Abstract
Immune checkpoint inhibitors (ICIs), represented by anti-CTLA-4 or anti-PD-1/anti-PD-L1 pathway antibodies, have led to a revolution in cancer treatment modalities. ICIs have unique clinical benefits, such as effectiveness against a broad range of tumor types, strong overall impact on survival, and persistent responses after the cessation of therapy. However, only a subset of patients responds to these therapies, and a small proportion of patients even experience rapid progression or an increased risk of death. Therefore, it is imperative to optimize patient selection for treatment. This review focuses on the mechanisms of tumor escape from immune surveillance, the composition and activity of a preexisting immune infiltrate, the degree of tumor foreignness (as reflected by the mutational burden, expression of viral genes, and driver gene mutations), and host factors (including peripheral blood biomarkers, genetic polymorphisms, and gut microbiome) to summarize current evidence on the biomarkers of responses to ICIs and explore the future prospects in this field.
Plain language summary
The significant differences in patients’ responses to immune checkpoint inhibitors (ICIs) have generated intense interest in identifying biomarkers to guide patient selection.
We summarize current potential biomarkers for the prediction of ICI efficacy, focusing on four levels (the mechanisms of tumor immune escape, the composition and activity of the immune system in the tumor, the foreignness of the tumor, and host factors).
Multivariate analyses must consider a variety of variables, including the aforementioned four aspects to identify the combinations of factors that predict patients’ response to ICIs.
Background
Cancer immunotherapy has undergone revolutionary progress in recent years, mainly due to the breakthrough regarding the extraordinary clinical outcomes associated with immune checkpoint inhibitors (ICIs) targeting the cytotoxic T-lymphocyte-associated antigen (CTLA-4) and programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway. Although the heterogeneity of somatic mutations in tumors raises challenges for the methods that target a single mutation, it also raises the possibility of using the large number of neoantigens to induce immune responses to kill tumor cells. However, the recognition and cytotoxicity functions of the innate and adaptive immune systems are inhibited by immune checkpoint pathways. Based on this theory, many ICIs, such as CTLA-4 and PD-1/PD-L1 pathway inhibitors, have emerged. CTLA-4 inhibitors mainly affect the early stage of the immune responses, during T-cell priming and activation, blocking the contact inhibition functions of regulatory T cells (Treg) on effector T cells (Teff) and thus enhancing Teff functions.Citation1,Citation2 PD-1/PD-L1 inhibitors mainly exert their effects primarily on immune responses within the tumor microenvironment (TME); they can reverse the status of Teff cell anergy and depletion to restore tumor cell killing functions and induce effective anti-tumor immune responses.Citation3,Citation4
Materials and methods
To summarize the recent research on the biomarkers of ICIs, we searched the PubMed database, using the following search terms “(((((checkpoint) OR PD-1) OR PD-L1) OR CTLA-4) AND ((inhibitor) OR blockade)) OR (((anti-PD-1) OR anti-PD-L1) OR anti-CTLA-4) AND (((biomarker) OR predictive) OR prediction) AND response”. PubMed was last searched in May 2018. A flow diagram of this review is presented in . Eligible trials in https://www.clini-caltrials.gov were also included in the survey. Additionally, reports from annual meetings of the American Society of Clinical Oncology and the European Society for Medical Oncology were searched through these organizations’ official websites at http://meetinglibrary.asco.org/ and http://www.europeancancercongress.org.
Figure 1 A flow diagram of this review.
Notes: aStudies of two or more factors included: mechanisms of tumor immune escape and tumor foreignness (n= 5); mechanisms of tumor immune escape and immune composition and activity in tumors (n=4); mechanisms of tumor immune escape, immune composition, and activity in tumors and tumor foreignness (n=2); tumor foreignness and host factors (n=1); immune composition and activity in tumors, tumor foreignness, and host factors (n=1). bOther factors included: studies about PET-CT, CT, and MRI parameters (n=4), and studies about clinical factors such as age, KPS, and so on (n=5).
Abbreviations: KPS, Karnofsky Performance Status; PET-CT, positron emission tomography-computed tomography.
Biomarkers to predict responses to ICIs
The advance of ICIs has revolutionized the approach of cancer treatment. The unique advantages of ICI therapy, such as crossing different histological types of tumors, significant elongation of the survival period, and persistent effectiveness after drug withdrawal, have generated widespread enthusiasm among patients, clinicians, and scientists. However, the heterogeneity of responses to ICIs has also generated new challenges. To date, anti-CTLA-4 therapy has shown reproducible activity only in patients with malignant melanoma (MM).Citation5,Citation6 In contrast, PD-1/PD-L1 inhibitors have a broad range of activity extending beyond MM.Citation7–Citation9 to an expanding list of cancers, including non-small-cell lung cancer (NSCLC),Citation10–Citation12 renal cell cancer (RCC),Citation13 head and neck squamous cancer (HNSCC),Citation14,Citation15 bladder cancer,Citation16,Citation17 and Hodgkin’s lymphoma.Citation18 However, certain types of cancer, such as prostate cancer and pancreatic cancer, have proven to be much more resistant to PD-1/PD-L1 inhibitors.Citation19 Champiat et alCitation20 even reported that a small group of patients (~10%) showed rapid progression after treatment with anti-PD-1/PD-L1 drugs. The US Food and Drug Administration (FDA) recently issued a statement requiring the cessation of trials of pembrolizumab in combination with dexamethasone and an immunomodulatory agent (lenalidomide or pomalidomide) for the treatment of patients with multiple myeloma due to the increased risk of death to patients in two recently halted clinical trials.Citation21 The above-mentioned facts underscore the need for biomarker development. Given the dynamic nature of the immune system and the complexity of immune responses, the identification of the biomarkers of ICIs is more challenging than the identification of the biomarkers of targeted therapy. Based on research performed to date, four prerequisites, namely, tumor antigen release, tumor antigen presentation, attenuated immune suppression, and tumor antigen-specific T-cell activation, need to be satisfied to achieve the optimal adaptive response. As such, we elucidate the current landscape and future directions of work on biomarkers for the prediction of ICI efficacy, focusing on the mechanisms of tumor immune escape, the composition and activity of the immune system in the tumor, the foreignness of the tumor, and host factors.
Mechanisms of tumor immune escape
To date, the detection of PD-L1 expression by immunohistochemistry (IHC) has been the most widely used clinical approach to predicting the efficacy of PD-1/PD-L1 inhibitors.Citation22 The FDA has approved the use of a relevant antibody (22c3) to quantify PD-L1 expression in tumor cells by IHC in NSCLC. An expression level >50% is required for using pembrolizumab in the first-line setting.Citation23 Regarding the target of PD-1/PD-L1 inhibitors, patients with high PD-L1 expression are expected to be more responsive to these inhibitors. Many studies have shown that both the objective response rate (ORR) and the overall survival (OS) of PD-L1-positive patients after ICI therapy were higher than those of PD-L1-negative patients.Citation15,Citation24,Citation25 Recently, atezolizumab was shown to result in a significant improvement in OS compared with docetaxel in stage IIIB or IV NSCLC (OAK trial), and patients with high levels of PD-L1 (≥50% on tumor cells or ≥10% on tumor-infiltrating lymphocytes [TILs]) derived the greatest benefit from atezolizumab.Citation24 In particular, the comparison between the Keynote 024 and Checkmate 026 clinical trials further suggested the significance of high PD-L1 expression in predicting the efficacy of the first-line treatment of metastatic NSCLC.Citation26–Citation28
However, there are many challenges related to using PD-L1 expression as a prediction biomarker. First, no definitive conclusion has been drawn regarding the association between PD-L1-positive tumors and ICI efficacy, and some contradictory results have even been obtained in some cancers, such as RCC, MM, and urothelium carcinoma.Citation13,Citation16,Citation17,Citation29–Citation31 Chae et alCitation32 performed a combined analysis of studies on ICI therapy biomarkers in NSCLC and concluded that there was still no consensus on the use of PD-L1 expression as an ideal marker for patient selection. Additionally, PD-L1-negative patients can still benefit from anti-PD-1/PD-L1 therapy. Taking the findings of the studies performed to date into consideration, it was shown that using only PD-L1 expression levels for the prediction of ICI efficacy is insufficient. Moreover, because of differences in the biological characteristics of tumors at different locations and the different types of antibodies used in IHC, it is more difficult to develop uniform IHC criteria for PD-LI evaluation.Citation33 Owing to the limitation presented by the semi-quantitative nature of IHC, some researchers used the Her-2 detection method in breast cancer to propose combining IHC and gene amplification to achieve qualitative and quantitative unification.Citation34 In this regard, Inoue et alCitation35 retrospectively analyzed 654 postoperative NSCLC patients and showed that the gene amplification number of PD-L1 could be used as a supplemental or alternative bio-marker of PD-L1 expression. Additionally, PD-L1 expression in tumor cells and immune cells is a dynamic process. Thus, the detection of PD-L1 expression occurring at a particular point in time may be insufficient.Citation36 Furthermore, the heterogeneity of PD-L1 expression in the same tumor tissue and between primary lesions and different metastatic tumors in the same patient also increases the difficulty of assessing PD-L1 expression levels.Citation37,Citation38 The details of PD-L1 detection in large Phase III trials performed to date are summarized in . However, the differences in their conclusions regarding PD-L1 expression and efficacy are probably related not only to the method of performing the PD-L1 assay but also to the complex interactions between tumors and the immune system, which along with tumor mutation burden (TMB) have been revealed as other potential biomarkers.
Table 1 Phase III clinical trials of ICIs with available efficacy results
CTLA-4 and PD-L2
Associations of other immunosuppressive molecules with the rate of response to ICI treatment have also been reported. It has been shown that the CTLA-4 mRNA expression level before treatment is correlated with the efficacy of both the anti-CTLA-4 antibody and the anti-PD-L1 antibody, which might be associated with the promotion of the inhibitory function by Tregs on Teffs via CTLA-4 in TME; however, this inhibitory function was weaker than that of PD-L1.Citation1,Citation39,Citation40 Moreover, Yearley et alCitation41 reported that PD-L2 status was also a significant predictor of progression-free survival (PFS) with pembrolizumab and that it operated independently of PD-L1 status in HNSCC. Although there are some limitations, tumor immune escape clearly plays a critical role in the mechanism of immune action and in the prediction of the biomarkers of ICIs.
Immune composition and activity in tumors
Tumor immunophenotypes
Chen et alCitation42 identified three tumor immunophenotypes: immune-inflamed, immune-excluded, and immune-desert phenotypes. Tumors with the immune-inflamed phenotype show immune cell infiltration at the tumor edge or in the tumor stroma, which is regarded as reflecting an inflammatory tumor. In this type of tumor, immune responses can be suppressed by the expression of immune checkpoints.Citation42 Therefore, ICIs can unleash the suppressed immunity and have better efficacy. The latter two types are non-inflammatory tumors. Owing to steric hindrance, effective immune responses are lacking inside these tumors; therefore, the function of ICIs is very limited in such cases. The classification of the above-mentioned immunophenotypes is based on the differences in the infiltration patterns of immune cells inside tumors. The proposed immunophenotypes provide a basis for personalized tumor immunotherapy. However, some immune-inflamed tumors may also not respond to ICIs, partly because the early Treg recruitment inhibits an effective anti-tumor immune response.Citation43 Additionally, several factors that influence immunophenotypes, such as TMB and the tumor microbial spectrum, might become biomarkers for the prediction of ICI efficacy.Citation42 Page et alCitation44 proposed that T-cell receptor (TCR) sequencing can provide additional information on TIL number and clonal diversity. The combination of TCR sequencing and IHC can assess TILs more comprehensively and accurately. However, these immunophenotypes focus on the numbers and aggregation patterns of TILs and ignore TIL functions. The use of a multi-parameter flow cytometer for the analysis of markers of TIL activation and depletion can compensate for this deficiency. Daud et alCitation45 analyzed 40 MM patients at the progressive stage treated with nivolumab or pembrolizumab and found that patients with CTLA-4highPD-1high expression in more than 20% of CD8+TILs had a better prognosis. Interestingly, the improved prognosis linked to ICI therapy was associated only with the CTLA-4highPD-1high double-positive population and was not associated with the single-positive one.Citation45 Other important biomarkers of exhaustion, including TIM-3, LAG-3, and VISTA, are usually co-expressed with PD-1 in excessively exhausted Teff cells.Citation42,Citation46 T cells that express many types of exhaustion/activation markers usually show a poor response to anti-PD-1/PD-L1 treatment.Citation42 The effects of the TIL infiltration patterns and exhaustion/activation markers on ICI efficacy require further studies with large sample sizes.
Immunosuppressive factors in TME
Some studies have shown that immunosuppressive factors, particularly Tregs in TME, are potentially involved in the lack of response to ICIs in specific subtypes of cancer that are heavily infiltrated with adaptive immune cells.Citation43,Citation47,Citation48 Enhancing the immune response to these tumors by depleting Tregs in addition to immune checkpoint inhibition impaired tumor growth and prolonged survival. Citation43 As Lowther et alCitation49 showed that PD-1-high Tregs in the TME and circulating blood were an exhausted type, it is reasonable to speculate that the function of ICIs may be impaired if PD-1 was preferentially expressed on these cells or if these Tregs were activated in the presence of ICIs.Citation43 In contrast, in an earlier Phase II trial of melanoma patients treated with ipilumab, higher infiltration of Foxp3+Tregs at baseline was significantly positively associated with clinical outcome.Citation50. More research on baseline Treg infiltration and the role of immune checkpoints on Tregs, such as CTLA-4 and PD-L1, is warranted. Some studies also showed that the depletion of Tregs during ICI treatment may be associated with ICI efficacy.Citation51,Citation52 Although some studies showed that eradicating or reprogramming other immunosuppressive factors, such as myeloid-derived suppressor cells (MDSCs), γδT cells, and macrophages, could enhance clinical responses to ICI treatment, few studies have demonstrated whether they can be a biomarker for predicting its efficacy.Citation48
Inflammatory gene signature
Inflammatory cells and proteins can participate in tumor metastasis, tumor growth, and angiogenesis.Citation53 Moreover, in some tumors, PD-L1 is not constitutively expressed but rather is induced in response to inflammatory signals produced by an active anti-tumor immune response, with expression induced on most tumor cells in response to IFN-γ.Citation54,Citation55 This interactive function allows Inflammatory gene signatures to be used as ICI biomarkers to select appropriate patient populations.Citation56 Ribas et al indicated that IFN-γ signaling-related genes may allow the improved selection of patients likely to respond to anti-PD-1 therapy with pembrolizumab.Citation57 In the exploratory analysis of the POPLAR study, patients with high Teff-IFN-γ-associated gene expression had improved OS with atezolizumab.Citation25 Additionally, several studies showed that the loss of IFN-γ signaling in tumor cells may represent a common mechanism for tumor resistance to ICIs.Citation58–Citation60 These studies indicated that consideration of the characteristics of IFN-γ-related genes in tumors would be useful in the ICI prognosis model.
Tumor foreignness
Tumor mutation spectrum and mutation burden
TMB refers to the number of somatic cell mutations in the tumor genome after removing germline mutations. Many studies have explored the association between TMB and ICI efficacy ().Citation27,Citation61–Citation68 Patients with a high TMB had significantly higher response rates, and longer PFS and OS than those with a lower TMB. Notably, most of these studies were retrospective and tested old biopsy specimens, which may not accurately reflect the current mutational burden of a tumor. Recently, Checkmate 227 showed that, in patients with advanced NSCLC and a tumor mutational burden of ≥10 per megabase, first-line treatment with nivolumab plus ipilimumab was associated with longer PFS than chemotherapy.Citation67 These results indicate that TMB is an important and independent biomarker in advanced NSCLC. Some other studies may indirectly support the use of TMB as a biomarker of ICI efficacy. For example, in studies about NSCLC and urothelial cancer, higher response rates were seen in current and former smokers than in non-smokers, which may be suggestive of the role played by a high mutational load. Citation67,Citation69,Citation70 A comparison among different types of tumors showed that tumors with higher TMB, such as MM, HNSCC, and bladder cancer, have a good effect on ICI therapy, with a response rate of more than 15%.Citation39,Citation71,Citation72 Tumors with low TMB, such as pancreatic cancer and prostate cancer, have a poor response to ICI therapy.Citation19 TMB can thus be used for cross-sectional analyses across many types of tumor to identify the patient population that can benefit from immunotherapy. However, TMB also has its limits. First, cancers are not static and can acquire mutations as they evolve. Issues related to the need for the dynamic monitoring of TMB and the timing required to detect TMB warrant further exploration. Second, immunogenic antigen expression is a necessary – but not a sufficient – condition for immune responses. Therefore, TMB can predict only the effectiveness of ICIs to some extent, and not all patients with high TMB can obtain obvious benefits after ICI therapy (immune tolerance might be caused by mechanisms other than PD-1/PD-L1 and CTLA-4).Citation73 Moreover, the effect of ICIs on some patients with a low mutation burden is not poor (the recognition of DNA damage-induced neoantigens by T cells is a relatively random process, and low mutation burden sometimes also produces strong neoantigens). Furthermore, a recent study suggested that not all neoantigens are positively correlated with prognosis. McGranahan et alCitation74 showed that the percentage of clonal neoantigens was positively correlated with ICI efficacy in lung adenocarcinoma, whereas the percentage of subclonal neoantigens was negatively correlated with efficacy. Therefore, if the majority of mutations were subclonal mutations, the presence of high TMB may not predict treatment efficacy. Thus, further classification of neoantigens might be necessary. TMB also has some problems, such as an unclear cut-off value, tumor heterogeneity, high cost of next-generation sequencing, and complicated data analysis. Nevertheless, a number of studies on the use of TMB as a biomarker for the prediction of ICI efficacy are now underway. The findings obtained thus far suggest the potential for including TMB analysis in the stratification of ICI clinical trials.
Table 2 Studies utilizing TMB as a predictor of response to treatment with ICIs
Mismatch repair deficiency (dMMR)
As with TMB, dMMR has recently become a marker for the prediction of ICI efficacy. Beyond the context of colorectal cancer, Le et alCitation75 expanded the application of dMMR across 12 different tumor types; in this study, 53% of patients showed an objective response, and 21% achieved a complete response. In May 2017, the FDA has approved pembrolizumab for the treatment of adult and pediatric cancers that progressed after prior treatment, which are dMMR or microsatellite instability high, irrespective of tumor type Citation76 DNA mismatch repair (MMR) is a critical mechanism in DNA repair. Its major function is to proofread mismatched bases in a timely manner to maintain genome stability.Citation77 dMMR results in many mutations that enhance tumor immunogenicity and induce more active immune responses.Citation78 Additionally, some studies have also confirmed that mutations in other genes involved in the DNA replication repair process (e.g., the POLE gene) are associated with ICI prognosis.Citation79 However, individuals with dMMR account for only a small percentage of patients. Some patients with a proficient MMR system can still benefit from ICI therapy.Citation80
Expression of viral genes
Recently, the association between the PD-1-PD-L1 pathway and virus infection in certain tumors, such as HPV-induced cervical cancer and HNSCC, and EBV-induced gastric cancer and nasopharyngeal carcinoma, has elicited considerable attention. First, PD-L1 expression is thought to play a role in the initiation and persistence of HPV infection by providing an immune-privileged site where T-cell activity is downregulated.Citation81–Citation83 Second, viral antigens that will generally not be lost or downregulated can trigger an immune response due to their exogenous nature. Moreover, virally mediated tumors develop in the context of chronic infection in which immune checkpoints may be activated over time. Many studies have demonstrated the positive correlation between PD-L1 expression and virus infection in various cancers, including HNSCC, cervical cancer, and EBV-induced malignant tumors.Citation81,Citation84–Citation87 Additionally, recent studies have shown that more T-cell infiltration was observed in virus-positive tumors than in the same type of virus-negative ones.Citation88
At present, study reports about ICI efficacy are limited to HNSCC. Both Keynote 012 and Checkmate 141 showed that HPV-positive tumors obtained more benefits from ICIs than HPV-negative ones.Citation15,Citation89 Data were insufficient in other types of virus-infected tumors, such as HPV-infected cervical cancer and EBV-induced malignant tumors. Keynote 028 showed the antitumor activity of pembrolizumab in PD-L1-positive cervical cancer, but it did not evaluate the association between the efficacy of pembrolizumab and HPV infection.Citation83 On the other hand, the preliminary results of Checkmate 358 showed that a response to nivolumab was observed regardless of PD-L1 or HPV status.Citation90 However, Checkmate 358 is a Phase I/II study including only 24 patients, the final results of which are yet to be published.Citation90 Further evaluation of the role of virus infection in ICI efficacy should be performed.
Driver gene mutation
Not all kinds of tumor cell gene mutations can enhance TIL-mediated immune responses. Recent studies have shown that tumor-associated driver gene mutations not only fail to enhance but also actually attenuate immune responses. The subgroup analysis in the Checkmate 057 trial showed that NSCLC patients with EGFR mutations or ALK rearrangements obtained relatively minor benefits from ICI therapy.Citation10 Currently, the mechanism underlying the effects of driver gene mutations on tumor local immunity and ICI efficacy is still unclear. It is speculated that tumors with driver gene mutations might have lower total mutation levels due to the lower mutation heterogeneity. A retrospective study showed that fewer NSCLC patients with EGFR mutations or ALK rearrangements exhibited both positive PD-L1 expression and high CD8+TIL infiltration.Citation91 Moreover, individuals with EGFR mutations with non-T790M-acquired drug resistance might benefit more from PD-1 inhibitors than patients with T790M-acquired drug resistance.Citation92 Based on these observations, recent studies on EGFR mutations have mainly adopted therapy of ICIs combined with tyrosine kinase inhibitors.Citation93 Although the Checkmate 142 trial showed that KRAS or BRAF mutations did not affect the efficacy of PD-1 inhibitors, some studies showed that KRAS and BRAF mutations or other mutations in the MAPK pathway attenuated immunity by reducing the transcription of major histocompatibility complex class I (MHC I) molecules. Citation94–Citation96 Additionally, β-catenin pathway activation and the direct or indirect loss of PTEN resulted in the reduction of CD8+TILs infiltration in melanoma.Citation97,Citation98 The effects of driver gene mutations on the immune microenvironment and on the efficacy of immunotherapy still require further research.
In summary, the T-cell immune response is closely associated with the increase of neoantigens that results from DNA damage, or repair system defects, and foreign antigens expressed by viral genes. DNA and RNA sequencing plays an important role in the evaluation of the tumor foreignness and can optimize the selection of patients for ICI therapy. However, the presence of immunogenic antigens is only one of the necessary conditions of immune responses in tumors. Furthermore, the effects of driver gene mutations on the immune microenvironment and the efficacy of immunotherapy are more complicated. Most studies have shown that, in patients with driver gene mutations, ICIs have poor efficacy. The use of ICIs combined with corresponding targeted therapy is a promising direction of future research for the treatment of these patients.
Host factors
Peripheral blood markers
Several studies have reported that the absolute counts of certain cell populations in peripheral blood (e.g., lymphocytes, monocytes, and neutrophils) were associated with ICI efficacy.Citation99–Citation106 However, some other studies cast doubt on this. Sun et alCitation107 reviewed all consecutive patients treated with anti-PD-1/PD-L1 monotherapy in Phase I trials performed at our institution between December 2011 and January 2014 and found that baseline absolute lymphocyte count (ALC) was not associated with response to anti-PD-1/PD-L1; thus, patients should not be excluded from early-phase clinical trials testing immune checkpoint blockers because of ALC. Additionally, a study by Subrahmanyam et alCitation108 also did not find that lymphocyte and monocyte frequencies had predictive value for ICI efficacy. However, they found differences in CD4+ and CD8+ memory T-cell subsets between responders and non-responders to anti-CTLA-4 and differences in specific NK cell subsets (CD69+ and MIP1β+ NK cell populations) in responders and non-responders to anti-PD-1. The distinct sets of candidate biomarkers for anti-CTLA-4 and anti-PD-1 therapies may be attributable to the different sites at which they function.Citation4 Moreover, some other subsets in peripheral blood, such as circulating MDSCs and CD14+CD16-HLA-DRhi monocytes, were reported as predictors of ICI efficacy.Citation109,Citation110 At present, the evidence that subsets of circulating blood cells can be used as predictors of ICI efficacy remains insufficient and this issue warrants further research.
Apart from these circulating immune cells, peripheral blood TCR diversity also plays an important role in CTLA-4 inhibitor therapy. CTLA-4 inhibitors can promote reconstruction of the TCR repertoire and increase its diversity.Citation111–Citation113 Cha et alCitation111 showed that the maintenance of high-frequency TCR clonotypes was associated with longer OS in patients following ipilimumab therapy; however, patients who lost more high-frequency clonotypes usually had shorter OS. These high-frequency TCR clonotypes might represent high-affinity T cells associated with anti-tumor responses.Citation111 Notably, Huang et alCitation114 recently developed a “reinvigoration score” by relating changes in circulating exhausted-phenotype CD8+ T cells to tumor burden to predict anti-PD-1 response. They found that these responding exhausted-phenotype CD8+ T cells in the blood contained TCR clonotypes shared with TILs, which may be the factor underlying this phenomenon. However, immune cell functions in TME clearly differ markedly from those in peripheral blood.
Genotypes of patients
Genotype may affect ICI efficacy; however, current evidence is limited to studies with small samples. Queirolo et alCitation115 analyzed 14 MM patients and found that the rate of response to ipilimumab was higher in patients with CTLA- 4-1577G/A and CT60G/A heterozygous genotypes. Another earlier study on the treatment of melanoma using ipilimumab showed that three types of CTLA-4 single-nucleotide polymorphisms (SNPs) (rs4553808, rs11571327, and missense SNP rs231775) were associated with the response to anti-CTLA-4-specific antibodies.Citation116 However, a Phase II clinical trial of MM did not reveal an association between CTLA-4 SNPs and treatment response.Citation50 Therefore, the association between SNPs and ICI efficacy still requires further verification.
Microbial spectrum
Several studies have demonstrated that manipulation of the microbiota may modulate the effect of cancer immunotherapy.Citation117–Citation119 For example, the transplantation of fecal microbiota from cancer patients who responded to ICI into germ-free or antibiotic-treated mice was reported to ameliorate the anti-tumor effects of ICIs.Citation117–Citation119 Moreover, Matson et alCitation120 recently analyzed baseline stool samples from MM patients before immunotherapy treatment and observed a significant association between commensal microbial composition and clinical response. Bacterial species that were more abundant in responders included Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium.Citation120 Similar to the previously mentioned results, Chaput et alCitation121 suggested that baseline gut microbiota enriched with Faecali bacteria and other Firmicutes is associated with a beneficial clinical response to ipilimumab. The search is underway for components of the microbiota that enhance the action of other immunotherapies. Discovery of the effect of gut microbiota on ICI efficacy has clearly opened up another direction for ICI biomarker discovery.
Overall, although some inspiring results have been obtained, few studies on host factors such as peripheral blood markers, gene polymorphisms, and gut microbiota have been performed thus far, and this work is still at the exploratory stage. It is challenging to identify the factors that actually predict treatment response and to separate them from the confounding factors.
Conclusion
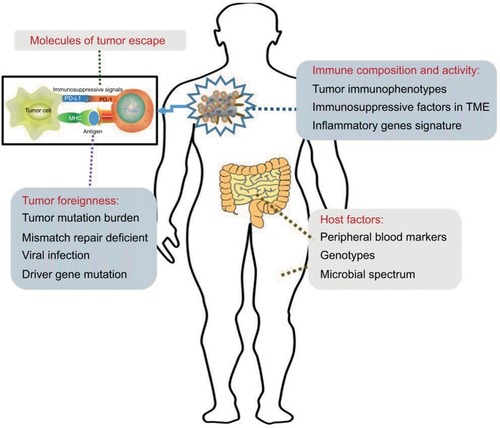
Our analyses showed that the main functions of ICIs are to unleash immune tolerance, which results from the activation of immune checkpoint pathways. The effectiveness of these therapies requires cooperation with all other aspects of the immune system. First, the expression of immunogenic antigens on tumor cells is an essential condition for the induction of anti-tumor immune responses. Therefore, evaluation of the tumor foreignness using methods such as gene analysis is necessary. Second, immune activities in the TME include the distribution and function of TILs and inflammatory gene expression and are also associated with ICI efficacy. Third, the specific mechanisms of tumor escape also play important roles in the effectiveness of ICIs. The detection of PD-L1 might require the use of combined measures. Furthermore, studies on peripheral blood markers, gene polymorphisms, and gut microbiota are still at an initial stage. These four classification methods provide a framework for our studies on ICI biomarkers ().
Figure 2 Graphical representation of distinct biomarkers for patient selection for treatment with immune checkpoint inhibitors.
Notes: Sensitivity to immune checkpoint inhibition is influenced by the following four variables: the molecules involved in tumor immune escape, the foreignness of the tumor, the composition and activity of the immune system in tumors, and host factors. As these four may be used in combination to determine the likelihood that an individual patient will respond to treatment, they are potential guides for treatment decisions.
Abbreviations: MHC, major histocompatibility complex; PD-1, programmed death receptor-1; PD-L1, programmed death receptor-ligand 1; TME, tumor microenvironment.
It is worth noting that the majority of the aforementioned factors were used as solitary subjects of study in most previous studies, especially in large Phase III trials (). The fact that most of them focused only on PD-L1 expression may have been due to the early stage at which these studies were performed. Few studies on their association and weights have been performed. The cancer immunogram proposed by Blank et alCitation122 is an approach involving the use of the above-mentioned methods, including many types of prediction markers, to predict ICI efficacy. It is imperative to perform multivariate predictive analyses that include tumor foreignness, immune composition, immune activity, tumor escape mechanisms, and some host factors. Additionally, many measures, including quantitative genetic analysis, IHC to determine the density and location of immune cell types, and flow cytometry for various cell surface markers, can be combined with some conventional laboratory examinations. With the implementation of large-scale ICI clinical studies and the emergence of some promising results, multivariate analyses can help us to optimize patient selection and possibly personalize cancer treatment using ICIs.
Acknowledgments
This work was supported by the grants from the Shandong Provincial Natural Science Foundation (ZR2015HZ004), the National Health and Family Planning Commission of China (201402011), and the National Science Foundation for Young Scientists of China (81602031).
Author contributions
YZ and ZL were responsible for the conception and design of the study. JY and FZ provided useful suggestions. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- PeggsKSQuezadaSAChambersCAKormanAJAllisonJPBlockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodiesJ Exp Med20092061717172519581407
- ShrikantPKhorutsAMescherMFCTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanismImmunity19991148349310549630
- KeirMEButteMJFreemanGJSharpeAHPD-1 and its ligands in tolerance and immunityAnnu Rev Immunol20082667770418173375
- MellmanICoukosGDranoffGCancer immunotherapy comes of ageNature201148048048922193102
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med201036371172320525992
- RobertCThomasLBondarenkoIIpilimumab plus dacarba-zine for previously untreated metastatic melanomaN Engl J Med20113642517252621639810
- RobertCLongGVBradyBNivolumab in previously untreated melanoma without BRAF mutationN Engl J Med201537232033025399552
- RobertCRibasAWolchokJDAnti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trialLancet20143841109111725034862
- WeberJSD’AngeloSPMinorDNivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trialLancet Oncol20151637538425795410
- BorghaeiHPaz-AresLHornLNivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancerN Engl J Med20153731627163926412456
- BrahmerJReckampKLBaasPNivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancerN Engl J Med201537312313526028407
- HerbstRSBaasPKimDWPembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trialLancet20163871540155026712084
- MotzerRJEscudierBMcDermottDFCheckMate, nivolumab versus everolimus in advanced renal-cell carcinomaN Engl J Med201557318031813
- ChowLQHaddadRGuptaSSeiwert, antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 Expansion CohortJ Clin Oncol2016343838384527646946
- FerrisRLBlumenscheinGJrFayetteJGillison, nivolumab for recurrent squamous-cell carcinoma of the head and neckN Engl J Med20163751856186727718784
- ApoloABInfanteJRBalmanoukianAAvelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib studyJ Clin Oncol2017352117212428375787
- MassardCGordonMSSharmaSSegal, safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancerJ Clin Oncol2016343119312527269937
- AnsellSMLesokhinAMBorrelloIArmand, PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphomaN Engl J Med201537231131925482239
- LawrenceMSStojanovPPolakPMutational heterogeneity in cancer and the search for new cancer-associated genesNature201349921421823770567
- ChampiatSDercleLAmmariSHyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1Clin Cancer Res2017231920192827827313
- FDA alerts healthcare professionals and oncology clinical investigators about two clinical trials on hold evaluating KEYTRUDA® (pembroli-zumab) in patients with multiple myeloma Available from: https://www.fda.gov/Drugs/DrugSafety/ucm574305.htmAccessed April 1, 2018
- PatelSPKurzrockRPD-L1 expression as a predictive biomarker in cancer immunotherapyMol Cancer Ther20151484785625695955
- Pembrolizumab (KEYTRUDA) Checkpoint Inhibitor Available from: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm526430.htm
- RittmeyerABarlesiFWaterkampDO.A.K.S. GroupAtezoli-zumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trialLancet201738925526527979383
- FehrenbacherLSpiraABallingerMP.S. GroupAtezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trialLancet20163871837184626970723
- Giroux LeprieurEDumenilCJulieCImmunotherapy revolutionises non-small-cell lung cancer therapy: results, perspectives and new challengesEur J Cancer201778162328407528
- CarboneDPReckMPaz-AresLCheckMate, first-line nivolumab in stage IV or recurrent non-small-cell lung cancerN Engl J Med20173762415242628636851
- ReckMRodriguez-AbreuDRobinsonAGPembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancerN Engl J Med20163751823183327718847
- KeeDMcArthurGImmunotherapy of melanomaEur J Surg Oncol20174359460327514721
- BalarAVGalskyMDRosenbergJEI.M.S. GroupAtezoli-zumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trialLancet2017389677627939400
- SharmaPCallahanMKBonoPRosenberg, Nivolumab mono-therapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trialLancet Oncol2016171590159827733243
- ChaeYKPanADavisAABiomarkers for PD-1/PD-L1 blockade therapy in non-small-cell lung cancer: is PD-L1 expression a good marker for patient selection?Clin Lung Cancer20161735036127137346
- HansenARSiuLLPD-L1 testing in cancer: challenges in companion diagnostic developmentJAMA Oncol20162151626562503
- MengXHuangZTengFXingLYuJPredictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapyCancer Treat Rev20154186887626589760
- InoueYYoshimuraKMoriKSugimura, Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancerOncotarget20167321133212827050074
- MukherjiDJabbourMNSaroufimMProgrammed death-ligand 1 expression in muscle-invasive bladder cancer cystectomy specimens and lymph node metastasis: a reliable treatment selection biomarker?Clin Genitourin Cancer20161418318726775720
- CalleaMAlbigesLGuptaMDifferential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinomaCancer Immunol Res201531158116426014095
- MadoreJVilainREMenziesAMPD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trialsPigment Cell Melanoma Res20152824525325477049
- HerbstRSSoriaJCKowanetzMPredictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patientsNature201451556356725428504
- Van AllenEMMiaoDSchillingBGenomic correlates of response to CTLA-4 blockade in metastatic melanomaScience201535020721126359337
- YearleyJHGibsonCYuNPD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancerClin Cancer Res2017233158316728619999
- ChenDSMellmanIElements of cancer immunity and the cancer-immune set pointNature201754132133028102259
- TaylorNAVickSCIglesiaMDTreg depletion potentiates checkpoint inhibition in claudin-low breast cancerJ Clin Invest20171273472348328825599
- PageDBYuanJRedmondDDeep sequencing of T-cell receptor DNA as a biomarker of clonally expanded TILs in breast cancer after immunotherapyCancer Immunol Res2016483584427587469
- DaudAILooKPauliMLTumor immune profiling predicts response to anti-PD-1 therapy in human melanomaJ Clin Invest20161263447345227525433
- LinesJLPantaziEMakJVISTA is an immune checkpoint molecule for human T cellsCancer Res2014741924193224691993
- TakeuchiYNishikawaHRoles of regulatory T cells in cancer immunityInt Immunol20162840140927160722
- LiXShaoCShiYHanWLessons learned from the blockade of immune checkpoints in cancer immunotherapyJ Hematol Oncol2018113129482595
- LowtherDEGoodsBALuccaLEPD-1 marks dysfunctional regulatory T cells in malignant gliomasJCI Insight20161e8593527182555
- HamidOSchmidtHNissanAA prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanomaJ Transl Med2011920422123319
- SelbyMJEngelhardtJJQuigleyMAnti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cellsCancer Immunol Res20131324224777248
- SimpsonTRLiFMontalvo-OrtizWFc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanomaJ Exp Med20132101695171023897981
- CoussensLMWerbZInflammation and cancerNature200242086086712490959
- PardollDMThe blockade of immune checkpoints in cancer immu-notherapyNat Rev Cancer20121225226422437870
- RemonJChaputNPlanchardDPredictive biomarkers for programmed death-1/programmed death ligand immune checkpoint inhibitors in nonsmall cell lung cancerCurr Opin Oncol20162812212926756384
- ParkerBSRautelaJHertzogPJAntitumour actions of interferons: implications for cancer therapyNat Rev Cancer20161613114426911188
- RibasAHodiFWolchokJResponse to PD-1 blockade with pembrolizumab (MK-3475) is Associated with an interferon inflammatory immune gene signatureASCO Annual MeetingMay 29–June 2, 2015Chicago, Illinois
- BenciJLXuBQiuYTumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockadeCell201616715401554 e151227912061
- ZaretskyJMGarcia-DiazAShinDSMutations associated with acquired resistance to PD-1 blockade in melanomaN Engl J Med201637581982927433843
- GaoJShiLZZhaoHLoss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapyCell2016167397404.e39927667683
- KowanetzMShamesDSCummingsCTumor mutation load assessed by FoundationOne (FM1) is associated with improved efficacy of atezolizumab (atezo) in patients with advanced NSCLCAnn Oncol20162777P
- GoodmanAMKatoSBazhenovaLTumor mutational burden as an independent predictor of response to immunotherapy in diverse cancersMol Cancer Ther2017162598260828835386
- YaghmourGPandeyMIrelandCRole of genomic instability in immunotherapy with checkpoint inhibitorsAnticancer Res20162640334038
- JohnsonDBFramptonGMRiothMJTargeted next generation sequencing identifies markers of response to PD-1 BlockadeCancer Immunol Res2016495996727671167
- RizviNAHellmannMDSnyderACancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancerScience201534812412825765070
- CampesatoLFBarroso-SousaRJimenezLComprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practiceOncotarget20156342213422726439694
- HellmannMDCiuleanuTEPluzanskiANivolumab plus Ipi-limumab in lung cancer with a high tumor mutational burdenN Engl J Med Epub2018416
- RosenbergJEHoffman-CensitsJPowlesTAtezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trialLancet20163871909192026952546
- BellmuntJde WitRVaughnDJPembrolizumab as second-line therapy for advanced urothelial carcinomaN Engl J Med20173761015102628212060
- GettingerSNHornLGandhiLOverall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancerJ Clin Oncol2015332004201225897158
- BrahmerJRTykodiSSChowLQSafety and activity of anti-PD-L1 antibody in patients with advanced cancerN Engl J Med20123662455246522658128
- TopalianSLHodiFSBrahmerJRSznol, safety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med20123662443245422658127
- SchumacherTNSchreiberRDNeoantigens in cancer immunotherapyScience2015348697425838375
- McGranahanNFurnessAJRosenthalRClonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockadeScience20163511463146926940869
- LeDTDurhamJNSmithKNMismatch repair deficiency predicts response of solid tumors to PD-1 blockadeScience201735740941328596308
- FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication Available from: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm560040.htmAccessed April 1, 2018
- IyerRRPluciennikABurdettVModrichPLDNA mismatch repair: functions and mechanismsChem Rev200610630232316464007
- SmyrkTCWatsonPKaulKLynchHTTumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinomaCancer2001912417242211413533
- HowittBEShuklaSAShollLMAssociation of polymerase e-mutated and microsatellite-instable endometrial cancers with neoan-tigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1JAMA Oncol201511319132326181000
- ChenKHYuanCTTsengLHShunCTYehKHCase report: mismatch repair proficiency and microsatellite stability in gastric cancer may not predict programmed death-1 blockade resistanceJ Hematol Oncol201692927012666
- Lyford-PikeSPengSYoungGDEvidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinomaCancer Res2013731733174123288508
- QinYEkmekciogluSForgetMACervical cancer neoantigen landscape and immune activity is associated with human papilloma-virus master regulatorsFront Immunol2017868928670312
- FrenelJSLe TourneauCO’NeilBVarga, safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trialJ Clin Oncol2017354035404129095678
- PartlovaSBoucekJKloudovaKDistinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinomaOncoimmunology20154e96557025949860
- MezacheLPanicciaBNyinawaberaANuovoGJEnhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancersMod Pathol2015281594160226403783
- DerksSLiaoXChiaravalliAMAbundant PD-L1 expression in Epstein-Barr virus-infected gastric cancersOncotarget20167329253293227147580
- MaCPatelKSinghiADProgrammed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated with Epstein-Barr Virus or Microsatellite InstabilityAm J Surg Pathol2016401496150627465786
- RooneyMSShuklaSAWuCJGetzGHacohenNMolecular and genetic properties of tumors associated with local immune cytolytic activityCell2015160486125594174
- SeiwertTYBurtnessBMehraRSafety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trialLancet Oncol20161795696527247226
- HollebecqueAMeyerTMooreKNAn open-label, multicohort, phase 1/2 study of nivolumab in patients with virus-associated tumors (CheckMate 358): efficacy and safety in recurrent or metastatic cervical, vaginal, and vulvar cancersPresented at American Society of Clinical Oncology Annual MeetingJune 2–6, 2017Chicago, IL Abstract 5504
- GainorJFShawATSequistLVEGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysisClin Cancer Res2016224585459327225694
- HarataniKHayashiHTanakaTTumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatmentAnn Oncol2017281532153928407039
- TabchiSKourieHRKattanJAdding checkpoint inhibitors to tyrosine kinase inhibitors targeting EGFR/ALK in non-small cell lung cancer: a new therapeutic strategyInvest New Drugs20163479479627562868
- OvermanMJMcDermottRLeachJLNivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 studyLancet Oncol2017181182119128734759
- BradleySDChenZMelendezBBRAFV600E co-opts a conserved MHC class I internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanomaCancer Immunol Res2015360260925795007
- AtkinsDBreuckmannASchmahlGEMHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinomaInt J Cancer200410926527314750179
- PengWChenJQLiuCLoss of PTEN promotes resistance to T cell-mediated immunotherapyCancer Discov2016620221626645196
- SprangerSBaoRGajewskiTFMelanoma-intrinsic beta-catenin signalling prevents anti-tumour immunityNature201552323123525970248
- FerrucciPFAsciertoPAPigozzoJBaseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumabAnn Oncol20162773273826802161
- LinGLiuYLiSElevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinomaOncotarget20167509635097126918355
- MartensAWistuba-HamprechtKGeukes FoppenMBaseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumabClin Cancer Res2016222908291826787752
- WeideBMartensAHasselJCBaseline biomarkers for outcome of melanoma patients treated with pembrolizumabClin Cancer Res2016225487549627185375
- ZaragozaJCailleABenetonNHigh neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanomaBr J Dermatol201617414615126343230
- JeyakumarGKimSBummaNNeutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapyJ Immunother Cancer201758229041991
- LalaniAAXieWMartiniDJChange in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinomaJ Immunother Cancer20186529353553
- RosnerSKwongEShoushtariANPeripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanomaCancer Med2018769069729468834
- SunRChampiatSDercleLBaseline lymphopenia should not be used as exclusion criteria in early clinical trials investigating immune checkpoint blockers (PD-1/PD-L1 inhibitors)Eur J Cancer20178420221128826073
- SubrahmanyamPBDongZGusenleitnerDDistinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patientsJ Immunother Cancer201861829510697
- KriegCNowickaMGugliettaSHigh-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapyNat Med20182414415329309059
- MeyerCCagnonLCosta-NunesCMFrequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumabCancer Immunol Immunother20146324725724357148
- ChaEKlingerMHouYImproved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patientsSci Transl Med20146238ra270
- McNeelDGTCR diversity – a universal cancer immunotherapy biomarker?J Immunother Cancer201646927879971
- RobertLTsoiJWangXCTLA4 blockade broadens the peripheral T-cell receptor repertoireClin Cancer Res2014202424243224583799
- HuangACPostowMAOrlowskiRJT-cell invigoration to tumour burden ratio associated with anti-PD-1 responseNature2017545606528397821
- QueiroloPMorabitoALaurentSAssociation of CTLA-4 polymorphisms with improved overall survival in melanoma patients treated with CTLA-4 blockade: a pilot studyCancer Invest20133133634523641913
- BreunisWBTarazona-SantosEChenRKileyMRosenbergSAChanockSJInfluence of cytotoxic T lymphocyte-associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockadeJ Immunother20083158659018528295
- VetizouMPittJMDaillereRAnticancer immunotherapy by CTLA-4 blockade relies on the gut microbiotaScience20153501079108426541610
- SivanACorralesLHubertNCommensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacyScience20153501084108926541606
- RoutyBLe ChatelierEDerosaLGut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumorsScience2018359919729097494
- MatsonVFesslerJBaoRThe commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patientsScience201835910410829302014
- ChaputNLepagePCoutzacCCarbonnel, baseline gut micro-biota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumabAnn Oncol2017281368137928368458
- BlankCUHaanenJBRibasASchumacherTNCANCER IMMUNOLOGY. The “cancer immunogram”Science201635265866027151852
