Abstract
Background
Primary adenosquamous carcinoma (ASC) of the lung is a rare and aggressive disease. The accurate diagnosis of ASC based on small biopsies is challenging because of the mixed components within the tumor, and this may lead to suboptimal treatment. Furthermore, information about the efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in lung ASC is limited.
Patients and methods
Data on a cohort of patients with lung ASC who underwent surgery between October 2008 and December 2016 at a single institution were retrospectively reviewed.
Results
This study analyzed 148 patients. Differences between the pre- and post-resection diagnosis were observed. Based on the results of preoperative biopsy, patients were diagnosed as having squamous cell carcinoma (n=26), adenocarcinoma (n=20), poorly differentiated carcinoma (n=20), and large cell carcinoma (n=1), and finally diagnosed as having ASC based on histopathological examination of the surgical specimens. Thirty patients (20.3%) with EGFR-sensitizing mutations (TKI group) were treated with EGFR-TKIs after surgery, whereas the remaining patients (79.7%) with unknown EGFR-mutation status received chemotherapy or chemoradiotherapy alone (non-TKI group). TKI treatment was associated with better median overall survival (OS) (HR=0.619; p=0.034). Multivariate analysis identified the presence of EGFR-TKI treatment as an independent prognostic factor for OS (HR=0.471; p=0.003).
Conclusion
Discrepancies between the pre- and post-operative diagnosis reflect the inadequacy of non-resection approaches to the diagnosis of ASC. ASC patients harboring EGFR-sensitizing mutations who were treated with EGFR-TKIs showed a significantly better prognosis than those receiving chemotherapy or chemoradiotherapy alone.
Introduction
Adenosquamous carcinoma (ASC) is a relatively rare subtype of non-small-cell lung cancer (NSCLC), accounting for 0.4%–4% of all lung cancers.Citation1 The overall prognosis is extremely poor, and most ASC patients with stage III disease cannot survive for more than 5 years.Citation2 The World Health Organization (WHO) Classification of Lung Tumors defines ASC as “a carcinoma showing components of both squamous cell carcinoma (SCC) and adenocarcinoma (AC), with each comprising at least 10% of the tumor”.Citation3 Although ASC is characterized by morphologically mixed tumors that contain adenomatous or squamous components, studies show that ASC has a more aggressive biological behavior and a poorer prognosis than pure AC or SCC,Citation1,Citation2,Citation4,Citation5 suggesting that ASC is not simply a mix of AC and SCC.Citation6,Citation7
The presence of mixed histological components within tumors potentially may lead to inaccurate tumor classification based on small biopsies, which are limited to a partial sampling of the lesion.Citation8–Citation10 This may affect the subsequent design of optimal treatment regimens. Accurate diagnosis is particularly important in patients with lung ASC because AC is a general indication for epidermal growth factor receptor (EGFR) mutation tests, and the survival advantage of pemetrexed treatment can only be achieved in patients with non-squamous histology.Citation11,Citation12 Patients with advanced-stage ASC who are misdiagnosed as SCC may miss out on the benefits of optimal treatment, highlighting the importance of an accurate diagnosis in lung cancer. In addition, excluding the presence of SCC histology is important because bevacizumab, which targets vascular endothelial growth factor, is associated with a high risk of bleeding in SCC and is approved only for the treatment of patients with non-squamous NSCLC.Citation13 As a result, ASC patients in whom only the AC component is identified may not be indicated for bevacizumab treatment.
The best treatment options for ASC patients have not been specifically studied. EGFR tyrosine kinase inhibitors (EGFR-TKIs) are currently recommended as the first-line treatment option for advanced AC with EGFR-sensitizing mutations; however, the efficacy of EGFR-TKIs in patients with ASC remains to be evaluated in detail. Studies show that the incidence of EGFR mutations in East Asian patients with ASC is similar to that in patients with AC;Citation14,Citation15 however, the effect of EGFR-TKIs on the prognosis of ASC patients with EGFR mutation remains unclear because of the rarity of the disease and the potential impact of mixed components.
The objectives of the present study were to investigate whether the mixed structure of ASC affects the accuracy of diagnosis in all patients who have undergone a surgical resection and whether EGFR-TKI treatment can benefit patients with EGFR-positive mutations.
Patients and methods
This retrospective study was approved by the Institutional Ethics Committee of Shanghai Chest Hospital (KS (Y) 1802). Written informed consent was obtained from all patients.
Patients
Post-operatively pathologically confirmed ASC patients who underwent complete tumor resection and lymphadenectomy between January 2008 and December 2015 in the Department of Thoracic Surgery of Shanghai Chest Hospital (Shanghai, China) were included in the study.
These resected samples were reassessed based on the diagnostic criteria proposed by the 2015 edition of the WHO classification system.Citation2
Subgroups
Each surgically resected tumor was systematically sampled according to standard procedures. Paraffin-embedded tumor specimens, which included the widest cross-sections, were reassessed by two senior clinical pathologists. The classification of adenomatous and squamous components within ASCs was performed by immunohistochemical staining of surgically resected tumors. Patients with ASC were subdivided into two groups according to post-surgical EGFR-TKI treatment as follows: the EGFR-TKI group, which included patients who received the TKI treatment in either an adjuvant or a recurrent setting, and the non-TKI group, which included patients who received only chemotherapy (CTx) or chemoradiotherapy (CRTx) over the entire treatment course. Specifically, in the EGFR-TKIs group, most patients received gefitinib (Iressa®, AstraZeneca; 250 mg once daily; n=27), whereas two patients received erlotinib (Tarceva®, Roche; 150 mg once daily; n=3). In the non-TKI group, CTx (cisplatin-based doublet) or CRTx (cisplatin-based doublet plus 45–70 Gy radiotherapy) was given.
Mutational analyses and clinical assessments
The mutational status of EGFR (exons 18–21) was determined using polymerase chain reaction-based direct sequencing and verified by DNA sequencing analysis. All mutations were verified by analysis of an independent polymerase chain reaction.
Objective tumor responses regarding complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) were retrieved from the medical records.Citation16
Follow-up
Follow-up information was acquired by review of the electronic medical records and by calls. The primary endpoints of the study were overall survival (OS), which was defined as the interval between the day of recurrence and the date of death by any cause or the last follow-up date, and post-operative OS, which was defined as the interval between the day of surgery and the date of death by any cause or the last follow-up date. Recurrence was defined as the proven detection of locoregional recurrence or distant metastasis.
Statistical analyses
Normally distributed continuous variables are presented as the mean ± SD or as the median and interquartile range, while categorical variables are presented as numbers and percentages. Categorical variables were compared using contingency table analysis and chi-squared tests. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. The variables found to be significant on univariate analysis (defined as a p-value of less than 0.15) were included in the multivariate analysis using the Cox proportional hazards model after backward stepwise Wald elimination. Other clinically relevant factors based on clinical knowledge and previous reports, such as age, gender, and smoking history, are also included in the Cox proportional hazards model. All tests were two sided and a p-value <0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS software (version 19.0; IBM-SPSS, Armonk, NY, USA).
Results
The study included 148 patients with ASC. The clinical characteristics of the patients are listed in . The mean age was 59.9±10.4 years. Most of the patients were men (70.7%) and had a smoking history (57.4%). Women (53.3% vs 25.4%, p=0.003), never-smokers (70.0% vs 35.6%, p=0.001), a central tumor (56.7% vs 28.8%, p=0.008), and extended lobectomy (46.7% vs 22.0%, p=0.007) were significantly more frequent in the EGFR-TKI treatment group than in the CTx/CRTx alone group.
Table 1 Clinical characteristics of patients with primary ASCs of the lung
Differential diagnosis between pre- and post-resection
A total of 67 patients (45.3%) were diagnosed pre-operatively with SCC (n=26), AC (n=20), poorly differentiated carcinoma (n=20), and large cell carcinoma (n=1), according to cytological and/or histological analysis, and finally diagnosed as ASC according to histopathological examination of the surgical specimens, which indicates a considerable inconsistency between pre- and post-operative diagnosis.
Efficacy of EGFR-TKI treatment
Thirty patients harboring sensitizing EGFR mutations, including DEL in exon 19 (n=16) and point mutation at codon 858 (L858R) in exon 21 (n=14), received EGFR-TKIs. Of those, 26 patients (86.7%) received EGFR-TKI treatment after recurrence, and four (13.3%) received TKIs as a postoperative adjuvant therapy. Of these 30 patients, 11 achieved PR and 12 had SD following EGFR-TKI treatment, accounting for a disease control rate of 76.7% (23/30). In the non-TKI group (n=118), only 15 patients had had a definitely prospective EGFR-mutation test, including 12 with a wild-type of EGFR and three with an EGFR-sensitizing mutation, and 89 patients never had the mutation detections. For the remaining 14 cases, the information on EGFR-mutation detection was missing.
In the non-TKI group, 21 patients (75.0%) with stage I, and all patients with stage II and IIIa disease received four to six cycles of CTx after surgery.
Survival analysis
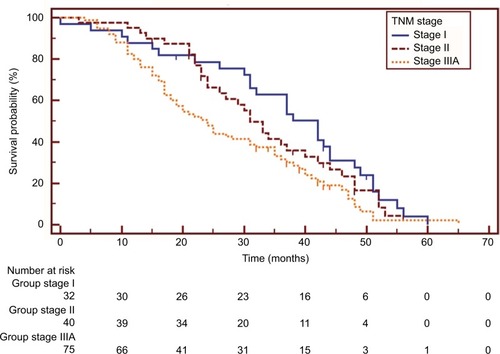
The survival data of one patient in the non-TKI group were missing. Overall, 131 patients (88.5%) had died at the last follow-up visit. The post-operative OS at 5 years and median OS were 1.6% and 31.0 (25.9–36.1) months, respectively. The median OS of patients with stage I, II, and IIIA disease was 42.0 (34.3–49.7), 31.0 (26.6–35.4), and 24.0 (18.9–29.1) months, respectively, and the difference was statistically significant (stage I vs IIIa: HR=0.579, 95% CI 0.385–0.872, p=0.011; stage II vs IIIa: HR=0.686, 95% CI 0.452–1.043, p=0.053) ().
Figure 1 Kaplan–Meier survival curves of overall survival analysis in patients with different disease stages (I vs IIIa: HR=0.579, 95% CI 0.385–0.872, p=0.011; II vs IIIa: HR=0.686, 95% CI 0.452–1.043, p=0.053).
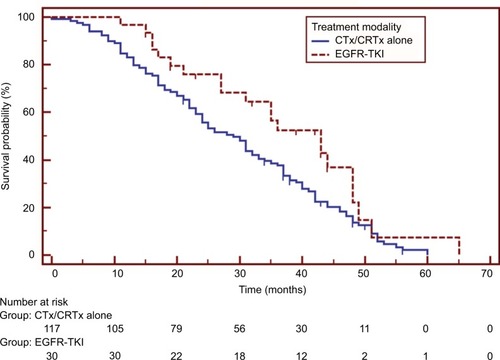
Patients in the EGFR-TKI treatment group had a significantly better median OS than those in the non-TKI group (HR=0.619, 95% CI 0.414–0.927, p=0.034), as shown in . Univariate analysis demonstrated that pathological stages and treatment modalities were significant prognostic factors for post-operative OS (). Further multivariate analysis using the Cox proportional hazards model identified pathological stage (stage I vs IIIa: HR=0.463, 95% CI 0.293–0.731, p=0.001; stage II vs IIIa: HR=0.565, 95% CI 0.366–0.871, p=0.010) and the presence of EGFR-TKI treatment (HR=0.471, 95% CI 0.286–0.775, p=0.003) as independent prognostic factors of post-operative OS ().
Table 2 Impact of individual variables on the survival of adenosquamous carcinoma patients estimated by univariate analysis
Table 3 Multivariate analysis
Figure 2 Survival comparisons between patients treated with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI group) and those with chemotherapy or chemoradiotherapy (CTx/CRTx alone group). Patients receiving EGFR-TKIs had a significantly longer survival (HR=0.619, 95% CI 0.414–0.927, p=0.034).
Discussion
Lung ASC is a highly aggressive disease. The role of CRx/CRTx is limited because of the aggressive nature of the disease and the mixed components of ASCs, and patients with lung ASC usually experience a very short disease-free time after surgery. However, information about lung ASC is limited and little progress has been made regarding the optimal management of ASC patients. This is partly attributed to the fact that there are very few patients with ASC, which, based on our study, may not be the case.
The ASC patients included in the present study underwent complete resections, which provided a sufficient number of specimens for diagnosis. By contrast, cytological analysis or small biopsy provides limited diagnostic information and thus may not be truly representative of lung cancer with a mixed histology.Citation8–Citation10,Citation17 Sixty-seven patients with post-operatively pathologically proven ASCs were pre-operatively misdiagnosed as having SCC (n=26), poorly differentiated SCC (n=20), AC (n=20), or large cell carcinoma (n=1) through cytology (diagnostic fine-needle aspiration or bronchial brush and lavage) or bronchial biopsy. This indicates a discrepancy in the diagnostic outcomes of ASC between pre-operative evaluations based on cytology or bronchial biopsy and post-operative diagnosis by immunohistochemistry, suggesting a diagnostic dilemma for ASC cases treated by non-resection approaches. This is an important issue in potential cases of ASC that are not surgical candidates, as patients with advanced-stage ASC who are misdiagnosed as SCC may miss out on the benefits of EGFR-mutation tests and TKI treatment. In addition, AC is one of the most frequent pre-operative diagnoses in our current series of ASC cases. Misdiagnosis can lead to severe complications, as SCC histology is associated with a high risk of pulmonary hemorrhage when treated with bevacizumab.Citation13 Therefore, the identification of specific biomarkers of ASC is essential. In addition, our study may provide a possible interpretation of the histological differentiation between pre- and post-treatment in lung cancer cases showing “transdifferentiation” between SCC and AC caused by drug treatment stress.Citation18,Citation19 Some of these cases are associated with the high heterogeneity of the ASC and the inadequate sampling, rather than real transdifferentiation.
The incidence of EGFR mutations and the efficacy of EGFR-TKIs have been mainly evaluated in lung AC. However, in patients with non-AC NSCLCs, the value of detecting EGFR mutations remains unclear.Citation20 In patients with ASC, genetic mutations are rarely evaluated, and research on the efficacy of EGFR-TKIs in ASC patients with EGFR mutations is limited. This is partly due to its rarity and the unclear value of assessing oncogenic mutations in ASCs. As a result, few relevant papers have been published, and most of these are retrospective evaluations of the frequency of EGFR mutations in ASCs.Citation21–Citation25 The present study compared EGFR-TKIs to conventional CTx in patients with lung ASCs. Survival comparisons between ASC patients with EGFR-sensitizing mutations who were treated with EGFR-TKIs and unselected ASC patients treated with CRx/CRTx alone after surgery indicated that ASC patients with EGFR-sensitizing mutations could benefit significantly from EGFR-TKI treatment.
The paucity of studies on the efficacy of EGFR-TKIs in lung ASC patients can be attributed to several factors, such as the relative rarity of ASC patients and the lack of awareness of the clinical significance of mutation tests in non-AC lung cancers. However, the insufficient diagnostic information provided by non-resected biopsies may be another contributory factor.
One of the major limitations of this study was the small sample size of ASC patients treated with TKIs. In addition, the frequency of EGFR mutations in the present cohort was not investigated. Therefore, both patients with mutant and wild-type EGFR may have been included in the non-TKI group, which may compromise the comparison of patient survival between the TKI and non-TKI groups. However, studies have shown no significant survival difference between adenosquamous lung cancer patients with wild-type EGFR and those with a mutation.Citation21,Citation23 In addition, the specific component within ASCs harboring EGFR mutations could not be identified because microdissection was not performed, and the mixed components were assessed separately. Finally, the effect of the different proportions of structural components within ASCs on the treatment response to TKIs was not evaluated because of the small sample size. Therefore, further studies are warranted.
Conclusion
The accuracy of non-resection approaches for the histological classification of lung ASC was relatively low, and a considerable number of potential ASC patients may miss out on optimal treatment owing to incorrect diagnostic information from the non-resected biopsies. EGFR mutation-positive ASC patients could achieve good disease control after EGFR-TKI treatment, and experience a significantly improved prognosis from EGFR-TKI treatment in comparison with unselected patients receiving standard CTx alone.
Acknowledgments
The authors are indebted to Keke Yu and Jie Xing (Department of Pathology of Shanghai Chest Hospital, Shanghai Jiaotong University) for pathological re-assessment.
Disclosure
The authors report no conflicts of interest in this work.
References
- CookeDTNguyenDVYangYSurvival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomyAnn Thorac Surg201090394394820732522
- GawrychowskiJBrulińskiKMalinowskiEPaplaBPrognosis and survival after radical resection of primary adenosquamous lung carcinomaEur J Cardiothorac Surg200527468669215784375
- TravisWDBrambillaEBurkeAPMarxANicholsonAGWHO classification of tumours of the lung, pleura, thymus and heartLyonInternational Agency for Research on Cancer2015
- MaedaHMatsumuraAKawabataTAdenosquamous carcinoma of the lung: surgical results as compared with squamous cell and adenocarcinoma casesEur J Cardiothorac Surg201241235736121737295
- FilossoPLRuffiniEAsioliSAdenosquamous lung carcinomas: a histologic subtype with poor prognosisLung Cancer2011741252921371773
- BastideKUgolinNLevaloisCBernaudinJFChevillardSAre adenosquamous lung carcinomas a simple mix of adenocarcinomas and squamous cell carcinomas, or more complex at the molecular level?Lung Cancer20106811920004040
- ZhaoHYangHYaoFImproved survival associated with a balanced structure between adenomatous and squamous components in patients with adenosquamous carcinoma of the lungEur J Surg Oncol201642111699170627365198
- SheltonDARanaDNHolbrookMTaylorPBaileySAdenosquamous carcinoma of the lung diagnosed by cytology?: a diagnostic dilemmaDiagn Cytopathol201240983083321416646
- ChenJGaoYDCaoYYangJLuoGWSurgical specimen histology revealed inadequacy of conventional transbronchial needle aspiration sample in the diagnosis of adenosquamous lung carcinomaJ Thorac Dis20157468068625973234
- ZhangCYangHZhaoHClinical outcomes of surgically resected combined small cell lung cancer: a two-institutional experienceJ Thorac Dis20179115115828203418
- ScagliottiGVParikhPvon PawelJPhase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancerJ Clin Oncol200826213543355118506025
- ScagliottiGHannaNFossellaFThe differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studiesOncologist200914325326319221167
- JohnsonDHFehrenbacherLNovotnyWFRandomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancerJ Clin Oncol200422112184219115169807
- JiaXLChenGEGFR and KRAS mutations in Chinese patients with adenosquamous carcinoma of the lungLung Cancer201174339640021592614
- SongZLinBShaoLZhangYTherapeutic efficacy of gefitinib and erlotinib in patients with advanced lung adenosquamous carcinomaJ Chin Med Assoc201376948148523769878
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- DragnevKHGehrGMemoliVATafeLJALK-rearranged adenosquamous lung cancer presenting as squamous cell carcinoma: a potential challenge to histologic type triaging of NSCLC biopsies for molecular studiesClin Lung Cancer2014153e37e4024524823
- JuknaAMontanariGMengoliMCSquamous cell carcinoma “transformation” concurrent with secondary T790M mutation in resistant EGFR-mutated adenocarcinomasJ Thorac Oncol2016114e49e5126746366
- LongoLMengoliMCBertoliniFSynchronous occurrence of squamous-cell carcinoma “transformation” and EGFR exon 20 S768I mutation as a novel mechanism of resistance in EGFR-mutated lung adenocarcinomaLung Cancer2017103242628024692
- SongXWangZClinical efficacy evaluation of tyrosine kinase inhibitors for non-adenocarcinoma lung cancer patients harboring EGFR-sensitizing mutationsOnco Targets Ther2017103119312228790845
- WangRPanYLiCAnalysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomasJ Thorac Oncol20149676076824481316
- PowrózekTKrawczykPRamlauREGFR gene mutations in patients with adenosquamous lung carcinomaAsia Pac J Clin Oncol201410434034524575772
- MorodomiYOkamotoTTakenoyamaMClinical Significance of Detecting Somatic Gene Mutations in Surgically Resected Adeno-squamous Cell Carcinoma of the Lung in Japanese PatientsAnn Surg Oncol20152282593259825373537
- ShiozawaTIshiiGGotoKClinicopathological characteristics of EGFR mutated adenosquamous carcinoma of the lungPathol Int2013632778423464964
- FanLYangHYaoFClinical outcomes of epidermal growth factor receptor tyrosine kinase inhibitors in recurrent adenosquamous carcinoma of the lung after resectionOnco Targets Ther20171023924528123305
