk of second cancersInt J Radiat Oncol Biol Phys20066511716618572
- SchneiderUBessererJMackAHypofractionated radiotherapy has the potential for second cancer reductionTheor Biol Med Model20107420149259
- JoostenABochudFBaechlerSLeviFMirimanoffROMoeckliRVariability of a peripheral dose among various linac geometries for second cancer risk assessmentPhys Med Biol201156165131515121775792
- KimDWChungWKShinDRisk of second cancer from scattered radiation of intensity-modulated radiotherapies with lung cancerRadiat Oncol201384723452670
- AoyamaHWesterlyDCMackieTRIntegral radiation dose to normal structures with conformal external beam radiationInt J Radiat Oncol Biol Phys200664396296716458781
- SharmaDSAnimeshDeshpandeSSPeripheral dose from uniform dynamic multileaf collimation fields: implications for sliding window intensity-modulated radiotherapyBr J Radiol20067933133516585727
- XuXGBednarzBPaganettiHA review of dosimetry studies on external-beam radiation treatment with respect to second cancer inductionPhys Med Biol20085313R193R24118540047
- MurrayLHenryAHoskinPSiebertFAVenselaarJBRAPHYQS/PROBATE Group of the GEC ESTROSecond primary cancers after radiation for prostate cancer: a review of data from planning studiesRadiat Oncol2013817223835163
- KirovaYMGambottiLDe RyckeYVilcoqJRAsselainBFourquetARisk of second malignancies after adjuvant radiotherapy for breast cancer: a large-scale, single-institution reviewInt J Radiat Oncol Biol Phys200768235936317379448
- GaoXFisherSGMohideenNEmamiBSecond primary cancers in patients with laryngeal cancer: a population-based studyInt J Radiat Oncol Biol Phys200356242743512738317
- BirgissonHPåhlmanLGunnarssonUGlimeliusBOccurrence of second cancers in patients treated with radiotherapy for rectal cancerJ Clin Oncol200523256126613116135478
- HashibeMRitzBLeADLiGSankaranarayananRZhangZFRadiotherapy for oral cancer as a risk factor for second primary cancersCancer Lett2005220218519515766594
- TravisLBFossåSDSchonfeldSJSecond cancers among 40,576 testicular cancer patients: focus on long-term survivorsJ Natl Cancer Inst200597181354136516174857
- ChuangSCHashibeMYuGPRadiotherapy for primary thyroid cancer as a risk factor for second primary cancersCancer Lett20062381425216039041
- WiltinkLMNoutRAFioccoMNo increased risk of second cancer after radiotherapy in patients treated for rectal or endometrial cancer in the randomized TME, PORTEC-1, and PORTEC-2 trialsJ Clin Oncol201533151640164625534376
- WallisCJMaharALChooRSecond malignancies after radiotherapy for prostate cancer: systematic review and meta-analysisBMJ2016352i85126936410
- PetersLJO’SullivanBGiraltJCritical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02J Clin Oncol201028182996300120479390
- ZhuXGeYLiTA planning quality evaluation tool for prostate adaptive IMRT based on machine learningMed Phys201138271972621452709
- AmitGPurdieTGLevinshteinAAutomatic learning-based beam angle selection for thoracic IMRTMed Phys20154241992200525832090
- GuidiGMaffeiNMeduriBA machine learning tool for re-planning and adaptive RT: A multicenter cohort investigationPhys Med201632121659166627765457
- AjithkumarTPriceSHoranGBurkeAJefferiesSPrevention of radiotherapy-induced neurocognitive dysfunction in survivors of paediatric brain tumours: the potential role of modern imaging and radiotherapy techniquesLancet Oncol2017182e91e10028214420
- DaşuAToma-DaşuIFranzénLWidmarkANilssonPSecondary malignancies from prostate cancer radiation treatment: a risk analysis of the influence of target margins and fractionation patternsInt J Radiat Oncol Biol Phys201179373874620472345
- DörrWHerrmannTCancer induction by radiotherapy: dose dependence and spatial relationship to irradiated volumeJ Radiol Prot2002223AA117A12112400959
- SchneiderUHälgRBessererJConcept for quantifying the dose from image guided radiotherapyRadiat Oncol20151018826377196
- ChengCSJongWLUngNMWongJHEvaluation of imaging dose from different image guided systems during head and neck radiotherapy: a phantom studyRadiat Prot Dosimetry2017175335736227940494
- AlaeiPSpeziEReynoldsMDose calculation and treatment plan optimization including imaging dose from kilovoltage cone beam computed tomographyActa Oncol201453683984424438661
- DingGXMunroPRadiation exposure to patients from image guidance procedures and techniques to reduce the imaging doseRadiother Oncol20131081919823830468
- YangWWangLReadPLarnerJShengKIncreased tumor radio-resistance by imaging doses from volumetric image guided radiation therapyMed Phys2009362808
- FlynnRTLoss of radiobiological effect of imaging dose in image guided radiotherapy due to prolonged imaging-to-treatment timesMed Phys20103762761276920632586
- PallottaSVanziESimontacchiGSurface imaging, portal imaging, and skin marker set-up vs. CBCT for radiotherapy of the thorax and pelvisStrahlenther Onkol2015191972673326087908
- WalshKEDoddKSSeetharamanKMedication errors among adults and children with cancer in the outpatient settingJ Clin Oncol200927689189619114695
- PortaluriMFucilliFIGianicoloEACollection and evaluation of incidents in a radiotherapy department: a reactive risk analysisStrahlenther Onkol20101861269369921140128
- GlitznerMFastMFde SennevilleBDReal-time auto-adaptive margin generation for MLC-tracked radiotherapyPhys Med Biol201762118620127991457
- NyholmTOlssonCAgrupMA national approach for automated collection of standardized and population-based radiation therapy data in SwedenRadiother Oncol2016119234435027102842
- MeadowsATFriedmanDLNegliaJPSecond neoplasms in survivors of childhood cancer: findings from the childhood cancer survivor study cohortJ Clin Oncol200927142356236219255307
- CardisEHoweGRonECancer consequences of the Chernobyl accident: 20 years onJ Radiol Prot200626212714016738412
- FriedmanDLWhittonJLeisenringWSubsequent neoplasms in 5-year survivors of childhood cancer: the childhood cancer survivor studyJ Natl Cancer Inst2010102141083109520634481
- ReulenRCFrobisherCWinterDLLong-term risks of subsequent primary neoplasms among survivors of childhood cancerJAMA2011305222311231921642683
- Cowens-AlvaradoRSharpeKPratt-ChapmanMAdvancing survivorship care through the National Cancer Survivorship Resource Center: developing American Cancer Society guidelines for primary care providersCA Cancer J Clin201363314715023512728
- WoodMEVogelVNgAFoxhallLGoodwinPTravisLBSecond malignant neoplasms: assessment and strategies for risk reductionJ Clin Oncol20123033734374523008293
- GriffioenGHDaheleMde HaanPFvan de VenPMSlotmanBJSenanSHigh-dose, conventionally fractionated thoracic reirradiation for lung tumorsLung Cancer201483335636224433824
- De RuysscherDFaivre-FinnCLe PechouxCPeetersSBelderbosJHigh-dose re-irradiation following radical radiotherapy for non-small-cell lung cancerLancet Oncol20141513e620e62425456380
- FoghSEAndrewsDWGlassJHypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomasJ Clin Oncol201028183048305320479391
- BartonMBAllenSDelaneyGPPatterns of retreatment by radiotherapyClin Oncol (R Coll Radiol)2014261061161824721443
- NiederCLangendijkJAGuckenbergerMGrosuALProspective randomized clinical studies involving reirradiation: Lessons learnedStrahlenther Onkol20161921067968627534408
- MartaGNHijalTde Andrade CarvalhoHReirradiation for locally recurrent breast cancerBreast20173315916528395234
- GurenMGUndsethCRekstadBLReirradiation of locally recurrent rectal cancer: a systematic reviewRadiother Oncol2014113215115725613395
- NewhauserWDDuranteMAssessing the risk of second malignancies after modern radiotherapyNat Rev Cancer201111643844821593785
- DetappeAThomasETibbittMWUltrasmall silica-based bismuth gadolinium nanoparticles for dual magnetic resonance-computed tomography image guided radiation therapyNano Lett20171731733174028145723
- EekersDBRoelofsEJelenUBenefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trialRadiother Oncol2016121338739427639891
- GustafssonCNordströmFPerssonEBrynolfssonJOlssonLEAssessment of dosimetric impact of system specific geometric distortion in an MRI only based radiotherapy workflow for prostatePhys Med Biol20176282976298928306555