Abstract
Introduction
The aim of this study was to elucidate the association between apolipoprotein A-I (Apo A-I) and overall survival (OS) as well as cancer-specific survival (CSS) in non-muscle-invasive bladder cancer (NMIBC) patients undergoing transurethral resection of bladder tumor (TURBT).
Patients and methods
We retrospectively collected data of 470 eligible patients diagnosed with NMIBC and who received TURBT between January 2004 and December 2011. Pretreatment blood indexes were examined. The association of Apo A-I with clinicopathological characteristics was further analyzed by dichotomizing our sample into those with Apo A-I ≤ 1.19 g/L (low Apo A-I group) and those with Apo A-I > 1.19 g/L (high Apo A-I group). OS and CSS were estimated by Kaplan–Meier analysis and the log-rank test was used to compare differences between groups. Univariate and multivariate Cox regression analyses were plotted to assess the prognostic value of Apo A-I in NMIBC patients. In addition, subgroup analyses were performed according to the risk classification of the International Bladder Cancer Group.
Results
In the overall population, patients in the high Apo A-I group had greater 5-year OS and 5-year CSS rates as compared to those in the low Apo A-I group. Kaplan–Meier survival analysis revealed that higher albumin, Apo A-I, and hemoglobin levels were associated with greater OS and CSS while elevated neutrophil–lymphocyte ratio was associated with worse OS and CSS in the overall and high-risk population rather than low- and intermediate-risk population. Furthermore, Apo A-I was shown to be an independent predictor in the overall population (for OS, hazard ratio [HR], 0.364, 95% confidence interval [CI], 0.221–0.598, p < 0.001; for CSS, HR, 0.328, 95% CI, 0.185–0.583, p < 0.001) and high-risk patients (for OS, HR, 0.232, 95% CI 0.121–0.443, p < 0.001; for CSS, HR, 0.269, 95% CI, 0.133–0.541, p < 0.001).
Conclusion
These results suggest that Apo A-I level could potentially serve as a useful prognostic indicator for therapeutic decision making in NMIBC patients.
Introduction
In 2012, an estimated 429,800 patients were diagnosed with, and 165,100 patients died from, bladder cancer worldwide.Citation1 NMIBC, which is confined to the mucosa or lamina propria, accounts for approximately 80% of all newly diagnosed bladder cancers.Citation2,Citation3 TURBT is a typical first-line treatment for NMIBC patients. However, 70% of patients may suffer from recurrence after TURBT and 5-year recurrence rates are as high as 80%.Citation4 As a result, many patients undergo TURBT a second time. In addition, prognosis is worse in high-risk NMIBC patients; for example, in those with T1G3, 5-year disease-specific death rates are as high as 11.3%.Citation5 Identifying factors that predict poor oncologic outcomes after TURBT might therefore improve therapeutic decision making for NMIBC patients.
As the major protein constituent of HDL, Apo A-I protein is vital for HDL assembly and plays a major role in its atheroprotective function by facilitating reverse cholesterol transport.Citation6 Accumulating evidence has revealed associations between Apo A-I and different types of cancer. Specifically, Apo A-I expression is inversely correlated with the risk of developing breast, lung, colon, and ovarian cancer.Citation7–Citation10 The US Food and Drug Administration has also approved the use of Apo A-I as a biomarker for detecting incipient tumors in patients with early-stage ovarian cancer.Citation11,Citation12 Additionally, elevated preoperative Apo A-I levels are an independent prognostic marker for longer OS in patients with renal cell, ovarian, colorectal, nasopharyngeal, and ureter urothelial carcinoma.Citation13–Citation17 This is consistent with animal studies demonstrating that increased Apo A-I levels suppress tumor growth and metastasis in malignant melanoma, Lewis lung, and ovarian tumor models.Citation18,Citation19
NLR is widely used as a predictor of oncologic outcomes in NMIBC patients. In this study, we examined the association between Apo A-I levels and prognosis in NMIBC patients to determine whether preoperative Apo A-I levels might also predict OS and CSS in NMIBC patients undergoing TURBT.
Patients and methods
Patient selection and data collection
We retrospectively examined data from 470 patients who were initially diagnosed with NMIBC and subsequently underwent TURBT at the Department of Urology, Xuanwu Hospital Capital Medical University, between January 2004 and December 2011. Patients without previous or coexisting tumors and for whom results of routine blood tests and blood biochemical indexes (including NEUT, LYMPH, PLT, NLR, PLR, HGB, TP, ALB, GLB, A/G, PAB, TG, TC, HDL-C, LDL-C, Apo A-I, and Apo B) prior to treatment were available were included in the study. Patients for whom clinical and pathological information was incomplete, who had non-urothelial bladder carcinoma, or who had other types of concomitant malignant tumors were excluded. Pathological stage was assessed based on the 2010 American Joint Committee on Cancer classification; the 2004 World Health Organization classifications were used to determine tumor grade. In addition, subgroup analyses were performed after categorizing patients based on IBCG risk classification.Citation20,Citation21 Low risk was defined as solitary, low-grade (LG) primary Ta tumors, intermediate risk as multiple or recurrent LG tumors, and high risk as T1 or Tis or high-grade (HG) tumors. NLR was the ratio of NEUT to LYMPH, and PLR was the ratio of PLT to LYMPH. Clinicopathological features included history of smoking; pathological tumor grade; presence of CIS; lymphovascular invasion; and stage, number, and size of tumors; oncologic outcomes examined included tumor recurrence, progression, OS, and CSS. Recurrence was defined as the first pathologically confirmed tumor relapse in the bladder or upper urinary tract, regardless of tumor stage. Progression was defined as an increase in T category or tumor grade or development of lymph node or distant metastasis.Citation20 A second TURBT was routinely performed in patients who were diagnosed with T1 or HG bladder cancer on initial TURBT. Patients underwent postoperative adjuvant intra-vesicle instillations of chemotherapy on the basis of tumor characteristics. Demographic data collected at the time of the TURBT procedure at our hospital were also extracted from patients’ records. This research was approved by the Ethics Committee of Xuanwu Hospital Capital Medical University. Written informed consent forms were obtained from all enrolled patients for the use of their medical data in this study.
Patient follow-up
Patients were routinely monitored every 3 months for the first 2 years after TURBT, twice a year for the following 2 years, and annually thereafter. Follow-up investigations consisted of history, physical examination, routine laboratory studies, and cystoscopy. Computed tomography scan was performed every year to assess bladder and upper tract urothelium recurrences.
Statistical analyses
Statistical analyses were conducted using SPSS statistical software package 22.0 (IBM, Armonk, NY, USA) and Med-Calc version 12.5 (MedCalc Software, Ostend, Belgium). Routine blood test results and blood biochemical indexes are presented as medians with ranges. Differences in continuous variables were assessed using unpaired t-tests. Categorical variables were analyzed using Pearson’s chi-square test. ROC curve analyses were used to calculate appropriate cut-off values for routine blood test results and blood biochemical indexes. Through dichotomized at each possible cut-off point, the optimal cut-off value was selected with the maximal value of Youden index, when the maximal sensitivity and specificity were obtained for predicting 5-year OS. In addition, Cox proportional hazard models were applied to these survival variables measured. The end points for this study were 5-year OS and CSS. OS was defined as the time interval (in months) between the date of surgery and date of death for any reason or last follow-up. CSS was measured as the time interval (in months) from the date of surgery to date of death attributed to NMIBC. OS and CSS were estimated by Kaplan–Meier analysis, and the log-rank test was used to assess differences between groups. Multivariate Cox regression analyses were performed to determine independent prognostic value of variables associated with differences in survival. Patients were treated as censored observations if they were alive at the time of last follow-up. Two-sided p-values of less than 0.05 were considered statistically significant.
Results
Clinicopathological characteristics of 470 NMIBC patients
Patient clinicopathological characteristics are shown in . Of the 470 patients included in this study, 354 (75.32%) were male and 188 (40.00%) had a history of smoking. The median age at diagnosis was 70 years with a range of 16 to 91. Most patients (n = 342, 72.77%) had LG carcinoma; only one had concomitant CIS. The median follow-up time was 89 months with a range of 10 to 154. The median Apo A-I level was 1.09 g/L (range 0.46–3.27 g/L) and the median NLR level was 2.01 (range 0.45–20). All patients received intravesical chemotherapy and none exhibited lymphovascular invasion. Additionally, 34 (7.23%) patients received intra-arterial chemotherapy. The 5-year OS and 5-year CSS rates for the overall patient population were 86.81% and 90.05%, respectively.
Table 1 Clinicopathological characteristics of 470 NMIBC patients treated by TURBT
Cut-off value selection for serum Apo A-I and other indexes for 5-year OS prediction
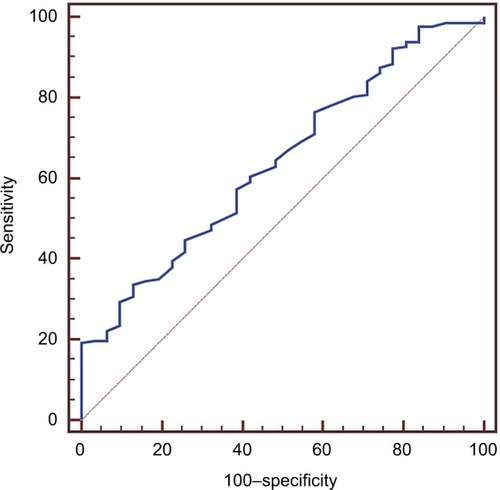
ROC curve analysis is shown . As described previously,Citation22 the optimal cut-off value of 1.19 g/L for Apo A-I level, with an area under the curve of 0.640 (95% CI, 0.575–0.700; p = 0.0062), was identified based on this analysis. Similarly, 22.28 kg/m2, 1.94 × 109/L, 1.58 × 109/L, 154 × 109/L, 133 g/L, 1.97 g/L, 127.27 g/L, 59.78 g/L, 41.11 g/L, 20.88 g/L, 1.68 mg/L, 248 mg/L, 1.10 mmol/L, 3.18 mmol/L, 0.83 mmol/L, 1.69 mmol/L, and 0.86 g/L were chosen for BMI, NEUT, LYMPH, PLT, HGB, NLR, PLR, TP, ALB, GLB, A/G, PAB, TG, TC, HDL-C, LDL-C, Apo B levels, respectively (data not shown). The Apo A-I cut-off value of 1.19 g/L was used to divide patients into low (≤1.19 g/L) and high (>1.19 g/L) Apo A-I groups. Accordingly, 326 (69.36%) patients were assigned to the low Apo A-I group and 144 (30.64%) to the high Apo A-I group.
Associations between preoperative Apo A-I level and clinicopathological characteristics
Comparisons of clinicopathological characteristics between the low and high Apo A-I groups are shown in . Patients with high Apo A-I levels were more likely to have higher ALB (p = 0.033), PAB levels (p = 0.003), and better 5-year OS (p = 0.001), 5-year CSS rates (p = 0.040) than those with low Apo A-I levels.
Table 2 Characteristics of 470 NMIBC patients grouped by Apo A-I
Association between Apo A-I, ALB, NLR, HGB and OS, CSS in the overall population
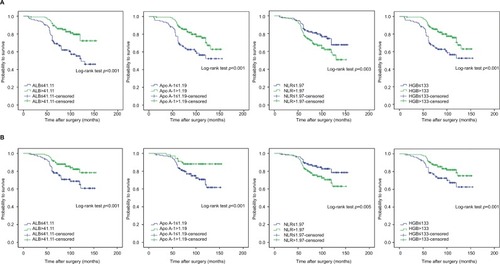
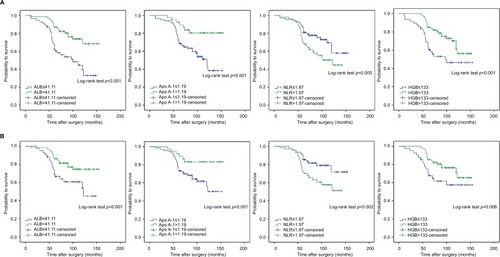
We examined whether Apo A-I, ALB, HGB levels, and NLR were associated with OS and CSS using Kaplan–Meier survival analysis. Patients were divided into two low and high groups based on preoperative Apo A-I (≤1.19 g/L vs. >1.19 g/L), ALB (≤41.11 g/L vs. >41.11 g/L), and HGB (≤133 g/L vs. >133 g/L) levels as well as NLR (≤1.97 vs. >1.97). Notably, OS and CSS were longer in patients in the high Apo A-I (>1.19 g/L), ALB (>41.11 g/L), and HGB (>133 g/L) groups. In contrast, elevated NLR (>1.97) was associated with poorer OS and CSS, as shown in .
Significant predictors of OS and CSS identified by univariate and multivariate analyses in the overall population
Results of univariate and multivariate Cox regression analysis of clinicopathological factors associated with OS and CSS are presented in and . Multivariate analysis revealed that Apo A-I level (HR, 0.364; 95% CI, 0.221–0.598; p < 0.001), age (HR, 2.388; 95% CI, 1.439–3.964; p = 0.001), BMI (HR, 0.580; 95% CI, 0.375–0.897; p = 0.014), tumor grade (HR, 1.678; 95% CI, 1.057–2.662; p = 0.028), tumor stage (HR, 1.772; 95% CI, 1.109–2.831; p = 0.017), TP level (HR, 0.373; 95% CI, 0.172–0.809; p = 0.013), ALB level (HR, 0.628; 95% CI, 0.411–0.962; p = 0.032), A/G (HR, 0.421; 95% CI, 0.261–0.678; p < 0.001), LDL level (HR, 2.310; 95% CI, 1.038–5.140; p = 0.040), and HGB level (HR, 0.590; 95% CI, 0.393–0.885; p = 0.011) were independent predictive factors for OS after adjusting for other confounding factors. Interestingly, NLR and PLR were of no apparent prognostic significance. Similarly, Apo A-I level (HR, 0.328; 95% CI, 0.185–0.583; p < 0.001) was also identified as an independent predictor of CSS in the overall patient population.
Table 3 Univariate and multivariate analyses to identify the predictive factors for OS in the overall population
Table 4 Univariate and multivariate analyses to identify the predictive factors for CSS in the overall population
Associations between Apo A-I, ALB, NLR, HGB and OS, CSS in subgroup population depending on risk status
The prognostic significance of Apo A-I, ALB, NLR, and HGB were further assessed in patient subgroups defined by IBCG risk classification. NMIBC patients in each subgroup were further subdivided into two groups according to the cut-off values for Apo A-I, ALB, NLR, and HGB levels. In high-risk patients, as in the overall patient population, elevated Apo A-I (>1.19 g/L), ALB (>41.11 g/L), and HGB levels (>133 g/L) were associated with better OS and CSS, while higher NLR (>1.97) was associated with worse OS and CSS (). In contrast, low- and intermediate-risk NMIBC patients had similar OS and CSS regardless of high or low Apo A-I, ALB, NLR, and HGB status, which may be due to their high probability of survival (data not shown).
Predictors of OS and CSS in subgroup population depending on risk status
Factors that predicted OS and CSS were then identified in the IBCG risk classification subgroups. In high-risk patients, age (HR, 2.842; 95% CI, 1.478–5.467; p = 0.002), BMI (HR, 0.444; 95% CI, 0.247–0.799; p = 0.007), tumor grade (HR, 2.451; 95% CI, 1.428–4.205; p = 0.001), TP level (HR, 0.247; 95% CI, 0.116–0.530; p < 0.001), ALB level (HR, 0.486; 95% CI, 0.274–0.860; p = 0.013), PAB level (HR, 3.404; 95% CI, 1.812–6.395; p < 0.001), Apo A-I level (HR, 0.232; 95% CI, 0.121–0.443; p < 0.001), PLT level (HR, 0.472; 95% CI, 0.223–0.997; p = 0.049), and HGB level (HR, 0.348; 95% CI, 0.207–0.584; p < 0.001) were independent predictive factors for OS (). Similarly, Apo A-I level (HR, 0.269; 95% CI, 0.133–0.541; p < 0.001) was also identified as an independent predictor for CSS in high-risk patients (). Although univariate analysis revealed that Apo A-I was significantly associated with OS and CSS in the low- and intermediate-risk patients, multivariate analysis indicated that Apo A-I was of no apparent prognostic significance after adjusting for the confounding effects of other variables (data not shown).
Table 5 Univariate and multivariate analyses to identify the predictive factors for OS in the high-risk population
Table 6 Univariate and multivariate analyses to identify the predictive factors for CSS in the high-risk population
Discussion
To the best of our knowledge, this retrospective study is the first to examine the prognostic value of Apo A-I levels in comparison to other blood indexes, NLR, and PLR in NMIBC patients who underwent TURBT. NLR is an indicator of systemic inflammatory responses and is a well-known predictor of oncologic outcomes in bladder cancer patients.Citation4 ALB is also an independent prognostic indicator in many malignancies, such as upper urinary tract urothelial, renal cell, and breast carcinomas.Citation23–Citation25 In agreement with these studies, we found here that elevated Apo A-I, ALB, and HGB levels were associated with better OS and CSS, while higher NLR was associated with worse OS and CSS. In addition, univariate analysis revealed that Apo A-I, ALB, and NLR were significantly associated with OS and CSS. However, only Apo A-I was of prognostic significance when all three indicators were included simultaneously in multivariate analysis. We also found that age and tumor grade were the most important prognostic factors for predicting OS and CSS, which was similar to a previous study examining prognostic indicators of OS in NMIBC patients.Citation26
Furthermore, analyses of patient subgroups defined by IBCG risk classification showed that elevated Apo A-I, ALB, and HGB levels were associated with better OS and CSS, while higher NLR was associated with worse OS and CSS, in high-risk NMIBC patients. However, in low- and intermediate-risk patients, Apo A-I was not associated with OS or CSS, which may result from their high probability of survival. Apo A-I level was also identified as an independent predictor of OS and CSS in high-risk patients after adjusting for the confounding effects of other variables.
As the major HDL-associated protein, Apo A-I is synthesized primarily in the liver and small intestines and shuttles redundant cholesterol from peripheral organs to the liver for excretion. Apo A-I has been extensively studied as a therapeutic agent for cardiovascular disease.Citation27–Citation29 Interestingly, Apo A-I has also been identified as an independent predictor of OS in several cancers.Citation13,Citation15–Citation17,Citation30–Citation32 Consistent with these studies, we found here that higher Apo A-I levels were strongly correlated with more favorable OS and CSS in the overall NMIBC patient population and in high-risk patients. Five-year OS and CSS rates were 83.44% and 87.94%, respectively, in patients with Apo A-I levels of 1.19 g/L or lower and 94.44% and 94.29%, respectively, in those with Apo A-I levels higher than 1.19 g/L. These findings also suggest that Apo A-I might serve as a valuable prognostic indicator in NMIBC patients undergoing TURBT.
Although the mechanisms responsible for the association between Apo A-I and antitumor properties are unclear, several plausible explanations have been proposed. Apo A-I mimetic peptides reduce levels of lysophosphatidic acid, a well-characterized promoter of ovarian cancer cell proliferation, in the serum by binding to it with high affinity.Citation19 Similarly, treatment of ID8 cells (a mouse epithelial ovarian cancer cell line) with Apo A-I mimetic peptides dramatically reduced cell viability and proliferation by upregulating the antioxidant enzyme MnSOD and decreasing HIF-1α expression.Citation33,Citation34 Apo A-I mimetic peptides can also function as novel antiangiogenesis agents as evidenced by their inhibition of human umbilical vascular endothelial cell proliferation, viability, migration, invasion, and tube formation,Citation35 though cancer-related angiogenesis may be limited in NMIBC patients. Furthermore, Apo A-I inhibits tumor-permissive features of the tumor microenvironment and promotes transformation of tumor-associated macrophages from a pro-tumor M2 to an antitumor M1 phenotype.Citation6,Citation18
Moreover, Apo A-I is upregulated in both primary and recurrent bladder cancer patients and may be a potential diagnostic biomarker for bladder cancer.Citation36–Citation39 Li et al found that Apo A-I levels were increased in urine samples from patients with bladder cancer; the authors also noted that the Apo A-I detected in urine from bladder cancer patients was not released from bladder tissue (whether cancerous or morphologically normal).Citation36 Investigations into the source of Apo A-I found in urine may improve our understanding of the role Apo A-I plays in bladder cancer. Here, we also found that preoperative Apo A-I levels increased with ALB and PAB levels, but not with NLR, in NMIBC patients. Hypoproteinemia is indicative of systemic inflammatory response, and ALB level is a well-known biomarker for malnutrition.Citation24 Low PAB levels also indicate depletion of protein resulting from LG chronic inflammation.Citation40
We think the elevated Apo A-I level may result from systemic inflammatory or immune response to bladder tumor, especially through macrophages. It has been found that tumor necrosis factor α could activate endogenous expression and secretion of Apo A-I in human macrophages, which was indeed mediated by mitogen-activated protein kinase cascades.Citation41 Moreover, Apo A-I could promote the transformation of tumor-associated macrophages from a pro-tumor M2 to an antitumor M1 phenotype and decrease the percentage of M2 macrophage by preventing their polarization.Citation6,Citation42 Interestingly, studies also demonstrated that M2 macrophage infiltration was negatively correlated with better CSS in patients with bladder cancer.Citation43 Therefore, we have reasons to believe that macrophages are involved in the association between Apo-I and survival of NMIBC patients. In addition, Apo A-I level was associated with longer survival in patients with renal cell, ovarian, colorectal, nasopharyngeal, and ureter urothelial carcinoma,Citation13–Citation17 rather than only in NMIBC patients, which may also indicate a systemic response. Undoubtedly, additional studies are still needed to determine the mechanisms that are responsible for the association between Apo A-I and inflammatory or immune processes in NMIBC patients.
In addition, two issues in this study should be noted: the low rate of concomitant CIS and patients with PUN-LMP included. The low rate of concomitant CIS may be attributed to the following reasons. First, some patients may be detected at a later stage and CIS may have progressed to muscle-invasive bladder cancer. For example, studies reported that approximately 54% of patients with CIS progress to muscle-invasive bladder cancer without treatment.Citation26 Second, concomitant CIS was found more in muscle-invasive bladder cancer patients than in NMIBC patients.Citation44 Third, the incidence of concomitant CIS was rare naturally and the number of patients was also limited in this study. Regarding the reasons for including patients with PUNLMP, there is no doubt that NMIBC patients comprise those with PUNLMP. Otherwise, a selection bias may result from excluding these patients. Another reason was that PUNLMP was essentially different from benign tumor with a 35% recurrence rate.Citation45 Moreover, numerous studies that attempted to investigate predictors of oncologic outcomes in NMIBC patients also included PUNLMP patients.Citation4,Citation46,Citation47 The retrospective, single-center design of this study, which inherently involves some degree of selection bias, limits the applicability of the results. For example, the optimal cut-off value determined by ROC curve analysis may be different from that calculated by other statistical methods and the limited number of patients may also result in a bias in cut-off value. Prospective, randomized trials are needed to validate our conclusions. In addition, NMIBC patients have relatively long survival times, and follow-up time points beyond 5 years are necessary to confirm these results. Moreover, other combined predictors were not included in our study, which probably should be adjusted as well. Finally, in-depth basic research and clinical studies are needed to identify the mechanisms responsible for the relationship between Apo A-I and survival time in NMIBC patients.
Conclusion
In this retrospective study of NMIBC patients who underwent TURBT, we found that elevated Apo A-I was associated with better OS and CSS and that higher preoperative serum Apo A-I levels were independent predictors of OS and CSS in the overall patient population and in high-risk patients. These results suggest that Apo A-I might serve as a valuable independent predictor of OS and CSS in NMIBC patients; measurement of Apo A-I levels during routine pretreatment evaluations might therefore improve therapeutic decision making for these patients.
Abbreviations
| A/G | = | albumin/globulin ratio |
| ALB | = | albumin |
| Apo A-I | = | apolipoprotein A-I |
| Apo B | = | apolipoprotein B |
| BMI | = | body mass index |
| CI | = | confidence interval |
| CIS | = | carcinoma in situ |
| CSS | = | cancer-specific survival |
| GLB | = | globulin |
| HDL-C | = | high-density lipoprotein–cholesterol |
| HG | = | high grade |
| HGB | = | hemoglobin |
| HR | = | hazard ratio |
| IBCG | = | International Bladder Cancer Group |
| LDL-C | = | low-density lipoprotein–cholesterol |
| LG | = | low grade |
| LYMPH | = | lymphocyte count |
| NEUT | = | neutrophil count |
| NLR | = | neutrophil–lymphocyte ratio |
| NMIBC | = | non-muscle-invasive bladder cancer |
| OS | = | overall survival |
| PAB | = | prealbumin |
| PLR | = | platelet–lymphocyte ratio |
| PLT | = | platelet count |
| PUNLMP | = | papillary urothelial neoplasm of low malignant potential |
| ROC | = | receiver-operating characteristic |
| TC | = | total cholesterol |
| TG | = | triglyceride |
| TP | = | total protein |
| TURBT | = | transurethral resection of bladder tumor |
Acknowledgments
The authors thank the staff of the medical record office of the Xuanwu Hospital Capital Medical University, who provided patients’ records, and all the staff who supported our study.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- TaeBSKimJKKangMPrognostic value of impaired estimated glomerular filtration rate in intravesical BCG-treated non-muscle-invasive bladder cancer patientsSci Rep201771138028469275
- Lopez-BeltranAMontironiRNon-invasive urothelial neoplasms: according to the most recent WHO classificationEur Urol200446217017615245809
- KangMJeongCWKwakCKimHHKuJHPreoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumorOncotarget201788128911290128039452
- CambierSSylvesterRJColletteLEORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance Bacillus Calmette-GuerinEur Urol2016691606926210894
- Zamanian-DaryoushMDiDonatoJAApolipoprotein A-I and cancerFront Pharmacol2015626526617517
- ChangSJHouMFTsaiSMThe association between lipid profiles and breast cancer among Taiwanese womenClin Chem Lab Med20074591219122317663634
- BorgquistSButtTAlmgrenPApolipoproteins, lipids and risk of cancerInt J Cancer2016138112648265626804063
- KimYWBaeSMLimHKimYJAhnWSDevelopment of multiplexed bead-based immunoassays for the detection of early stage ovarian cancer using a combination of serum biomarkersPLoS One201279e4496022970327
- van DuijnhovenFJBueno-De-MesquitaHBCalligaroMBlood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and NutritionGut20116081094110221383385
- ClarkeCHYipCBadgwellDProteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein III enhance the sensitivity of CA125 for detecting early stage epithelial ovarian cancerGynecol Oncol2011122354855321708402
- NosovVSuFAmneusMValidation of serum biomarkers for detection of early-stage ovarian cancerAm J Obstet Gynecol20092006639e631e63519285648
- Tuft StavnesHNymoenDAHetland FalkenthalTEKaernJTropeCGDavidsonBAPOA1 mRNA expression in ovarian serous carcinoma effusions is a marker of longer survivalAm J Clin Pathol20141421515724926085
- QuanQHuangYChenQImpact of serum apolipoprotein A-I on prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer: a propensity score-matched analysisTransl Oncol201710228829428292509
- GuoSHeXChenQThe effect of preoperative apolipoprotein A-I on the prognosis of surgical renal cell carcinoma: a retrospective large sample studyMedicine (Baltimore)20169512e314727015197
- JiangRYangZHLuoDHElevated apolipoprotein A-I levels are associated with favorable prognosis in metastatic nasopharyngeal carcinomaMed Oncol20143188025023050
- WenWJChenMKQinZKSerum apolipoprotein A-1 predicts superior prognosis in upper tract urothelial carcinoma after rapid surgeryChin J Endourol (Electronic Edition)201603193197 Chinese [with English abstract].
- Zamanian-DaryoushMLindnerDTallantTCThe cardioprotective protein apolipoprotein A-1 promotes potent anti-tumorigenic effectsJ Biol Chem201328829212372125223720750
- SuFKozakKRImaizumiSApolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancerProc Natl Acad Sci U S A201010746199972000221041624
- ManoRBanielJShoshanyONeutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancerUrol Oncol201533267e61e67
- BrausiMWitjesJALammDA review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer GroupJ Urol201118662158216722014799
- Hajian-TilakiKReceiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluationCaspian J Intern Med20134262763524009950
- KoKParkYHLeeJWKuJHKwakCKimHHInfluence of nutritional deficiency on prognosis of renal cell carcinoma (RCC)BJU Int2013112677578024028765
- CuiJYuMZhangNPrognostic scores based on the preoperative plasma fibrinogen and serum albumin level as a prognostic factor in patients with upper urinary tract urothelial carcinomaOncotarget2017840689646897328978171
- LisCGGrutschJFVashiPGLammersfeldCAIs serum albumin an independent predictor of survival in patients with breast cancer?JPEN J Parenter Enteral Nutr2003271101512549592
- BabjukMBohleABurgerMEAU Guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016Eur Urol201771344746127324428
- CamontLChapmanMJKontushABiological activities of HDL subpopulations and their relevance to cardiovascular diseaseTrends Mol Med2011171059460321839683
- DegomaEMRaderDJNovel HDL-directed pharmacotherapeutic strategiesNat Rev Cardiol20118526627721243009
- LibbyPRidkerPMHanssonGKProgress and challenges in translating the biology of atherosclerosisNature2011473734731732521593864
- WangXPLiXHZhangLHigh level of serum apolipoprotein A-I is a favorable prognostic factor for overall survival in esophageal squamous cell carcinomaBMC Cancer20161651627444612
- QuanQChenQChenPDecreased apolipoprotein A-I level indicates poor prognosis in extranodal natural killer/T-cell lymphoma, nasal typeOnco Targets Ther201691281129027051293
- LuoXLZhongGZHuLYSerum apolipoprotein A-I is a novel prognostic indicator for non-metastatic nasopharyngeal carcinomaOncotarget2015641440374404826503474
- GanapathyESuFMeriwetherDD-4F, an apoA-I mimetic peptide, inhibits proliferation and tumorigenicity of epithelial ovarian cancer cells by upregulating the antioxidant enzyme MnSODInt J Cancer201213051071108121425255
- GaoFChattopadhyayANavabMApolipoprotein A-I mimetic peptides inhibit expression and activity of hypoxia-inducible factor-1alpha in human ovarian cancer cell lines and a mouse ovarian cancer modelJ Pharmacol Exp Ther2012342225526222537771
- GaoFVasquezSXSuFL-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathwaysIntegr Biol (Camb)20113447948921283904
- LiCLiHZhangTLiJLiuLChangJDiscovery of Apo-A1 as a potential bladder cancer biomarker by urine proteomics and analysisBiochem Biophys Res Commun201444641047105224661883
- BrachaSMcNamaraMHilgartIA multiplex biomarker approach for the diagnosis of transitional cell carcinoma from canine urineAnal Biochem2014455414724704347
- LeiTZhaoXJinSMengQZhouHZhangMDiscovery of potential bladder cancer biomarkers by comparative urine proteomics and analysisClin Genitourin Cancer2013111566222982111
- FrantziMvan KesselKEZwarthoffECDevelopment and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center studyClin Cancer Res201622164077408627026199
- CaiWKongWDongBPretreatment serum prealbumin as an independent prognostic indicator in patients with metastatic renal cell carcinoma using tyrosine kinase inhibitors as first-line target therapyClin Genitourin Cancer2017153e437e44628188047
- ShavvaVSMogilenkoDANekrasovaEVTumor necrosis factor alpha stimulates endogenous apolipoprotein A-I expression and secretion by human monocytes and macrophages: role of MAP-kinases, NF-kappaB, and nuclear receptors PPARalpha and LXRsMol Cell Biochem Epub2018213
- PengMZhangQChengYApolipoprotein A-I mimetic peptide 4F suppresses tumor-associated macrophages and pancreatic cancer progressionOncotarget2017859996939970629245934
- AljaberyFOlssonHGimmOJahnsonSShaboIM2-macrophage infiltration and macrophage traits of tumor cells in urinary bladder cancerUrol Oncol Epub20171226
- KarlAGrimmTJokischFGaisaNTStiefCGNichtmuskelinvasives Harnblasenkarzinom: Aktuelles zu Diagnoseverfahren, lokalen Therapieoptionen und zum Update der WHO-Klassifikation 2016. [Non-muscle invasive bladder cancer: Current aspects of diagnostics, local therapy options and the update of the 2016 WHO classification]Urologe A201655912471258 German [with English abstract]27518790
- ChengLMontironiRLopez-BeltranATERT promoter mutations occur frequently in urothelial papilloma and papillary urothelial neoplasm of low malignant potentialEur Urol201771349749828040359
- MbeutchaAShariatSFRiekenMPrognostic significance of markers of systemic inflammatory response in patients with non-muscle-invasive bladder cancerUrol Oncol20163411483.e417e483.e424
- SylvesterRJOosterlinckWHolmangSSystematic review and individual patient data meta-analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa-T1 urothelial carcinoma of the bladder: which patients benefit from the instillation?Eur Urol201669223124426091833