Abstract
Objective
To explore the value of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) in predicting downstaging to stage 0–I cancer after neoadjuvant chemoradiotherapy (nCRT) in locally advanced rectal cancer.
Materials and methods
We respectively investigated pretreatment CEA, pretreatment CA19-9, posttreatment CEA, posttreatment CA19-9, pre–post-CA19-9 ratio, and pre–post-CEA ratio in 674 patients with locally advanced rectal cancer receiving nCRT and determined the patients’ thresholds by using the receiver operating characteristic curve analysis. The association between downstaging (stage 0–I after nCRT), pathological complete response, and clinicopathological parameters was evaluated using the Pearson χ2 test. The clinicopathological parameters which were found to be significantly associated with downstaging were analyzed by logistic regression models and were incorporated into a scoring system.
Results
Multivariate analysis showed that pretreatment CA19-9 level, posttreatment CEA level, pre–post-CEA ratio, and pre–post-CA19-9 ratio were significantly correlated with downstaging. Area under the curve of the scoring system was higher than that of parameters alone.
Conclusion
The 4-factor scoring system with CA19-9 level, posttreatment CEA level, pre– post-CEA ratio, and pre–post-CA19-9 ratio is of more value in predicting downstaging to stage 0–I patients with locally advanced rectal cancer after nCRT than using the parameters alone.
Introduction
Neoadjuvant chemoradiotherapy (nCRT) followed by radical surgery and adjuvant chemotherapy has become the standard treatment for locally advanced rectal cancer.Citation1–Citation4 After nCRT, 10%–30% patients could achieve pathological complete response (pCR) and 2%–50% patients could achieve complete clinical response.Citation5–Citation7 However, among the pCR or complete clinical response patients, outcomes of patients with a wait-and-see policy were similar to those who underwent surgery.Citation6,Citation8 According to previous studies, selective patients with stage ypT0-2N0 are considered eligible candidates for organ preservation.Citation9–Citation11 Accurate prediction of the response to nCRT is still under investigation. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) are cancer antigens that might elevated in the serum of patients with colorectal cancer and has been routinely used in the staging of patients with rectal cancer.Citation12–Citation14 There have been some reports investigating whether pretreatment CEA and CA 19-9 level could predict pathological responses to nCRT in rectal cancer.Citation15–Citation17 However, the combination of or the changes in the level of CEA and CA19-9 have not been investigated among locally advanced rectal cancer patients with stage ypT0-2N0 after nCRT. The purpose of this study was to assess the predictive role of CEA and CA19-9 in tumor response of nCRT for individualized treatment strategy, especially for organ-sparing management after nCRT and for patients on a wait-and-see policy.
Materials and methods
Ethics statement
Although patients’ consents were not specifically obtained for this analysis, all information was retrospectively extracted in the context of compliance with the ethical standards of the institutional and/or national research committees and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Patient medical records were analyzed retrospectively, with no individual patient identifiable information used. Therefore, The Fujian Medical University Union Hospital Ethic Review Board deemed patients’ consents unnecessary.
Patients and clinical parameters
The retrospective study was approved by the Fujian Medical University Union Hospital Ethic Review Board. Clinicopathological parameters of 674 patients were obtained from our maintained database. These patients underwent nCRT followed by total mesorectal excision (TME) at our institution between September 2007 and April 2016. The clinicopathological parameters include age, gender, levels of CEA, levels of CA19-9, treatment modality, clinical tumor-node-metastasis staging (cTNM), pathological tumor-node-metastasis staging after the neoadjuvant treatment (ypTNM). The pretreatment parameters were collected one week before the nCRT began, and the post-treatment parameters were defined as changes in the levels of the preoperative parameters 6–8 weeks after nCRT. Patients were staged according to the seventh edition of the American Joint Committee on Cancer Staging Manual. Clinical T and N staging classification were determined according to magnetic resonance imaging and endoscopic ultrasonography.
Treatment
The definitions of gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) have been published previously.Citation18 The GTV was calculated based on clinical information, including digital rectal examination, endoscopy ultrasound, and abdominopelvic magnetic resonance imaging. The CTV included a minimum of a 3 cm craniocaudal margin to the GTV in addition to the entire mesorectum, presacral, and internal iliac lymph node drainage regions. The neoadjuvant radiotherapy regimen consists of 3-dimensional conformal radiotherapy and intensity-modulated radiation therapy. Forty-five Gy was delivered to PTV of CTV in 25 fractions. A dose of 50.4 Gy was delivered to PTV of GTV with 3-dimensional conformal radiotherapy in 28 fractions, while 50 Gy was delivered to PTV of GTV with intensity-modulated radiation therapy in 25 fractions. The chemotherapeutic regimens with dosages were as follows: 1) FOLFOX4: oxaliplatin 85 mg/m2 intravenous (IV), Day 1, leucovorin 200 mg/m2 IV ×2 days, 5-FU 400 mg/m2 IV bolus ×2 days, then 600 mg/m2/d ×2 days continuous infusion. This was repeated every 2 weeks for a total of 6 months of perioperative therapy. 2) mFOLFOX6: oxaliplatin 85 mg/m2 IV Day 1, leucovorin 400 mg/m2 IV, 5-FU 400 mg/m2 IV bolus on Day 1, then 1,200 mg/m2/d ×2 days continuous infusion. This was repeated every 2 weeks for a total of 6 months of perioperative therapy. 3) CapeOX: oxaliplatin 130 mg/m2 IV, Day 1, capecitabine 1,000 mg/m2 twice daily, Days 1–14 every 3 weeks. This was repeated every 3 weeks for a total of 6 months of perioperative therapy. The neoadjuvant 5-FU based or capecitabine-based chemotherapy was initiated on the first day of radiotherapy. Also, TME was performed 6–8 weeks after the end of nCRT.
Data analysis
In this study, we defined pre–post-CEA ratio as pretreatment CEA to posttreatment CEA ratio and pre–post-CA19-9 ratio as pretreatment CA19-9 to posttreatment CA19-9 ratio. Downstaging was defined as a transition from the pretreatment clinical stage II–III (cT3-4NanyM0) to the pathologic staging of surgical specimen ypStage 0–I (ypT0-2N0M0). pCR was defined as the absence of tumor cells. The receiver operating characteristic (ROC) curve was generated and Youden’s index was calculated to evaluate the ideal cut-off values of pretreatment CEA, pretreatment CA19-9, posttreatment CEA, posttreatment CA19-9, pre–post-CA19-9 ratio, and pre–post-CEA ratio for tumor response prediction. Patients were assigned into 2 groups according to the cut-off value. The association between downstaging, pCR, and clinicopathological parameters was evaluated using the Pearson χ2 test. A logistic regression analysis was used to identify significant independent parameters for downstaging. Significant independent parameters were incorporated into a scoring system. P-value <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS software 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients’ general characteristics
Six hundred and seventy-four patients with locally advanced rectal cancer receiving nCRT were enrolled. Among the 674 patients, 444 patients were male, and 230 patients were female, with a median age of 56 at diagnosis. The median pretreatment CA 19-9 and CEA levels were 11.855 and 4.1 U/mL. Baseline characteristics are detailed in .
Table 1 Characteristics of locally advanced rectal cancer patients
ROC curves of CEA and CA19-9 for downstaging and pCR
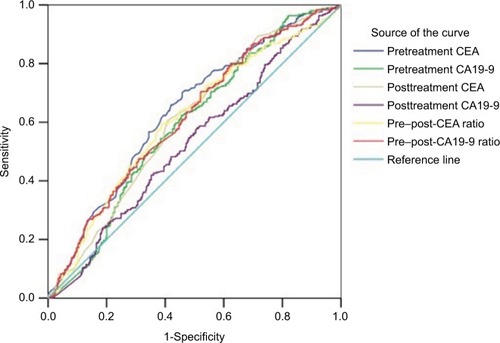
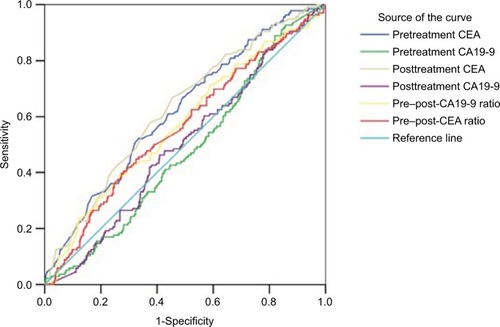
ROC curve analysis was used to evaluate cut-off values of CEA and CA19-9 for predicting downstaging and pCR. The optimum cut-off values defined by ROC curve for pretreatment CEA, pretreatment CA19-9, posttreatment CEA, post-treatment CEA, pre–post-CEA ratio, and pre–post-CA19-9 ratio to discriminate between patient’s downstaging were 5.05 U/mL (area under the curve [AUC]: 0.637, 95% CI: [0.595–0.679], P<0.0001), 12.6 U/mL (AUC: 0.59, 95% CI: [0.547–0.633], P<0.0001), 2.45 U/mL (AUC: 0.604, 95% CI: [0.562–0.647], P<0.0001), 1.56 U/mL (AUC: 0.608, 95% CI: [0.565–0.651], P<0.0001), and 1.28 U/mL (AUC: 0.615, 95% CI: [0.572–0.657], P<0.0001), respectively. The posttreatment CA19-9 was not a predictive factor of down-staging (). Meanwhile, the optimum cut-off value for pretreatment CEA, posttreatment CEA, pre–post-CEA ratio, and pre–post-CA19-9 ratio to discriminate between patients’ pCR were 2.85 U/mL (AUC: 0.619, 95% CI: [0.568–0.670], P<0.0001), 2.45 U/mL (AUC: 0.628, 95% CI: [0.577–0.679], P<0.0001), 1.07 U/mL (AUC: 0.573, 95% CI: [0.518–0.627], P=0.009), and 0.92 U/mL (AUC: 0.615, 95% CI: [0.506–0.615], P=0.029), respectively, but pretreatment CA19-9 and posttreatment CA19-9 were not predictive factors of pCR ().
Correlations of characteristic parameters with downstaging and pCR
Pearson χ2 test was performed to investigate whether CEA and CA19-9 and other characteristic parameters were associated with downstaging. This revealed that pretreatment CEA, pretreatment CA19-9, posttreatment CEA, pre–post-CEA ratio, and pre–post-CA19-9 ratio were significantly associated with downstaging (). Meanwhile, pretreatment CEA, pretreatment CA19-9, pre–post-CEA ratio, and pre– post-CA19-9 ratio were significantly associated with pCR ().
Table 2 Characteristics for downstaging with locally advanced rectal cancer after nCRT
Table 3 Characteristics for pCR with locally advanced rectal cancer after nCRT
ROC curves of a 4-factor scoring system for downstaging
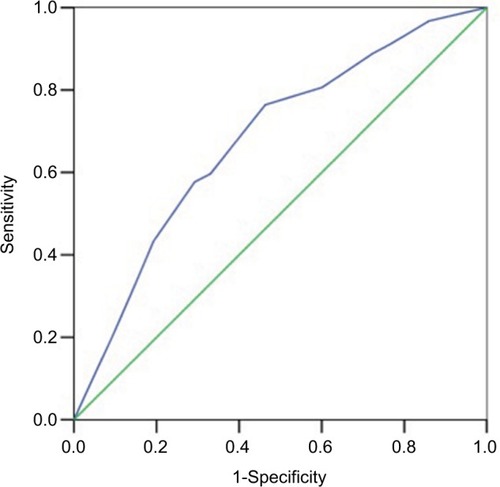
The logistic analysis showed that pretreatment CA19-9, posttreatment CEA, pre–post-CEA ratio, and pre–post-CA19-9 ratio were significantly associated with downstaging (). A 4-factor scoring system which assigns points to various variables was constructed. According to the regression coefficient values in the logistic analysis, 5 points, 9 points, 6 points, and 5 points were added for pretreatment CA19-9 level lower than 12.6 U/mL, posttreatment CEA level lower than 2.45 U/mL, pre–post-CEA ratio lower than 1.56, and pre–post-CA19-9 ratio lower than 1.28, respectively. The cut-off value of the 4-factor scoring system calculated by ROC curve was 10.5 (AUC: 0.680, 95% CI: [0.64–0.721], P<0.0001, sensitivity 76.4%, specificity 53.7%) (). AUC of the scoring system was higher than either of the parameters. These results implied that the scoring system was a significant predictor that can be superior to the use of either CEA or CA19-9 alone.
Table 4 Multivariate analyses of predictors for downstaging with locally advanced rectal cancer after nCRT
Discussion
Local control and overall survival rate of rectal cancer has markedly improved after TME. However, TME is associated with poor functional outcome and decrease of the quality of life, especially among patients with distal rectal cancer.Citation8,Citation19 nCRT reduced the rate of local recurrence and improved survival. The recurrence rate of ypT2 patients was not higher than the ypT1 or ypT0/Tis patients.Citation11,Citation20 Local excision after nCRT would be a simple and safe alternative to TME in selected patients with stage ypT0-2N0 while preserving the quality of life.Citation11,Citation20–Citation23 Accurate prediction of the response to nCRT potentially assists in individualized treatment. However, there is a lack of an ideal model that could accurately screen the stage ypT0-2N0 for organ-sparing management.
The serum CEA level is widely used as a tumor marker in rectal cancer patients. Some studies have evaluated the value of CEA in predicting the response to nCRT in rectal cancer.Citation24–Citation29 However, the cut-off point was defined by the upper limit of normal value as 5 ng/mL rather than using the ROC curve analysis, as was done in the most previous studies. Also, most of the previous studies analyzed the feasibility of pretreatment CEA level or posttreatment CEA level as the indicator for pCR, but few analyzed the combination of pretreatment CEA levels and posttreatment CEA level as the indicator of stage ypT0-2N0 cancer. Our study suggested that pretreatment CEA, posttreatment CEA and pre–post-CEA ratio was significantly associated with the stage ypT0-2N0, and pretreatment CEA and pre–post-CEA ratio was significantly associated with pCR.
Meanwhile, CA 19-9 is another widely used tumor marker in gastrointestinal tumor and is a significant predictor of survival for rectal cancer.Citation30–Citation32 However, few studies analyzed the association between CA19-9 and nCRT response. Our study suggested that pretreatment CA19-9 and pre–post-CA19-9 ratio were significantly associated with stage ypT0-2N0 tumor, and pre–post-CA19-9 ratio was significantly associated with pCR. The result was similar to a previous study.Citation17
Previous studies failed to build a scoring system for organsparing management among patients with stage ypT0-2N0 after nCRT. In the present study, multivariate analysis showed pretreatment CA19-9 level, posttreatment CEA level, pre–post-CEA ratio, and pre–post-CA19-9 ratio were significantly correlated with downstaging. A 4-factor scoring system which assigns points to various variables was constructed according to the regression coefficient values in the logistic analysis. The 4-factor scoring system with CA19-9 level, posttreatment CEA level, pre–post-CEA ratio, and pre–post-CA19-9 ratio is a better predictive biomarker related to downstaging to stage 0–I tumor after nCRT in locally advanced rectal cancer patients than using any of the parameters alone.
There are still some limitations in this study. First, the study is subject to selection bias due to retrospective nature of the analysis. Second, the data were derived from a single institution. Last but not least, the discrimination threshold in this study was determined using ROC analysis. However, it should be validated in different cohorts of patients.
Conclusion
The 4-factor scoring system with CA19-9 level, posttreatment CEA level, pre–post-CEA ratio, and pre–post-CA19-9 ratio is a better predictive model related to downstaging to stage 0–I cancer after nCRT in locally advanced rectal cancer patients than using either CEA or CA19-9 alone. Further studies are needed to validate the result.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SauerRBeckerHHohenbergerWGerman Rectal Cancer Study GroupPreoperative versus postoperative chemoradiotherapy for rectal cancerNew Engl J Med2004351171731174015496622
- SauerRLierschTMerkelSPreoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 yearsJ Clin Oncol201230161926193322529255
- RodelCLierschTBeckerHPreoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trialLancet Oncol201213767968722627104
- NCCNNCCN Clinical Practice Guidelines in Oncology Rectal Cancer Version 32017Fort Washington, PANCCN Available from: https://wwwnccnorg/professionals/physician_gls/pdf/rectal.pdfAccessed July 19, 2018
- ParkIJYouYNAgarwalANeoadjuvant treatment response as an early response indicator for patients with rectal cancerJ Clin Oncol201230151770177622493423
- Habr-GamaAPerezRONadalinWOperative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term resultsAnn Surg20042404711717 discussion 717–71815383798
- GerardJPChamoreyEGourgou-BourgadeSClinical complete response (cCR) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer. Findings from the ACCORD 12/PRODIGE 2 randomized trialRadiother Oncol2015115224625225921382
- MaasMBeets-TanRGLambregtsDMWait-and-see policy for clinical complete responders after chemoradiation for rectal cancerJ Clin Oncol201129354633464022067400
- WolthuisAMPenninckxFHaustermansKEctorsNVan CutsemED’HooreAOutcome standards for an organ preservation strategy in stage II and III rectal adenocarcinoma after neoadjuvant chemoradiationAnn Surg Oncol201118368469020842458
- LeeLKellyJNassifGJChemoradiation and local excision for T2N0 rectal cancer offers equivalent overall survival compared to standard resection: a National Cancer Database analysisJ Gastrointest Surg201721101666167428819913
- ShinYSYoonYSLimSBPreoperative chemoradiotherapy followed by local excision in clinical T2N0 rectal cancerRadiat Oncol J201634317718527730804
- Vukobrat-BijedicZHusic-SelimovicASoficACancer antigens (CEA and CA 19-9) as markers of advanced stage of colorectal carcinomaMed Arch201367639740125568506
- ZhangSYLinMZhangHBDiagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinomaInt J Clin Exp Pathol2015889404940926464695
- DressenKHermannNManekellerSDiagnostic performance of a novel multiplex immunoassay in colorectal cancerAnticancer Res20173752477248628476816
- Moureau-ZabottoLFarnaultBde ChaisemartinCPredictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancerInt J Radiat Oncol Biol Phys201180248349121093174
- FarnaultBMoureau-ZabottoLde ChaisemartinCPredictive factors of tumour response after neoadjuvant chemoradiation for locally advanced rectal cancer and correlation of these factors with survivalCancer Radiother2011154279286 French21515083
- YeoSGKimDYKimTHCarbohydrate antigen 19-9 levels associated with pathological responses to preoperative chemoradiotherapy in rectal cancerAsian Pac J Cancer Prev201415135383538725041006
- XuBChenYGuoYPretreatment tumor thickness as a predictor of pathologic complete response to neoadjuvant chemoradiation therapy for stage II/III rectal adenocarcinomaAm J Clin Oncol201841660160627672742
- JuulTBattersbyNJChristensenPValidation of the English translation of the low anterior resection syndrome scoreColorectal Dis2015171090891625807963
- Garcia-AguilarJRenfroLAChowOSOrgan preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trialLancet Oncol201516151537154626474521
- VerseveldMde GraafEJVerhoefCChemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study)Br J Surg2015102785386025847025
- ShinYSYuCSParkJHTotal mesorectal excision versus local excision after favorable response to preoperative chemoradiotherapy in “Early” clinical T3 rectal cancer: a propensity score analysisInt J Radiat Oncol Biol Phys201799113614428816139
- PerezROHabr-GamaASao JuliaoGPTransanal local excision for distal rectal cancer and incomplete response to neoadjuvant chemoradiation – does baseline staging matter?Dis Colon Rectum201457111253125925285691
- WallinURothenbergerDLowryALuepkerRMellgrenACEA – a predictor for pathologic complete response after neoadjuvant therapy for rectal cancerDis Colon Rectum201356785986823739192
- RestivoAZorcoloLCoccoIMElevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancerAnn Surg Oncol201320386487123010737
- LiQWZhengRLLingYHPrediction of tumor response after neoadjuvant chemoradiotherapy in rectal cancer using (18)fluorine-2-deoxy-D-glucose positron emission tomography-computed tomography and serum carcinoembryonic antigen: a prospective studyAbdom Radiol (New York)201641814481455
- YeoSGKimDYChangHJReappraisal of pretreatment carcinoembryonic antigen in patients with rectal cancer receiving preoperative chemoradiotherapyTumori2013991939923549007
- BuijsenJvan StiphoutRGMenheerePPLammeringGLambinPBlood biomarkers are helpful in the prediction of response to chemo-radiation in rectal cancer: a prospective, hypothesis driven study on patients with locally advanced rectal cancerRadiother Oncol2014111223724224746569
- YangKLYangSHLiangWYCarcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgeryRadiat Oncol201384323452434
- MikiHAkiyoshiTOguraAPretreatment serum carbohydrate antigen 19–9 concentration is a predictor of survival of patients who have undergone curative resection of stage IV rectal cancerDig Surg Epub201791
- ZhangLNOuYangPYXiaoWWElevated CA19-9 as the most significant prognostic factor in locally advanced rectal cancer following neoadjuvant chemoradiotherapyMedicine20159445e179326559251
- ZhangLNXiaoWWXiSYPathological assessment of the AJCC tumor regression grading system after preoperative chemoradiotherapy for Chinese locally advanced rectal cancerMedicine2016953e227226817863