Abstract
Background
The purpose of this study was to investigate the prognostic values of Nutritional Risk Screening 2002 (NRS-2002) and hematologic inflammation markers in patients with esophageal squamous cell carcinoma (ESCC) receiving curative esophagectomy.
Materials and methods
A total of 277 patients with ESCC treated with standard curative esophagectomy were retrospectively analyzed. These patients were grouped for further analysis according to the systemic inflammation score (SIS), the combination of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (CNP) score and NRS-2002 score. The Kaplan–Meier method and log-rank test were adopted to calculate and compare the progression-free survival (PFS) and overall survival (OS) rates with these parameters. The Cox proportional hazards model was used to carry out univariate and multivariate analyses. Receiver operating characteristic (ROC) curves were applied to verify the accuracy of SIS, CNP and NRS-2002 for survival prediction.
Results
In univariate analysis, the following factors were significantly associated with poor PFS and OS: sex, T stage, N stage, TNM stage, SIS, CNP and NRS-2002 (all P<0.05). Furthermore, multivariate Cox regression analysis showed that CNP (hazard ratio [HR]=1.602; 95% confidence interval [CI] 1.341–1.913; P=0.000), NRS-2002 (HR=2.062; 95% CI 1.523–2.792; P=0.000) and TNM stage (HR=1.194; 95% CI 1.058–1.565; P=0.048) were independent prognostic factors for PFS. Correspondingly, CNP (HR=1.707; 95% CI 1.405–2.074; P=0.000), NRS-2002 (HR=2.716; 95% CI 1.972–3.740; P=0.000) and TNM stage (HR=1.363; 95% CI 1.086–1.691; P=0.036) were also independent prognostic factors for OS. Finally, the results of ROC curves indicated that CNP and NRS-2002 were superior to SIS as a predictive factor for PFS or OS in patients with ESCC receiving surgery.
Conclusion
This study demonstrated that CNP combined with NRS-2002 is promising as a predictive marker for predicting clinical outcomes in patients with ESCC receiving surgery.
Introduction
Esophageal cancer (EC) is the eighth most common malignancy and the fifth most common cause of cancer death all over the world.Citation1 People’s Republic of China accounts for about half of the world’s total cases of EC,Citation2 and esophageal squamous cell carci noma (ESCC) is the most lethal pathological type.Citation3 Despite significant improvements in the diagnosis and treatment, the prognosis of ESCC is still poor due to its aggressive biological behavior.Citation4 At present, surgical resection remains the best curative method for non-metastatic EC patients. Nevertheless, most of the patients still develop local relapse or distant metastasis after esophagectomy, and so the 5-year overall survival (OS) rate is still unfavorable and ranges from 26.2% to 49.4%.Citation5 Therefore, it is critical to search some biomarkers for distinguishing patients who are likely to develop recurrence following surgery from patients who are not easy to relapse.
Recently, there is increasing evidence that the survival of cancer patients is determined not only by tumor itself, but also by host-related factors, such as the preoperative nutritional and inflammatory status. Essentially, EC patients have a high risk of being malnourished prior to treatment, and there is accumulating evidence demonstrating that poor nutritional status is associated with inferior clinical prognosis in patients who underwent esophagectomy.Citation6–Citation8 Therefore, pretreatment nutritional condition is important for the prognosis of ESCC in patients receiving surgery. At present, there are many assessment methods applied to nutritional evaluation;Citation9–Citation11 among these, Nutritional Risk Screening 2002 (NRS-2002) was a new evaluation system, published by the European Society for Clinical Nutrition and Metabolism (ESPEN) in 2002 and was based on 128 randomized controlled trials. It was the first system in the world that was developed via evidence-based medicine with a great advantage of predicting malnutrition risk,Citation11 especially in patients with carcinoma. Chen et alCitation12 found that the standard of the NRS-2002 was feasible in China.
In addition, there are several studies demonstrating that the presence of a systemic inflammatory response and malnutrition were associated with a worse prognosis in various malignancies,Citation13–Citation16 and the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte– monocyte ratio (LMR) have been studied in EC.Citation17–Citation19 Recently, systemic inflammation score (SIS), a novel prognostic score consisting of serum albumin and LMR, and CNP (the combination of NLR and PLR) may be better predictive factors for postoperative clinical outcome in malignancies. To the best of our knowledge, SIS and CNP have been well documented in other types of human malignancies, including EC,Citation20–Citation22 but the combination of nutritional status and hematological markers has rarely been studied in ESCC patients. Therefore, we conducted this retrospective study, attempting to investigate the correlations of preoperative NRS-2002, CNP and SIS with their prognostic impacts on progression-free survival (PFS) and OS in ESCC patients.
Materials and methods
Patients
Between January 2010 and December 2013, a total of 277 esophageal carcinoma patients who underwent esophagectomy and lymph node dissections at the Department of Thoracic Surgery, The First Affiliated Hospital of Soochow University, were recruited in this retrospective research. The inclusion criteria were as follows: 1) curative esophagectomy with R0 resection and no presence of preoperative adjuvant therapy; 2) histologically proven ESCC; 3) normal liver and renal function, without severe dysfunction of important organs, and overall performance status of 0 or 1; 4) complete record of pretreatment hematological variables; 5) no presence of distant metastasis; 6) without second primary cancers before or at diagnosis; 7) patients with complete follow-up time; and 8) no presence of infection or inflammatory conditions, such as rheumatologic conditions, connective tissue disorders or heart diseases. Finally, 277 patients were enrolled and analyzed in this study. Clinicopathological features were obtained from the patients’ medical records. The hematological and laboratory parameters were routinely examined in all patients within 1 or 2 weeks prior to surgery. All patients were staged according to the American Joint Committee on Cancer staging manual (seventh edition, 2010).Citation23 This research was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University. Informed written consent was obtained from all individual participants included in this study.
Surgery
Esophagectomy with thoracic and abdominal dissection was required in each surgical procedure, including the left thoracotomy with standard lymphadenectomy and the cervico-thoraco-abdominal approach with extended lymphadenectomy. In this research, 168 patients (61%) underwent two-field lymphadenectomy. In this cohort of patients, thoracoabdominal lymphadenectomy was performed, including the subcarinal, paraesophageal, pulmonary ligament, diaphragmatic and pericardial lymph nodes, as well as those located along the lesser gastric curvature, the origin of the left gastric artery, the celiac trunk, the common hepatic artery and the splenic artery. For 109 (39%) patients, the three-field lymphadenectomy was performed, and in this group, the cervical lymph nodes were thought to be abnormal according to preoperative imaging evaluation.
Nutritional assessment
Nutritional risk was assessed by NRS-2002 within 1 week before surgery.Citation11 NRS-2002 consists of impaired nutritional status (low, moderate or severe) and severity of disease (low, moderate or severe), with an adjustment for age ≥70 years. Nutritional status was evaluated by three variables: body mass index (BMI), recent weight loss and food intake during 1 week before treatment. For severity of disease, as an indicator of stress metabolism and increased nutritional requirements, a score between 1 and 3 was given according to the recommendations. A data collection sheet was used to obtain information about changes in the body weight, food intake and severity of disease according to the ESPEN guidelines.Citation24 A total score exceeding 3 suggested nutritional risk, whereas that below 3 suggested no nutritional risk temporarily.
Hematological parameters calculation and follow-up
The following pretreatment hematological parameters were collected within 1 week prior to the initial treatment: serum albumin, neutrophil count, lymphocyte count, monocyte count and platelet count. NLR, PLR and LMR were calculated by division of the absolute values of the corresponding hematological parameters. The median values of serum albumin, NLR, PLR and LMR were as the optimum cutoff value. Then the SIS was scored as follows: patients with both elevated serum albumin and elevated LMR were assigned a score of 0, patients with either decreased serum albumin or decreased LMR were assigned a score of 1 and patients with both decreased serum albumin and decreased LMR were assigned a score of 2. Correspondingly, the CNP was established based on the combination of NLR and PLR: patients with both an elevated NLR and PLR were allocated a score of 2, and patients showing one or neither were allocated a score of 1 or 0, respectively.
After the completion of treatment, all patients were asked to return to the hospital for examination every 3 months for the first year, every 6 months for the next 2 years and then annually. The duration of follow-up was calculated from the day of treatment to the day of death or March 2018.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Science program (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL, USA). CNP and SIS were divided into CNP 0, SIS 0 and CNP1/2, SIS 1/2 groups by corresponding score, respectively. The relationships between clinical characteristics and CNP, SIS and NRS-2002 were examined by chi-square test or Fisher’s exact test. The end points for this study were 5-year PFS and 5-year OS. PFS was defined as the length of time after surgery during which the patient survived with no sign of tumor recurrence. OS was calculated from date of surgery to the date of individual’s death or last follow-up. The Kaplan–Meier method and log-rank tests were used for 5-year PFS and 5-year OS analyses. Univariate and multivariate analyses of Cox regression proportional hazard model were used to evaluate the influence of each variable on PFS and OS with the enter method. Hazard ratio (HR) with 95% confidence interval (CI) was used to quantify the strength of the association between predictors and survival. Receiver operating characteristic (ROC) curves were also plotted to verify the accuracy of CNP, SIS and NRS-2002 for survival prediction. A 2-tailed P-value ≤0.05 was considered statistically significant.
Results
Clinicopathological characteristics of patients
The basic characteristics of the enrolled patients are shown in . Among the 277 patients, 62 (22%) were females and 215 (78%) were males. The median age prior to surgery was 62 years (range 40–82 years). The location of the tumors mostly occurred in the middle third (179/277, 65%) and the lower third (88/277, 31%) of the esophagus. In our cohort, 109 (39%) patients underwent esophagectomy alone and 168 (61%) received postoperative chemotherapy or radiotherapy. None of these patients received neoadjuvant therapy before surgery. The median follow-up period was 36 months (range 6–72 months). During the follow-up period, 223 (80%) patients had tumor recurrences (48 cases with surgical anastomosis recurrences, 118 cases with locally regional lymph node metastasis and 57 cases with distant metastasis).
Table 1 Clinicopathological characteristics of 277 patients with esophageal squamous cell carcinoma following surgery
Associations of NRS-2002 and inflammation-based markers with clinicopathological characteristics
The relationships of CNP, SIS and NRS-2002 with clinicopathological characteristics are shown in . We determined the cutoff value of 42.20 g/L for serum albumin, 3.01 for NLR, 133.33 for PLR and 3.66 for LMR according to the corresponding median values. As already mentioned, the CNP was established based on the combination of NLR and PLR and the SIS was established based on the combination of serum albumin and LMR; then, 100 (36%) and 77 (28%) patients were assigned a score of 0 in CNP and SIS, respectively; 74 (27%) and 119 (43%) patients were assigned a score of 1 in CNP and SIS, respectively; and 103 (37%) and 81 (29%) patients were assigned a score of 2 in CNP and SIS, respectively.
Table 2 Characteristics of 277 ESCC patients stratified by CNP, SIS and NRS-2002 scores
As shown in , we identified a close relationship between CNP, SIS, NRS-2002 and TNM stage (all P<0.05), that is to say, high CNP, SIS and NRS-2002 score, compared with low ones, were significantly correlated with advanced TNM staging. Furthermore, we found that the high scores in SIS and CNP were significantly correlated with more advanced N status (P<0.05). In addition, the NRS-2002 ≥3.0 group was related to advanced T stage and elder age.
PFS and OS according to CNP, SIS and NRS-2002 status
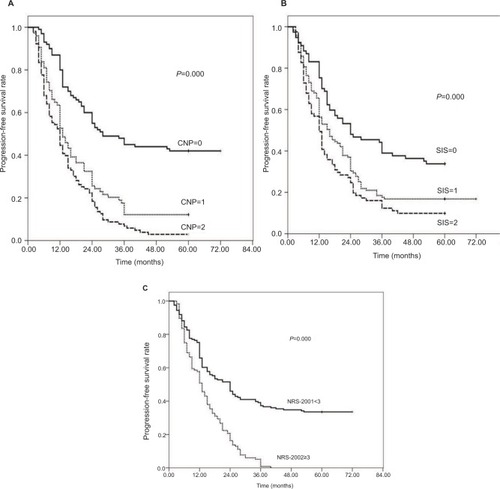
Among the 227 patients,, the median PFS time was 15 months (range: 2–72 months); the PFS rates at the 1-, 3- and 5-year period were 59.6%, 22.0% and 19.5%, respectively; as shown in , in the CNP=0 group, the 1-, 3- and 5-year PFS rates were 80.0%, 45.0% and 42.0%, respectively; in the CNP=1 group, the PFS rates were 52.7%, 12.2% and 12.2%, respectively; and in the CNP=2 group, the PFS rates were 44.7%, 6.8% and 2.9%, respectively (; χ2=60.348, P=0.000). In the SIS=0 group, the 1-, 3- and 5-year PFS rates were 75.3%, 39.0% and 33.8%, respectively; in the SIS=1 group, the PFS rates were 56.3%, 17.6% and 16.8%, respectively; and in the SIS=2 group, the PFS rates were 49.4%, 12.3% and 9.9%, respectively (; χ2=19.057, P=0.000). In the NRS-2002 <3.0 group, the 1-, 3- and 5-year PFS rates were 65.8%, 37.3% and 33.5%, respectively, while in the NRS-2002 ≥3.0 group, the PFS rates were 50.9%, 5.20% and 0.00%, respectively (; χ2=48.702, P=0.000).
Figure 1 Kaplan–Meier survival curves for progression-free survival (PFS) in patients with esophageal squamous cell carcinoma (ESCC) after surgery. (A) 1-, 3- and 5-year PFS of patients with combination of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (CNP)=0 were longer than those with CNP=1 or 2. (P=0.000, log-rank). (B) 1-, 3- and 5-year PFS of patients with systemic inflammation score (SIS)=0 were obviously different from those with SIS=1 or 2. (P=0.000, log-rank). (C) 1-, 3- and 5-year PFS of patients with Nutritional Risk Screening 2002 (NRS-2002) <3 were obviously improved compared with patients in NRS-2002 ≥3. (P=0.000, log-rank).
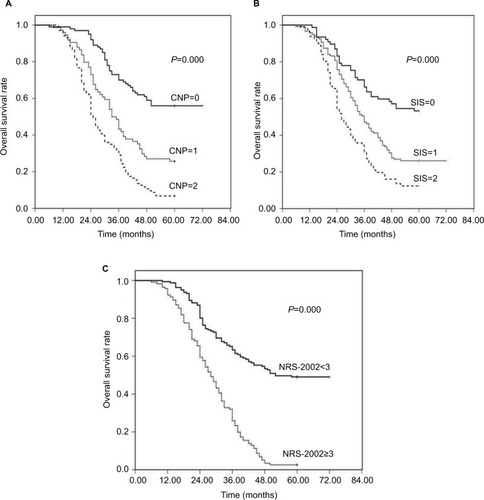
Correspondingly, in our cohort, the median OS time was 36 months (range: 6–72 months); the OS rates at the 1-, 3- and 5-year time were 96.4%, 47.7% and 29.6%, respectively; the OS grouped according to CNP, SIS and NRS-2002 status. Additionally, the 1-, 3, and 5-year OS rates were 99.0%, 70.0%, and 56.0% in the CNP=0 group, 95.9%, 43.2% and 25.7% in the CNP=1 group, and 94.2%, 29.1% and 6.8% in the CNP=2 group, separately (; χ2=73.982, P=0.000). The 1-, 3- and 5-year OS rates were 98.7%, 66.2% and 53.2% in the SIS=0 group, 95.8%, 47.1% and 26.1% in the SIS=1 group, and 93.8%, 30.9% and 12.3% in the SIS=2 group (; χ2=36.552, P=0.000), respectively. Furthermore, in the NRS-2002 <3.0 group, the 1-, 3- and 5-year OS rates were 98.8%, 63.4% and 49.1% separately, while in the NRS-2002 ≥3.0 group, the OS rates were 92.2%, 25.9% and 2.6% respectively (; χ2=83.427, P=0.000). On a whole, PFS and OS of patients in the CNP=0, SIS=0 and NRS-2002 <3.0 group were obviously improved compared with patients in the CNP=1/2, SIS=1/2 and NRS-2002 ≥3.0 groups.
Figure 2 Kaplan–Meier survival curves for overall survival (OS) in patients with esophageal squamous cell carcinoma (ESCC) after surgery. (A) 1-, 3- and 5-year OS of patients with combination of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (CNP)=0 were longer than those with CNP=1 or 2. (P=0.000, log-rank). (B) 1-, 3- and 5-year OS of patients with systemic inflammation score (SIS)=0 were obviously different from those with SIS=1 or 2 (P=0.000, log-rank). (C) 1-, 3- and 5-year OS of patients with Nutritional Risk Screening 2002 (NRS-2002) <3 were obviously improved compared with patients in NRS-2002 ≥3. (P=0.000, log-rank).
Univariate and multivariate survival analyses
The results of univariate analysis of the factors related to PFS and OS are shown in . In univariate analysis, the following factors were significantly associated with poor PFS and OS: sex, T stage, N stage, TNM stage, CNP, SIS and NRS-2002 (all p<0.05). shows the results of multivariate Cox regression analysis of the factors related to PFS and OS. This analysis showed that CNP (HR=1.602; 95% CI 1.341–1.913; P=0.000), NRS-2002 (HR=2.062; 95% CI 1.523–2.792; P=0.000) and TNM stage (HR=1.194; 95% CI 1.058–1.565; P=0.048) were independent prognostic factors for PFS in patients with ESCC after surgery. Correspondingly, CNP (HR=1.707; 95% CI 1.405–2.074; P=0.000), NRS-2002 (HR=2.716; 95% CI 1.972–3.740; P=0.000) and TNM stage (HR=2.363; 95% CI 1.086–1.691; P=0.036) were also independent prognostic factors for OS in ESCC patients following surgery ()
Table 3 Univariate analysis of survival of esophageal squamous cell carcinoma treated by surgery
Table 4 Multivariate analysis of survival of esophageal squamous cell carcinoma treated by surgery
ROC curve for survival prediction
shows the ROC curves analysis of CNP, SIS and NRS-2002 for PFS and OS prediction. As shown in , the area under the curve (AUC) for CNP, SIS and NRS-2002 was 0.788 (95% CI: 0.727–0.850, P=0.000), 0.654 (95% CI: 0.573–0.736, P=0.003) and 0.760 (95% CI: 0.704–0.816, P=0.000), respectively. The results indicated that CNP and NRS-2002 were superior to SIS as predictive factors for PFS in patients with ESCC receiving surgery.
Figure 3 Receiver operating characteristic (ROC) curves of pretreatment combination of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (CNP), systemic inflammation score (SIS) and Nutritional Risk Screening 2002 (NRS-2002) for predicting progression-free survival (PFS) (A) and overall survival (OS) (B) in patients with esophageal squamous cell carcinoma (ESCC) after surgery.
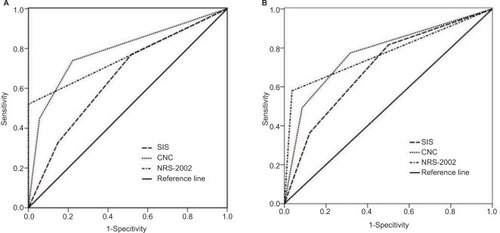
ROC curves for OS were also plotted; as shown in , AUC was 0.774 (95% CI: 0.715–0.832, P=0.000) for CNP, 0.699 (95% CI: 0.632–0.766, P=0.045) for SIS and 0.771 (95% CI: 0.717–0.826, P=0.000) for NRS-2002, indicating that CNP and NRS-2002 were also superior to SIS as predictive factors for OS in patients with ESCC after surgery.
Discussion
Malnutrition and systemic inflammatory response are common in various malignancies and are correlated with poor prognosis. In this clinical research, we explored the importance for the survival prediction of pretreatment NRS-2002, CNP and SIS scores in patients with ESCC receiving surgery. The present study demonstrated that CNP and NRS-2002 were not only the significant risk factors for PFS, but also the independent prognostic factors for OS in ESCC patients following surgery. To the best of our knowledge, this is the first report to demonstrate the clinical significance of CNP and SIS combined with NRS-2002 in patients with ESCC by curative surgery.
Malnutrition has been considered as a significant prognostic factor in cancer patients since 1980, when Dewys et alCitation25 discovered a shorter survival in malnourished patients compared with well-nourished ones. Since then, the correlation between nutritional risk and clinical prognosis has also been demonstrated in a variety of patients, including different types of malignancies.Citation26 Liu et alCitation27 demonstrated that preoperative nutritional status, a novel nutritional-based prognostic score, was independently associated with OS in gastric cancer. Wu et alCitation6 showed that pre-therapeutic serum albumin level was a significant prognostic factor for survival outcomes in patients who underwent esophagectomy. Therefore, nutritional assessment is critical to the efficacy and prognosis of anti-neoplastic therapy, and it should be taken into consideration along with other well-defined prognostic factors for better preoperative assessment and prognostic evaluation.
At present, there are many assessment methods applied to nutritional evaluation; among these, patient-generated subjective global assessment (PG-SGA) is widely used as a golden standard for subjective assessment of nutritional status in cancer patients.Citation28–Citation29 On the other hand, NRS-2002 is a valid method for identifying risk patients and those who will benefit from nutritional treatment.Citation11 A previous study has shown that 28% of patients were at nutritional risk based on NRS-2002, and 34% of patients with head and neck cancer were malnourished according to PG-SGA.Citation30 These results suggested that NRS-2002 seems to be a reliable indicator of malnutrition. Because PG-SGA required specialized nurses to implement and needed long- time evaluation in everyday clinical practice, in contrast, NRS-2002 was the first one developed via evidence-based medicine in the world, with a great advantage of the prediction of malnutrition risk, and it was applicable for a preoperative assessment for patients with ESCC receiving surgery, with the characteristics of non-invasiveness, objective evaluation, convenience and generalization. Therefore, our present study cohort adopts NRS-2002 as nutritional risk assessment tool to stratify patients in malnourished and well-nourished groups. The results showed that PFS and OS of ESCC patients in the NRS-2002 <3.0 group were obviously improved compared with those of patients in the NRS-2002 ≥3 groups. These results indicated that NRS-2002 might be an excellent instrument in predicting the association between nutritional risk and clinical outcome; consequently, preoperative nutritional support is necessary in ESCC patients with a preoperative nutritional score (NRS-2002) ≥3.0.
In the case of hematologic inflammation markers, a high CNP score was significantly associated with poor PFS and OS in our ESCC patients receiving curative esophagectomy with R0 resection. Since the pathologist Rudolf Virchow first discovered leukocytes in malignant tissue specimens about 150 years ago,Citation31 the prognostic values of pretreatment hematologic markers have been highlighted. Compelling evidence suggested that there were statistically significant differences in the survival rates grouped by NLR, PLR and LMR levels for several types of malignancies.Citation16–Citation19 However, the current study also showed that hematologic parameters were controversial in the prediction of prognosis in esophagus carcinoma. Duan et alCitation32 reported that preoperative serum NLR is a useful prognostic marker to complement TNM staging for operable ESCC patients, particularly in patients with stage IIIA disease; on the contrary, Rashid et alCitation33 found that NLR did not prove to be a significant predictor of number of involved lymph nodes, disease recurrence or death. Furthermore, survival time was not significantly different between patients with high (≥3.5) or low (<3.5) NLR (P=0.49). This controversy might result from the optimal cutoff points for NLR and PLR to predict overall survival. In our present study, therefore, the median values of NLR and PLR were as the cutoff point, which were 3.01 and 133.33, respectively, and then the CNP score was established based on the combination of NLR and PLR, consisting of more prognostic information than single NLR or PLR; the results indicated that pretreatment CNP score was an independent risk factor for PFS and OS in ESCC following surgery; nevertheless, there was no prognostic association found for SIS in multivariate analyses.
The limitations of this study are as follows: first, not all hematologic markers of inflammation were used in the analysis, because some biomarkers were not routinely examined, such as C-reactive proteinCitation34 and fibrinogen.Citation35 Second, it was a single-institution, retrospective study. Third, relying on recalled weight, height and food intake from the medical record might have caused bias in assessing BMI and weight change, and ultimately had some effect on NRS-2002 rating; finally, 277 patients with ESCC were enrolled in this study and the sample size is relatively small and may be insufficient to strengthen our results. Given these limitations, future larger randomized trials are needed to clarify these results.
In conclusion, this study demonstrated that CNP combined with NRS-2002 is promising as a predictive marker for predicting clinical outcomes in patients with ESCC receiving surgery. However, considering the retrospective nature of this study, large-scaled prospective trials are still warranted to verify our results.
Acknowledgments
This work was supported by the grant from Suzhou Cancer Clinical Medical Center (grant no. Szzx201506).
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- ChenWQHeYTZhengRSEsophageal cancer incidence and mortality in China, 2009J Thorac Dis201351192623372946
- VizcainoAPMorenoVLambertRParkinDMTime trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973–1995Int J Cancer200299686086812115489
- PennathurAGibsonMKJobeBALuketichJDOesophageal carcinomaLancet2013381986440041223374478
- LiuJXieXZhouCPengSRaoDFuJWhich factors are associated with actual 5-year survival of oesophageal squamous cell carcinoma?Eur J Cardiothorac Surg2012413e7e1122219482
- WuNChenGHuHPangLChenZLow pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomasNutr Cancer201567348148525706773
- WatanabeMIshimotoTBabaYPrognostic impact of body mass index in patients with squamous cell carcinoma of the esophagusAnn Surg Oncol201320123984399123797753
- YoshidaNHaradaKBabaYPreoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancerLangenbecks Arch Surg2017402233334128138759
- DetskyASMcLaughlinJRBakerJPWhat is subjective global assessment of nutritional status?JPEN J Parenter Enteral Nutr19871118133820522
- VellasBVillarsHAbellanGOverview of the MNA—its history and challengesJ Nutr Health Aging2006106456463 discussion 463–46517183418
- KondrupJRasmussenHHHambergOStangaZAd Hoc ESPEN Working GroupNutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trialsClin Nutr200322332133612765673
- ChenWJiangZZhangYEvaluation of European nutritional risk screening method in Chinese hospitalized patients practicesChin J Clin Nutr200513137141
- JiangXHikiNNunobeSPrognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancerBr J Cancer2012107227527922713657
- GuthrieGJCharlesKARoxburghCSHorganPGMcMillanDCClarkeSJThe systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancerCrit Rev Oncol Hematol201388121823023602134
- SchütteKTippeltBSchulzCMalnutrition is a prognostic factor in patients with hepatocellular carcinoma (HCC)Clin Nutr20153461122112725434576
- GaliziaGLietoEZamboliANeutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: a propensity score-matched analysisSurgery2015158111212025818659
- XieXLuoKJHuYWangJYChenJPrognostic value of preoperative platelet–lymphocyte and neutrophil–lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancerDis Esophagus2016291798525410116
- FengJFHuangYChenQXPreoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinomaWorld J Surg Oncol2014125824641770
- HanLHJiaYBSongQXWangJBWangNNChengYFPrognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinomaAsian Pac J Cancer Prev20151662245225025824745
- FengJFHuangYLiuJSCombination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinomaOnco Targets Ther201361605161224403837
- HanLSongQJiaYThe clinical significance of systemic inflammation score in esophageal squamous cell carcinomaTumour Biol20163733081309026423404
- ChangYAnHXuLSystemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinomaBr J Cancer2015113462663326135896
- RiceTWBlackstoneEHRuschVW7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junctionAnn Surg Oncol20101771721172420369299
- KondrupJAllisonSPEliaMVellasBPlauthMEducational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN)ESPEN guidelines for nutrition screening 2002Clin Nutr200322441542112880610
- DewysWDBeggCLavinPTPrognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology GroupAm J Med19806944914977424938
- SorensenJKondrupJProkopowiczJEuroOOPS Study GroupEuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcomeClin Nutr200827334034918504063
- LiuXQiuHKongPZhouZSunXGastric cancer, nutritional status, and outcomeOnco Targets Ther2017102107211428442919
- OtteryFDDefinition of standardized nutritional assessment and interventional pathways in oncologyNutrition199612Suppl 1S15S198850213
- BauerJCapraSFergusonMUse of the scored patient-generated subjective global assessment (PG-SGA) as a nutrition assessment tool in patients with cancerEur J Clin Nutr200256877978512122555
- Orell-KotikangasHÖsterlundPSaarilahtiKRavascoPSchwabUMäkitieAANRS-2002 for pre-treatment nutritional risk screening and nutritional status assessment in head and neck cancer patientsSupport Care Cancer20152361495150225370893
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- DuanHZhangXWangFXPrognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinomaWorld J Gastroenterol201521185591559725987784
- RashidFWaraichNBhattiIA pre-operative elevated neutrophil: lymphocyte ratio does not predict survival from oesophageal cancer resectionWorld J Surg Oncol20108120053279
- ThurnerEMKrenn–PilkoSLangsenlehnerUThe elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapyEur J Cancer201551561061925618827
- KijimaTArigamiTUchikadoYCombined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinomaCancer Sci2017108219319927889946
