Abstract
Introduction
Hepatocellular carcinoma (HCC) has a close relationship with lipid metabolism. Peroxisome proliferator-activated receptor α (PPARα) plays a crucial role in the regulation of fatty acid oxidation in the liver. However, the role of PPARα in HCC remains unclear.
Methods
A total of 804 HCC specimens were collected to construct a tissue microarray and for immunohistochemical analysis. The relationship between PPARα expression and clinical features of HCC patients was analyzed. Kaplan–Meier analysis was conducted to assess the prognostic value of PPARα expression levels.
Results
The expression of PPARα in HCC was noticeably decreased in HCC tissues. HCC patients with high levels of PPARα expression in cytoplasm had smaller tumors (P=0.027), less vascular invasion (P=0.049), and a higher proportion of complete involucrum (P=0.038). Kaplan–Meier analysis showed that HCC patients with low PPARα expression in the cytoplasm had significantly worse outcomes in terms of overall survival (P<0.001), disease-free survival (P=0.024), and the probability of recurrence (P=0.037). Similarly, overall survival was significantly shorter in HCC patients with negative PPARα expression in the nucleus (P=0.034). Multivariate Cox analyses indicated that tumor size (P=0.001), TNM stage (P<0.001), vascular invasion (P<0.001), and PPARα expression in the cytoplasm (P<0.001) were found to be independent prognostic variables for overall survival.
Conclusion
Our data revealed that PPARα expression was decreased in HCC samples. High PPARα expression was correlated with longer survival times in HCC patients, and served as an independent factor for better outcomes. Our study therefore provides a promising biomarker for prognostic prediction and a potential therapeutic target for HCC.
Introduction
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer death worldwide.Citation1 Due to the extensive heterogeneity in clinical presentation and tumor biology, the classification for HCC therapy is complicated.Citation2,Citation3 One of the heterogeneities of HCC is tumor metabolic reprogramming. Although most tumors share common metabolic transformations like aerobic glycolysis,Citation4 the metabolic phenotypes of cancer cells are highly diverse because of the tumor microenvironment. For example, primary ovarian cancer cells have highly activated lipogenesis in order to supply the lipid required for uncontrolled cell proliferation. However, when ovarian cancer metastasizes to omental fat that contains a microenvironment abundant in adipocytes, the cancer cells are metabolically reprogrammed to favor more lipid oxidation using adipocyte-derived fatty acids.Citation5 In HCC, due to the high rate of nutrient consumption and lack of vasculature, HCC cells frequently experience a stressful metabolic microenvironment, which is characterized by oxygen and nutrient deficiency.Citation6 There may therefore be adaptive metabolic reprogramming that develops within HCC cells that allows them to cope with the stressful metabolic microenvironment. A study conducted by Wang et al revealed that HCC cells display distinct lipid levels, which are positively correlated with HCC cell survival in stressful metabolic microenvironments.Citation7 In addition, acetyl-coenzyme A carboxylase alpha plays an important role in HCC cells by promoting fatty acid synthesis and thereby increasing the lipid content of these cells.
Another important approach to increasing the lipid content in HCC cells, in addition to increasing fatty acid synthesis, is to inhibit fatty acid oxidation. Previous studies have confirmed that peroxisome proliferator-activated receptors (PPARs) play a crucial role in regulating fatty acid oxidation and the upstream rate-limiting enzyme of fatty acid oxidation, carnitine palmitoyltransferase 1.Citation8 Despite similar structures, the three PPAR isotypes α, β, and γ vary greatly in their tissue distribution, pharmacology, type of endogenous ligand, and biological effects. Especially in the liver, PPARα acts as a master regulator of liver metabolism. PPARα-regulated processes are thought to be involved in all liver diseases.Citation9 Owing to the importance of PPARα in metabolism, many studies have examined the role of PPARα in tumorigenesis, with some studies implicating it in the promotion and development of cancer, with others presenting evidence for an anti-tumorigenic role.Citation10 PPARα activation increases proliferation in breast cancer cell lines and in a renal cell carcinoma cell line,Citation11,Citation12 and its persistent activation causes liver cancer in rodents, whereas PPARα null mice have been shown to be resistant to the hepatocarcinogenic effects of PPARα agonists.Citation13 Despite these findings, the role of PPARα as either a tumor suppressor or inducer in HCC remains unclear, and may differ in different species.
In this study, we investigated the role of PPARα in human HCC. We showed that PPARα expression was positively correlated with overall survival in patients with HCC, and therefore it provides a promising biomarker for prognostic prediction and is a potential therapeutic target for the clinical management of HCC.
Subjects and methods
Subjects
A total of 804 paraffin-embedded HCC specimens collected between January 2000 and December 2010 were obtained from the archives of the Department of Pathology of Sun Yat-sen University Cancer Center. None of the patients received any chemotherapy or radiotherapy prior to surgery. The follow-up period was defined as the interval from the date of surgery to the date of death or the last follow-up. This study was approved by the Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center. All tissues were anonymous and the requirement of obtaining informed consent was waived by the Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center.
Tissue microarray (TMA) construction and immunohistochemistry (IHC)
The TMA slides included 804 HCC tissues along with their adjacent normal tissues. Using a tissue array instrument (Minicore; Excilone, Elancourt, France), each tissue core was punched (diameter: 0.6 mm) from the marked areas and re-embedded. All specimens were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24 hours and embedded in paraffin wax. Following this, the paraffin-embedded HCC sections were sliced into 4-μm slices and mounted onto glass slides. After dewaxing, the slides were treated with 3% hydrogen peroxide in methanol and blocked using a biotin blocking kit (Dako Denmark A/S, Glostrup, Denmark). After blocking, the slides were incubated with a PPARα antibody (ab8934, 1:1000; Abcam, Cambridge, UK) overnight in a moist chamber at 4°C. After washing three times in PBS, the slides were incubated with biotinylated goat anti-mouse antibodies for 1 hour. The slides were then stained with 3,3′-diaminobenzidine tetrahydrochloride. Finally, the slides were counterstained with Mayer’s hematoxylin and observed under a microscope.
The level of PPARα protein expression was determined by semiquantitative IHC detection. Intensity was scored according to the standard: “0” (negative staining); “1” (weak staining); “2” (moderate staining); and “3” (strong staining). The final score was calculated by multiplying the percentage of positive expression by the intensity score. The scores were independently determined by two pathologists. The median IHC score was chosen as the cut off value for defining high and low expression.
Statistical analysis
Statistical analysis was performed using SPSS (version 16.0; SPSS Inc, Chicago, IL, USA). Student’s t-test and Pearson’s χ2 test, or Fisher’s exact test, were chosen for examining the correlations between PPARα expression level and the clinical and pathological variables. Survival curves were constructed using the Kaplan–Meier method (log-rank test). A multivariate Cox proportional hazards regression model was used to evaluate the independence of PPARα in predicting outcomes. Differences were defined as significant for P-values <0.05.
Results
Expression of PPARα in HCC TMA tissues
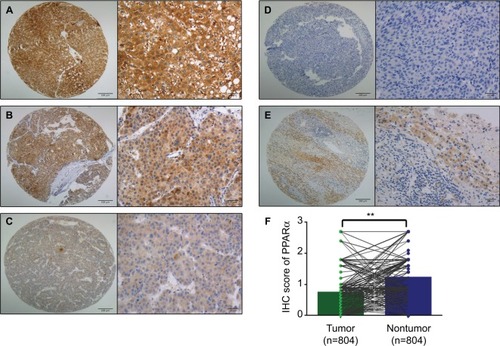
First, paraffin-embedded HCC tissues were collected to detect PPARα expression (n=804). PPARα was mainly expressed in the cytoplasm of HCC liver cancer cells, with only a small fraction of cells showing expression in the nucleus. The PPARα IHC score for HCC tissue was 0.74±0.61, being significantly lower than normal liver tissue which had a score of 1.22±0.65 (P<0.001, ).
Figure 1 PPARα is mainly expressed in the cytoplasm with only a few tissues showing nuclear expression.
Notes: Representative images of PPARα (cytoplasm) expression in HCC tissues with strong (A), moderate (B), weak (C), and negative (D) expression are shown. Representative images of PPARα expression in a nontumor sample are also shown (E) (left panel: magnification ×100; right panel: magnification ×400). PPARα expression is decreased in HCC tissues compared with the corresponding nontumor tissue as assessed by IHC (**P<0.001) (F).
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma; IHC, immunohistochemistry.
Association of cytoplasmic PPARα with HCC clinical features
To determine the potential clinical significance of PPARα in HCC, the relationship between PPARα and the clinical features of HCC patients was evaluated. Using the median IHC score in the tumor tissue (IHC score 0.9), a high level of PPARα expression was found in 15.3% (123/804) of cases. HCC patients with high levels of PPARα expression had smaller tumor sizes (P=0.027), less vascular invasion (P=0.049), and a higher proportion of complete involucrum (P=0.038), as shown in . Since HCC is a male-dominant liver disease, we also compared the expression of PPARα in male and female. However, we did not observe a significant difference of PPARα expression between male and female (0.75±0.62 vs 0.69±0.56, P=0.42).
Table 1 Association between PPARα expression in the cytoplasm and the clinical features of hepatocellular carcinoma
Association of PPARα expression in the nucleus and clinical features in HCC
There were only 24 tissues among the 804 samples that showed positive PPARα expression in the nucleus. Accordingly, we also compared tissues with negative and positive PPARα expression in the nucleus. As shown in , data showed that HCC patients with negative PPARα expression in the nucleus often had larger tumors (P=0.014). Among the HCC patients with positive PPARα expression in the nucleus, a significantly lower proportion had poor undifferentiated tumors (P<0.001), were TNM stage III–IV (P=0.036) and had an incomplete involucrum (P=0.004). We also compared the PPARα between virus-induced HCC and non-virus-induced HCC. We found no difference of PPARα expression between them either in the cytoplasm (0.71±0.55 vs 0.75±0.63, P=0.47) or in the nuclei (0.01±0.08 vs 0.01±0.07, P=0.53).
Table 2 Association between PPARα expression in the nucleus and the clinical features of hepatocellular carcinoma
Association of PPARα expression with clinical outcomes in HCC patients
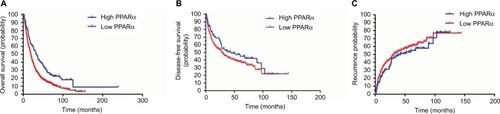
To determine the prognostic impact of PPARα expression on HCC patients, we conducted a Kaplan–Meier survival analysis using data from the 804 HCC patients enrolled in the study. In HCC patients with low PPARα expression in the cytoplasm, the Kaplan–Meier analysis revealed that the patients had significantly worse outcomes in terms of overall survival (P<0.001). Similar trends were observed for disease-free survival and the probability of recurrence, which showed that HCC patients with low PPARα expression in the cytoplasm had significantly worse outcomes than those with high PPARα expression in the cytoplasm, with worse disease-free survival (P=0.024), and a higher probability of recurrence (P=0.037), as shown in .
Figure 2 Low PPARα (cytoplasm) expression is correlated with an unfavorable prognosis in 804 HCC patients.
Notes: Kaplan–Meier analysis shows significant differences in overall survival between postoperative HCC patients with high and low PPARα (cytoplasm) expression (A). A similar trend was observed in HCC patients with high and low PPARα (cytoplasm) expression comparing disease-free survival (B) and the probability of recurrence (C).
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma.
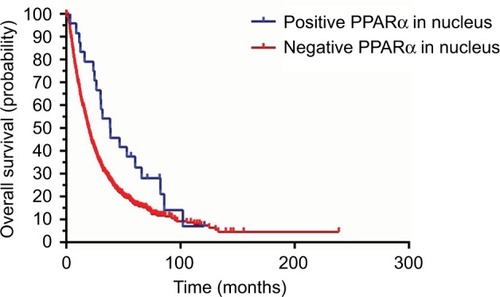
We also conducted a Kaplan–Meier survival analysis to explore the differences in prognosis for HCC patients with both positive and negative PPARα expression in the nucleus. The data indicated that HCC patients with negative PPARα expression in the nucleus always had poorer outcomes, as shown in . The overall survival was significantly shorter in HCC patients with negative PPARα expression in the nucleus (P=0.034). However, we did not find a prognostic impact of PPARα expression in the nucleus in HCC patients with respect to disease-free survival and the probability of recurrence (data not shown).
Figure 3 Positive PPARα (nucleus) expression is correlated with favorable prognosis in HCC patients. Kaplan–Meier analysis shows significant differences in overall survival between HCC patients with positive and negative PPARα (nucleus) expression. However, there was no significant difference observed with positive and negative PPARα (nucleus) expression comparing disease-free survival and the probability of recurrence.
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma.
Univariate and multivariate analyses of prognostic variables in HCC
To evaluate whether PPARα expression was an independent risk factor for outcomes in HCC, both univariate and multivariate analyses were conducted. Age, serum α-fetoprotein level, tumor size, tumor multiplicity, tumor differentiation, TNM stage, vascular invasion, involucrum, and PPARα expression in the cytoplasm and nucleus were all shown to be prognostic variables for overall survival in HCC patients. In the multivariate analysis, only tumor size (P=0.001), TNM stage (P<0.001), vascular invasion (P<0.001), and PPARα expression in the cytoplasm (P<0.001) were found to be independent prognostic variables for overall survival ().
Table 3 Univariate and multivariate analyses of prognostic variables for overall survival
Subgroup analyses of prognostic value of PPARα expression in the cytoplasm in HCC
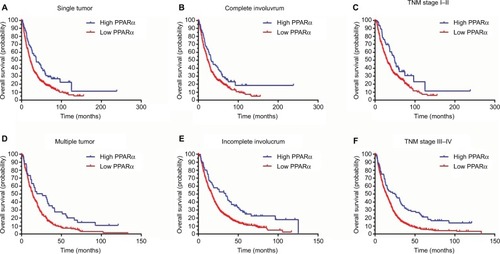
A stratified survival analysis was also conducted to further reveal the prognostic significance of PPARα expression in the cytoplasm among HCC patients. The Kaplan–Meier survival analysis showed that PPARα expression in the cytoplasm was associated with overall survival in both single and multiple HCCs (single HCCs: P=0.001, multiple HCCs: P=0.003), in complete and incomplete involucrum HCCs (complete involucrum HCCs: P=0.027, incomplete involucrum HCCs: P<0.001), and in TNM stage I–II HCCs and III–IV HCCs (TNM stage I–II HCCs: P=0.021, TNM stage III–IV HCCs: P<0.001), as shown in .
Figure 4 Low PPARα (cytoplasm) expression is associated with unfavorable outcomes in subgroups of HCC patients.
Notes: A stratified survival analysis was conducted for PPARα (cytoplasm) expression and unfavorable outcomes in single (A) and multinodular HCC (D), in HCCs with complete (B) and incomplete involucrum (E), in HCCs with TNM stage I–II (C) and stage III–IV (F).
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma.
Discussion
HCC is an end-stage liver disease with chronic viral infection accounting for most of the HCC etiology worldwide, especially in Asia.Citation14–Citation16 Recently, the rate of nutrient-associated HCC such as non-alcohol fatty liver disease-induced HCC has been found to be increasing.Citation17,Citation18 In addition, the reprogramming of energy metabolism is one of the hallmarks of cancer.Citation19 Increasing evidence suggests that solid tumors might be also reliant on non-glucose carbon sources.Citation19 Cancer cells show increased expression of enzymes involved in de novo fatty acid synthesis, which is very important for cellular biosynthesis such as in cell membranes.Citation20 The more lipid content in tumor cells, the higher the invasiveness.Citation21 However, to increase lipid content for cellular biosynthesis, inhibition of lipid oxidation is a potential approach besides increasing de novo lipogenesis. PPARα plays the central role in the lipid oxidation pathway in hepatocytes. Hence, we evaluated the expression of PPARα in HCC and explored the relationship of PPARα with HCC prognosis.
The PPAR family belongs to the nuclear hormone receptor superfamily. Like other nuclear hormone receptors, PPARs have four functional domains: amino-terminal domain, DNA binding domain, transcriptional activity regulation domains, and ligand binding domains.Citation22,Citation23 PPAR is located in the cytoplasm and when activated by ligands, it transforms into a heterodimer with retinoic X receptors (RXRs) or glucocorticoid receptor and then enters the nucleus and binds to the peroxisome proliferator response element to activate target gene transcription.Citation22,Citation24,Citation25 Peroxisome proliferator response element is a specific DNA sequence located upstream of PPAR target genes. PPARα is a member of the PPAR family, which contains three members: PPARα, PPARδ, and PPARγ. As the first protein identified in the PPAR family, PPARα is mostly expressed in the liver and brown adipose tissue, followed by heart, kidney, and skeletal muscle, where it plays an important role in lipid metabolism. Previous reports have shown that PPARα plays an important role in regulating cell proliferation and maintaining the metabolic balance in cells, regulating tumorigenesis, as well as other cell biology processes.Citation23 However, there are controversial data surrounding the role of PPARα in tumorigenesis.
In colon cancer, it is well-established that sustained chronic colon inflammation can promote the occurrence of colon cancer. One study reported that increased PPARα expression could inhibit the expression of pro-inflammatory cytokines such as interleukin-17 and interferons γ, which implies an anti-tumorigenic effect of PPARα in colon cancer.Citation26 In breast cancer, one study found lack of PPARα expression in patients with basal-like breast cancer.Citation27 In human breast cancer (MCF-7) and human cervical cancer (A2780) cell lines, PPARα reduces the expression of VEGF by promoting the ubiquitination of hypoxia inducible factor alpha.Citation28 On the other hand, studies have also revealed the tumorigenic impact of PPARα in breast cancer. PPARα can be activated by leukotrienes produced by regulatory B cells and in this way promote cancer metastasis.Citation29 Activating PPARα can also lead to the upregulation of the expression of cyclin E and the promotion of breast cancer proliferation.Citation30 The role of PPARα in cancer is still unknown and requires further exploration. It is possible that PPARα activation could reduce or promote tumorigenesis, depending on the type of tissue and different PPARα ligands. Especially in HCC, since PPARα is primarily expressed in liver tissue, the role of PPARα in HCC tumorigenesis is still unknown. Activation of PPARα has effects on fatty acid catabolism, hepatocyte proliferation, hepatomegaly, and is closely related to the occurrence of HCC.Citation31 However, fenofibrate, an oral agonist of PPARα, can inhibit the proliferation of Huh7 cells, an HCC cell line, by inhibiting the Akt pathway.Citation32 It is also well-known that sustained activation of PPARα induces HCC in rodents.Citation33
In this study, we found that the expression of PPARα in HCC tissue was significantly lower than in normal liver tissue. Interestingly, we found that PPARα is mainly expressed in the cytoplasm of liver cancer cells, with only a small fraction of cancer cells expressing PPARα in the nucleus. In the cytoplasm, following activation by ligands such as fatty acids, PPARα forms a heterodimer after which it translocates from the cytoplasm to the nucleus,Citation34 where the heterodimer acts as a transcription factor by binding to peroxisome proliferator response elements located upstream of target genes, thus activating target gene transcription. In this way, PPARα regulates lipid oxidation, inflammation, and immune-related gene expression.Citation34 Based on the results of our study, we believe that PPARα activation is inhibited in HCC cells. The mechanism underlying this may be that inhibition of PPARα activation will inhibit fatty acid oxidation, thereby increasing the intracellular lipid content, which can then be used to synthesize important cellular constituents such as cell membranes, or for use as an energy store to deal with metabolic stress, as has previously been reported.Citation7 However, this hypothesis needs to be explored further.
Based on the results of our study, we found that a high level of PPARα expression in the cytoplasm was associated with smaller tumor sizes, less vascular invasion, and a higher proportion of complete involucrum. Similarly, positive PPARα expression in the nucleus was often accompanied with a smaller tumor size and a significantly lower proportion of poor, undifferentiated tumors, tumors at TNM stage III–IV, and an incomplete involucrum. Previous studies have reported that activating PPARα can inhibit cancer cell growth and reduce vessel formation.Citation28,Citation35 In HCC, PPARα may play a similar role in reducing HCC cell proliferation and vessel formation, since our data indicated that high PPARα expression in the cytoplasm and positive PPARα expression in the nucleus are accompanied by smaller tumor sizes and less vascular invasion. The specific role of PPARα in the development of HCC needs to be studied further to confirm this hypothesis.
The prognostic implications of PPARα expression in HCC have not been previously reported. In our study, PPARα was identified as an independent factor for overall survival in a large cohort of 804 patients with HCC. Patients with high levels of PPARα expression usually survived for a longer period. These data suggest that PPARα expression has clinical implications in predicting outcomes in HCC patients. PPARα is a major regulator of lipid metabolism in the liver; the relationship between liver fat content and PPARα expression needs to be further explored. In addition, in our study, only PPARα expression in HCC cell cytoplasm was prognostic for disease-free survival and the probability of recurrence. To further confirm the prognostic effect of PPARα expression in the nucleus for disease-free survival and the probability of recurrence among HCC patients, further study will be required involving more patients positive for PPARα expression in the HCC cell nucleus.
Retinoids and their receptors have a close relationship with PPAR.Citation36,Citation37 RXRs and retinoic acid receptors (RAR) are two main nuclear receptors binding with retinoids. Retinoids are a group of structural and functional analogs of vitamin A and play an important role in the regulation of cell proliferation and differentiation.Citation38,Citation39 Both RXR and RAR are composed of three subtypes, α, β, and γ. RAR binds with 9-cis retinoic acid and all-trans-retinoic acid while RXR only binds with 9-cis retinoic acid. A study reported that RXRα was decreased not only in HCC, but also in the early stage of liver carcinogenesis.Citation40,Citation41 In addition, studies have also revealed that hepatocarcinogenesis is accompanied by the accumulation of a phosphorylated form of RXRα, which is an inactive form of RXRα and abolishes its ability to form heterodimers with other nuclear receptors.Citation42 Evidence focusing on the relationship between RAR and HCC is still limited. Studies show that RARb is a tumor suppressor gene.Citation43 Enhanced RARβ expression correlates with the growth inhibitory effect of cancer cells and the absence of RARβ expression is accompanied with tumor progression.Citation44 In addition, overexpression RARβ by vector induce drug sensitivity of tumor cells and suppresses proliferation.Citation45 However, the specific role of RXR subtypes and RAR subtypes in HCC still needs further exploration.
In summary, our data demonstrate a role for PPARα in the development of HCC. Data reveal that PPARα expression is decreased in HCC samples and, unlike PPARα in rodent liver, PPARα in human HCC may have an antitumor effect. Increases in PPARα expression were significantly correlated with improved tumor differentiation and less vascular invasion. High PPARα expression was correlated with longer survival times in HCC patients and served as an independent factor for better outcomes. Collectively, our data suggest that PPARα is a promising biomarker for the prognosis of patients with HCC.
Acknowledgments
This study was supported by grants from the National Key R&D Program of China (2017YFC1309000), National Natural Science Foundation of China (nos 81572405, 81572406, 81502079, 81602135), and Science and Technology Program of Guangzhou (201707020038).
Disclosure
The authors report no conflicts of interest in this work.
References
- FornerAReigMBruixJHepatocellular carcinomaLancet20183911301131429307467
- CaiSHLuSXLiuLLZhangCZYunJPIncreased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinomaTherap Adv Gastroenterol201710761771
- ChenSLLiuLLLuSXHBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasisMol Oncol2017111225124028580773
- VanderHMCantleyLCThompsonCBUnderstanding the Warburg effect: the metabolic requirements of cell proliferationScience20093241029103319460998
- NiemanKMKennyHAPenickaCVAdipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growthNat Med2011171498150322037646
- HirayamaAKamiKSugimotoMQuantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometryCancer Res2009694918492519458066
- WangMDWuHFuGBAcetyl-coenzyme A carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patientsHepatology2016631272128626698170
- ChungSKimYJYangSJLeeYLeeMNutrigenomic functions of PPARs in obesogenic environmentsPPAR Res20162016479457628042289
- MelloTMaterozziMGalliAPPARs and mitochondrial metabolism: from NAFLD to HCCPPAR Res20162016740323028115925
- YoussefJBadrMPeroxisome proliferator-activated receptors and cancer: challenges and opportunitiesBr J Pharmacol2011164688221449912
- SuchanekKMMayFJRobinsonJAPeroxisome proliferator-activated receptor alpha in the human breast cancer cell lines MCF-7 and MDA-MB-231Mol Carcinog20023416517112203367
- AbuAOWetterstenHIWeissRHInhibition of PPARα induces cell cycle arrest and apoptosis, and synergizes with glycolysis inhibition in kidney cancer cellsPLoS One20138e7111523951092
- PetersJMCattleyRCGonzalezFJRole of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643Carcinogenesis199718202920339395198
- CaiSCaoJYuTXiaMPengJEffectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pretreated with interferon compared with de novo therapy with entecavir and telbivudineMedicine (Baltimore)201796e702128562554
- CaiSYuTJiangYZhangYLvFPengJComparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week resultClin Exp Med20161642943626164128
- CaiSHLvFFZhangYHJiangYGPengJDynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHBBMC Infect Dis2014148524528480
- OuHCaiSLiuYXiaMPengJA noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis BTherap Adv Gastroenterol201710207217
- ShaohangCZejinODuanLRisk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndromeUnited Eur Gastroenterol J20186558566
- PavlovaNNThompsonCBThe emerging hallmarks of cancer metabolismCell Metab201623274726771115
- SunamiYRebeloAKleeffJLipid metabolism and lipid droplets in pancreatic cancer and stellate cellsCancers (Basel)201710E329295482
- LiZKangYLipid metabolism fuels cancer’s spreadCell Metab20172522823028178563
- HanLShenWJBittnerSKraemerFBAzharSPPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-alphaFuture Cardiol20171325927828581332
- GaoJYuanSJinJShiJHouYPPARα regulates tumor progression, foe or friend?Eur J Pharmacol201576556056426409040
- HuangJYBiYZuoGWFengTEffects of HBx on localization of PPARγ in HepG2 cell lineActa Academiae Medicinae Militaris Tertiae20081816931696
- MoranEPMaJXTherapeutic effects of PPAR alpha on neuronal death and microvascular impairmentPPAR Res2015201559542625705219
- LeeJWBajwaPJCarsonMJFenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient miceGastroenterology200713310812317631136
- BakerBGBallGRRakhaEALack of expression of the proteins GMPR2 and PPARα are associated with the basal phenotype and patient outcome in breast cancerBreast Cancer Res Treat201313712713723208589
- ZhouJZhangSXueJActivation of peroxisome proliferator-activated receptor α (PPARα) suppresses hypoxia-inducible factor-1α (HIF-1α) signaling in cancer cellsJ Biol Chem2012287351613516922932900
- WejkszaKLee-ChangCBodogaiMCancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor alphaJ Immunol20131902575258423408836
- ChangNWWuCTChenDRYehCYLinCHigh levels of arachidonic acid and peroxisome proliferator-activated receptor-alpha in breast cancer tissues are associated with promoting cancer cell proliferationJ Nutr Biochem20132427428122902323
- ScaleraATarantinoGCould metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease?World J Gastroenterol2014209217922825071314
- YamasakiDKawabeNNakamuraHFenofibrate suppresses growth of the human hepatocellular carcinoma cell via PPARα-independent mechanismsEur J Cell Biol20119065766421514001
- TanakaNMoriyaKKiyosawaKKoikeKGonzalezFJAoyamaTPPARα activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in miceJ Clin Invest200811868369418188449
- AdeghateEAdemAHasanMYTekesKKalaszHMedicinal chemistry and actions of dual and pan PPAR modulatorsOpen Med Chem J20115939821966330
- MartinassoGOraldiMTrombettaAInvolvement of PPARs in cell proliferation and apoptosis in human colon cancer specimens and in normal and cancer cell linesPPAR Res200720079341617389773
- WagnerCEJurutkaPWMarshallPAHeckMCRetinoid X receptor selective agonists and their synthetic methodsCurr Top Med Chem20171774276727320333
- TripathySChapmanJDHanCYAll-trans-retinoic acid enhances mitochondrial function in models of human liverMol Pharmacol20168956057426921399
- MarkMGhyselinckNBChambonPFunction of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesisAnnu Rev Pharmacol Toxicol20064645148016402912
- ChambonPA decade of molecular biology of retinoic acid receptorsFASEB J1996109409548801176
- HoshikawaYKankiKAshlaAAc-Jun N-terminal kinase activation by oxidative stress suppresses retinoid signaling through proteasomal degradation of retinoic acid receptor α protein in hepatic cellsCancer Sci201110293494121272161
- Matsushima-NishiwakiRShidojiYNishiwakiSYamadaTMoriwakiHMutoYAberrant metabolism of retinoid X receptor proteins in human hepatocellular carcinomaMol Cell Endocrinol19961211791908892319
- YoshimuraKMutoYShimizuMPhosphorylated retinoid X receptor alpha loses its heterodimeric activity with retinoic acid receptor betaCancer Sci2007981868187417900311
- MooreDMKalvakolanuDVLippmanSMRetinoic acid and interferon in human cancer: mechanistic and clinical studiesSemin Hematol1994313137
- LippmanSMLotanRAdvances in the development of retinoids as chemopreventive agentsJ Nutr2000130479S482S10721933
- SunSYLotanRRetinoids and their receptors in cancer development and chemopreventionCrit Rev Oncol Hematol200241415511796231
