Abstract
Introduction
The purpose of this study was to analyze the efficacy and safety of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy for N3 stage nasopharyngeal carcinoma (NPC).
Methods
This study included 44 N3 stage NPC patients treated with concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy. The intensity-modulated radiation therapy doses were planning target volume (PTV) 70–72 Gy for gross disease in the nasopharynx and 66–70 Gy for positive lymph nodes. The doses for high-risk- and low-risk region PTV were 60–62 and 54–56 Gy in 31–33 fractions. All patients received a concurrent chemotherapy program consisting of cisplatin 100 mg/m2, day 1, and the cycle repetition was every 21 days. The adjuvant chemotherapy program consisted of 4 cycles of S-1. The dose of S-1 was determined according to the body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2≤BSA<1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2. S-1 was given on days 1–28, given 6 weeks apart.
Results
All 44 patients completed at least 2 cycles of concurrent chemotherapy and 4 cycles of adjuvant chemotherapy. The total efficiency of therapy was 100.0%. The 3-year overall survival (OS), distant metastasis-free survival (DMFS), local-regional control, and progression-free survival rates were 86.4%, 84.1%, 97.7%, and 81.8%, respectively. There were no differences in the OS, DMFS, and efficiency between fast-fading group (reaching partial response before the second cycle of concurrent chemotherapy) and general-fading group (the rest of the group). The incidence of rash in the entire group was low, and there was also no association with prognosis.
Conclusion
In patients with N3 stage NPC, concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy yielded an excellent survival benefit, and the toxicities were mild and tolerable. Distant metastasis was the main cause of treatment failure.
Introduction
More than 60,000 cases of nasopharyngeal carcinoma (NPC) are newly diagnosed each year in China, with >34,000 deaths.Citation1 However, the incidence of NPC in other countries of the world continued to decline >20% in the past 30 years. As the intensity-modulated radiation therapy (IMRT) technology is widely used in clinical applications, locoregional control (LRC) rate of NPC has been significantly improved. Currently, distant metastasis is the main reason for treatment failure, and the ratio of distant metastasis to locoregional failure is 9 to 1. Therefore, to increase the efficacy of NPC treatment, basically, the incidence of distant metastases has to be reduced. The lymph-node stage was the most important prognosis factor affecting the distant metastasis-free survival (DMFS). The 5-year DMFS of N3M0 stage NPC is 50%–70%.Citation2,Citation3
The monoclonal antibody of immune checkpoint PD-1 has proved that the objective response rate of treatment was 25.9% in a Phase Ib study of recurrent or metastatic NPC.Citation4 Immunodetection point PD-L1-positive expression rate was 25% in head and neck squamous cell carcinoma (HNSCC).Citation5 A number of Phase III clinical studies of PD-1 monoclonal antibody have been proved to achieve negative results in HNSCC.Citation6 These results told us that it may still return to chemotherapy to improve the efficacy of N3M0 stage NPC treatment.
Concurrent chemoradiotherapy plus adjuvant chemotherapy have become the standard treatment for local advanced NPC, as stated in the National Comprehensive Cancer Network Guidelines. But adjuvant chemotherapy would increase acute and late complications. Therefore, the toxicities were intolerable for the majority of NPC patients. S-1 is an oral anticancer drug of fluorouracil derivative and can be converted to 5-Fu in vivo, with the advantages of convenient, efficient, and light side effects.Citation7 The aim of the study was to investigate whether N3M0 stage NPC treatment could be optimized by concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy.
Materials and methods
Ethical statement
This study was reviewed and approved by the Ethics Committee of Hainan General Hospital, Haikou, China. Written informed consent was obtained from each patient.
Patients
We retrospectively analyzed data from 44 patients with N3M0 stage NPC who were treated in our clinic between May 2013 and September 2014. These patients had histologically confirmed nonkeratinizing carcinoma without distant metastasis. Clinical and laboratory examinations included plasma Epstein–Barr virus DNA load, magnetic resonance imaging (MRI) of head and neck, chest and abdominal computed tomography, electroconvulsive therapy (ECT), and positron emission tomography (PET) for exclusion of distant metastases. The NPC patients were classified according to the 7th Edition of American Joint Committee on Cancer tumor–node–metastasis classification. The clinical characteristics of the patients included in the study are shown in .
Table 1 Clinical characteristics of N3M0 stage nasopharyngeal carcinoma patients
IMRT
IMRT was delivered by using a simultaneous integrated boost technique. The gross tumor volume (GTV) included the nasopharynx gross tumor volume (GTVnx) and positive neck lymph nodes (GTVnd) as measured by MRI. The high-risk clinical tumor volume (CTV1) included the entire nasopharyngeal mucosa, retropharyngeal lymph nodes, skull base, parapharyngeal space, pterygopalatine fossa, sphenoid sinus, posterior third of nasal cavity, and maxillary sinus. The low-risk clinical tumor volume (CTV2) included those without lymph-node metastasis in the cervical lymph drainage area. The planning target volume (PTV) was created, based on GTVnx and each CTV with an additional 3 mm margin, and GTVnd with an additional 3–5 mm margin. PTV approaching the brain and spinal cord were shrunk accordingly. The prescribed doses were PTV 70–72 Gy for gross disease in nasopharynx, and 66–70 Gy for positive lymph nodes in 31–33 fractions. The prescribed doses for high-risk- and low-risk region PTV were 60–62 and 54–56 Gy in 31–33 fractions, respectively.
Chemotherapy
All patients received a concurrent chemotherapy program consisting of cisplatin 100 mg/m2, day 1, and the cycle repetition was every 21 days. The adjuvant chemotherapy was given after IMRT and 3 weeks from the last synchronous chemotherapy, and the program consisted of 4 cycles of S-1. The dose of S-1 was determined according to the body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2≤BSA<1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2. S-1 was received for 28 days, after stopping for 14 days, given 6 weeks apart. Throughout the whole course of chemotherapy, liver and renal function tests and routine blood and serum tests were performed.
Follow-up
Patients were examined every 3 months for the first 2 years, every 6 months for the 3 following years, and annually thereafter. Each follow-up included chest X-ray, abdominal ultrasound, and endoscopy. MRI and ECT were performed every 6 months. PET was optional in high-risk cases. Toxicities were observed and scored according to the Toxicity Criteria of the Radiation Therapy Oncology Group. Efficacy was determined using the RECIST solid tumor efficacy evaluation criteria.
Statistical analysis
Data were processed using SPSS 21.0 software. Overall survival (OS), DMFS, LRC, and progression-free survival (PFS) were analyzed by Kaplan–Meier model and follow-up periods were tabled using Excel. Comparison between groups for OS and DMFS was performed using the log-rank test. Comparison between groups for the frequency of data was carried out using the χ2-test. P-values <0.05 were considered statistically significant.
Results
Response rates
Three months after IMRT, the total efficiency of therapy was 100.0%. There were 34 (77.3%) and 10 (22.7%) cases of complete response (CR) and partial response (PR), respectively. The CR rates in the groups with lymph nodes >3 cm were inferior to those of <3 cm (P<0.05). MRI showed that lymph nodes with liquefaction necrosis subsided significantly slower than solid lymph nodes (P<0.01). Furthermore, fixed lymph nodes subsided more slowly than active lymph nodes when assessed by palpation (P<0.05).
Patterns of failure
Patients were monitored for 12–53 months, with a median follow-up duration of 40 months. There were 6 deaths, including recurrent NPC (1 case) and distant metastasis (5 cases). The overall failure rate was 20.5% (9 patients). Distant metastasis was the main cause of failure. There were 8 patients with distant metastases (18.2%); the common metastasis sites were bone (4 cases), lung (2 cases), liver (1 case), and multiple locations (1 case). The median distant metastasis time was 18 months (range, 6–43 months).
Survival analysis
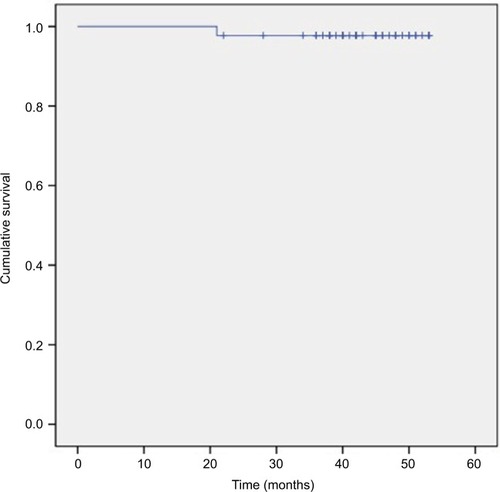
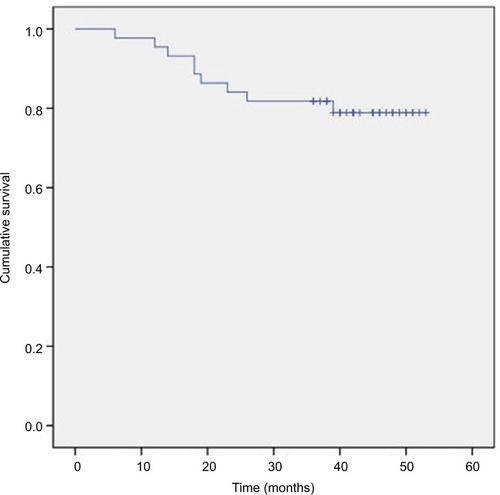
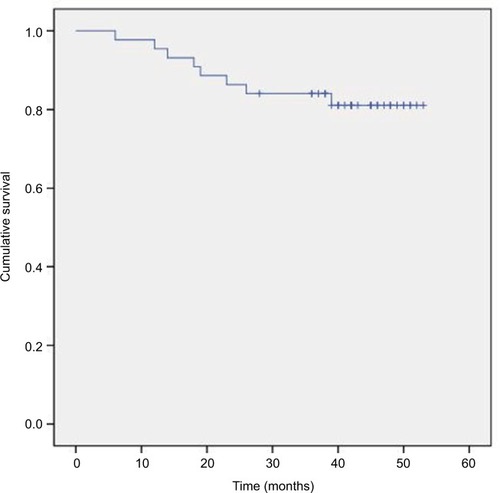
The 3-year OS, DMFS, LRC, and PFS rates were 86.4%, 84.1%, 97.7%, and 81.8%, respectively (–). We divided the tumor regression rate into fast-fading group (reaching PR before the second cycle of concurrent chemotherapy) and general-fading group (the rest of all), and there were no differences in the OS, DMFS, and efficiency between the 2 groups (all P>0.05).
Figure 1 Kaplan–Meier estimate of overall survival for N3 stage nasopharyngeal carcinoma patients treated with concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy.
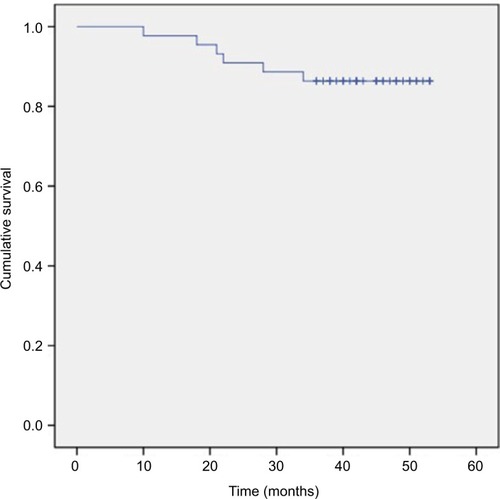
Figure 2 Kaplan–Meier estimate of distant metastasis-free survival for N3 stage nasopharyngeal carcinoma patients treated with concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy.
Toxicities
All 44 patients completed at least 2 cycles of concurrent chemotherapy and 4 cycles of adjuvant chemotherapy. The acute toxicities included myelosuppression, mucositis, xerostomia, and dermatitis (, ). Because the incidence of rash in the entire group was low, there was no association with prognosis. The total incidence of severe (≥ grade III) acute toxicities was 9.1% (4 cases). The chronic toxicities mainly were grade I (23 cases) and grade II (9 cases) radioactive xerostomia. In addition, there were 16 cases (36.4%) of hearing loss and 19 cases (43.2%) of neck skin fibrosis.
Table 2 Acute toxicities during concurrent chemoradiotherapy
Table 3 Acute toxicities during S-1 adjuvant chemotherapy
Discussion
NPC is a rare disease in the world, but is the most common head and neck cancer in Southeastern Asia. The induction chemotherapy has demonstrated improved LRC and DMFS. However, it is unclear whether OS will benefit from the induction chemotherapy.Citation8 IMRT is superior to conventional techniques in radiotherapy, with respect to technical advantages, LRC, minimizing damage to critical organs, and treatment outcomes. IMRT produces superior dose distributions with improved tumor coverage and lower doses to normal tissues. Considering the last time fraction of IMRT is completed, the tumor often does not completely disappear. So, adjuvant chemotherapy is an effective method to improve LRC and DMFS.
In recent years, oral 5-Fu has been proven to have very satisfactory results in multiple solid tumors. Twu et al and Liu et al reported that oral tegafur–uracil as adjuvant chemotherapy has achieved very good efficacy and the toxicities with tegafur–uracil were very mild in NPC.Citation9,Citation10 Stockler et al and Simkens et al concluded that oral capecitabine was effective and safe, and maintenance treatment with capecitabine was particularly suitable for overall management for advanced breast cancer and metastatic colorectal cancer.Citation11,Citation12 Capecitabine has a wider and longer range of applications. The metronomic use of capecitabine may also be a good choice for NPC, considering its low toxicities and high efficacy. A large Phase III trial evaluating the value of single-agent capecitabine as adjuvant in NPC was started in January 2017, which may provide significant results (NCT02958111). The choice of adjuvant chemotherapy was S-1 in this study, mainly to consider its advantages, including being economic and convenient, having mild toxicities (especially hand–foot syndrome and abdominal pain), blood concentration quickly reaching steady state, continuing to play a role in killing tumors.
S-1 is a combination of tegafur and gimeracil (CDHP) and oltiracetam (OXO) in a molar ratio of 1:0.4:1. Among them, tegafur is a prodrug of 5-Fu. After tegafur is administered into the body, it turns into an anticancer drug after being transformed into 5-Fu by the liver P450 enzyme. CDHP can enhance the efficacy and OXO can reduce the toxicity of tegafur, respectively.Citation13,Citation14 Since inception, S-1 has been frequently used in gastrointestinal tumors. But more and more research has demonstrated that S-1 can provide significant effects on the survival rates in head and neck cancer. However, S-1 is rarely used in NPC and a few studies have focused on the treatment of recurrent or metastatic NPC.Citation15,Citation16
In the present study, the total efficiency of the group was 100.0%, in which 34 cases (77.3%) reached CR. The efficiencies and survival rates observed in this study were better than the former results of induction chemotherapy in our unit.Citation17 We suspect that such a good result may be due to the maintenance of the blood concentration of S-1, which is more conducive to the suppression of tumor proliferation after IMRT. MRI showed that lymph nodes with liquefaction necrosis subsided significantly more slowly than solid lymph nodes. As a patient with the longest withdrawal time, the neck lymph node completely subsided 14 months after IMRT, because of refusal to do a neck dissection. We divided the tumor regression rate into fast-fading group (reaching PR before the second cycle of concurrent chemotherapy) and general-fading group (the rest of all), and there were no differences in the OS, DMFS, and efficiency between the 2 groups. Because the incidence of rash in the entire group was low, there was no association with prognosis. In the course of treatment, we asked all patients to avoid taking cold food and high-fiber diet for 3–5 days to reduce the incidence of abdominal pain and distension before taking S-1. The total incidence of severe (≥ grade III) acute toxicities was 9.1% (4 cases), the incidence and extent of mucositis were tolerable, and late adverse reactions were also acceptable. Our results on treatment failure indicate that distant metastasis was the major factor affecting treatment outcome.
How can we further improve the efficacy of NPC treatment? We believe that long-term use of oral S-1 as adjuvant chemotherapy should be further evaluated in NPC, and S-1 may be better used for about 1 year after IMRT. Besides this, not only N3 patients, but also other high-risk locoregionally advanced NPCs such as N2 and T4N1 patients may benefit from oral S-1. Because the tumor volumes are often huge for high-risk locoregionally advanced NPCs, tumor clonogenic cells showed resistance to radiation, which cannot be commonly completely killed by routine radiation dose. There were still small residual lesions after radical radiotherapy, and these lesions would proliferate to form recurrence lesions and metastasis. Therefore, it could help to cause the death of the remaining tumor cells by maintaining the S-1 blood concentration in vivo for a long time.
In summary, concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy in N3 stage NPC were feasible and resulted in a better local control rate and distant metastasis-free survival. The side effects of treatment were generally tolerated. Future studies should extend the cycles of S-1 adjuvant chemotherapy to 6 or 8 cycles in large-scale prospective clinical studies.
Acknowledgments
This study was supported by the Natural Science Foundation of Hainan Province, China (No. 805102). The authors thank all the patients and the research staff for their contributions to this project.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiangXYangJGaoTNasopharynx Cancer Epidemiology in ChinaChina Cancer20162511835840 Chinese
- ZongJLinSLinJImpact of intensity-modulated radiotherapy on nasopharyngeal carcinoma: validation of the 7th edition AJCC staging systemOral Oncol201551325425925467207
- GuoQPanJZongJSuggestions for lymph node classification of UICC/AJCC staging system: a retrospective study based on 1197 nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapyMedicine (Baltimore)20159420e80825997052
- HsuCLeeSHEjadiSSafety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 studyJ Clin Oncol201735364050405628837405
- StratiAKoutsodontisGPapaxoinisGPrognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinomaAnn Oncol20172881923193328838214
- AzourySCGilmoreRCShuklaVMolecularly targeted agents and immunotherapy for the treatment of head and neck squamous cell cancer (HNSCC)Discov Med20162111850751627448787
- WenLYouCLuXZhangLPhase II trial of concurrent chemoradiotherapy with S-1 versus weekly cisplatin for locoregionally advanced nasopharyngeal carcinomaMol Clin Oncol20153368769126137288
- WangJShiMHsiaYFailure patterns and survival in patients with nasopharyngeal carcinoma treated with intensity modulated radiation in Northwest China: a pilot studyRadiat Oncol201272922233756
- TwuCWWangWYChenCCMetronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein-Barr virus deoxyribonucleic acidInt J Radiat Oncol Biol Phys2014891212924725686
- LiuYCWangWYTwuCWPrognostic impact of adjuvant chemotherapy in high-risk nasopharyngeal carcinoma patientsOral Oncol201764152128024719
- StocklerMRHarveyVJFrancisPACapecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancerJ Clin Oncol2011294498450422025143
- SimkensLHJTinterenHVMayAMaintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer GroupLancet201538599801843185225862517
- FengtongJJiangtaoFYatingWLiliWJianboCXiaofeiWEffects of S-1 combined with radiotherapy in the treatment of nasopharyngeal cancer: a meta-analysis based on randomized controlled trialsOpen Med (Wars)20171210711428730169
- PengPJChengHOuXQSafety and efficacy of S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy: multi-institutional retrospective analysisDrug Des Devel Ther201414810831087
- PengPJLvBJWangZHMulti-institutional prospective study of nedaplatin plus S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-containing regimensTher Adv Med Oncol201792687428203299
- PengPOuXLiaoHPhase II study of gemcitabine plus S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapyTher Adv Med Oncol20168315315927239233
- ZhangSLinSHuLLobaplatin combined with docetaxel neoadjuvant chemotherapy followed by concurrent lobaplatin with intensity-modulated radiotherapy increases the survival of patients with high-risk lymph node positive nasopharyngeal carcinomaJ BUON201621116116727061544