Abstract
Background
Prognosis of patients with hepatocellular carcinoma (HCC) who undergo transcatheter intra-arterial therapies, including transcatheter arterial chemoembolization and transcatheter arterial infusion chemotherapy, is affected by many clinical factors including liver function and tumor progression. However, the effect of body composition such as skeletal muscle and visceral and subcutaneous adipose tissues (VAT and SAT, respectively) on the prognosis of these patients remains unclear. We investigated the prognostic value of body composition in HCC patients treated with transcatheter intra-arterial therapies.
Patients and methods
This study retrospectively evaluated 100 HCC patients treated with transcatheter intra-arterial therapies between 2005 and 2015. Areas of skeletal muscle, VAT, and SAT were measured on computed tomography images at third lumbar vertebra level and normalized by the height squared to calculate the skeletal muscle index, VAT index, and SAT index (SATI). The visceral to subcutaneous adipose tissue area ratio was also calculated. Overall survival (OS) was compared between high- and low-index groups for each body composition. Furthermore, prognostic significance was assessed by univariate and multivariate analyses using Cox proportional hazards models.
Results
Among the body composition indexes, only SATI could significantly differentiate OS (p=0.012). Multivariate analysis showed that SATI (low- vs. high-SATI: HR, 2.065; 95% CI, 1.187–3.593; p=0.010), serum albumin (<3.5 vs. ≥3.5 g/dL; HR, 2.007; 95% CI, 1.037–3.886; p=0.039), serum alpha-fetoprotein (<20 vs. ≥20 ng/mL; HR, 0.311; 95% CI, 0.179–0.540; p<0.001), and Modified Response Evaluation Criteria in Solid Tumors assessment (complete response+partial response+stable disease vs. progressive disease; HR, 0.392; 95% CI, 0.221–0.696; p=0.001) were indicated as independent prognostic factors for OS.
Conclusion
High SAT volume is associated with better survival outcomes in HCC patients treated with transcatheter intra-arterial therapies. Elucidation of the mechanisms regulating SAT volume may offer a new therapeutic strategy for these patients.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world and a leading cause of cancer death.Citation1 Transcatheter intra-arterial therapies, including transcatheter arterial chemoembolization (TACE) and transcatheter arterial infusion chemotherapy (TAI), are endorsed in practice guidelines as major treatment options for patients with unresectable HCC.Citation2–Citation6 However, it is often difficult to exactly predict the prognosis of HCC patients treated with transcatheter intra-arterial therapies in clinical practice, because outcomes are affected by various factors, including the etiology of underlying liver disease; hepatic reserve function; tumor-specific factors, such as number, diameter, and distribution in the liver; and the efficacy of treatment.Citation7–Citation10 Furthermore, a close association between sarcopenia and clinical outcomes of HCC has been shown in recent studies, and, thus, body composition changes related to skeletal muscle loss may also affect prognosis.Citation11,Citation12 It is important to identify all contributors to the prognosis for precise prediction.
Body mass index (BMI) is a widely used anthropometric index to assess the degree of obesity and is associated with clinical outcomes of malignancies including HCC.Citation13 In addition, recent studies have revealed that quantification of several body compositions, such as skeletal muscle mass and visceral and subcutaneous adipose tissue (VAT and SAT, respectively) volumes, is also useful to predict the prognosis of HCC patients treated with various methods, including surgical resection, radiofrequency ablation, and tyrosine kinase inhibitors.Citation14–Citation17 However, there has been no study that specifically focused on the association between body composition and outcomes of HCC patients treated with transcatheter intra-arterial therapies.
In this study, we retrospectively measured the area of skeletal muscle, VAT, and SAT using cross-sectional computed tomography (CT) images in HCC patients treated with trans-catheter intra-arterial therapies. We statistically analyzed the association between these body compositions and outcomes and evaluated the prognostic value of the body compositions.
Patients and methods
Patients
We retrospectively analyzed consecutive HCC patients who underwent transcatheter intra-arterial therapies as initial treatment at Niigata University Medical and Dental Hospital between January 2005 and December 2015. The diagnosis of HCC was confirmed on the basis of typical enhancement patterns on dynamic CT or dynamic magnetic resonance imaging, i.e., contrast enhancement in the arterial phase and subsequent washout in the equilibrium phase.Citation18 When the typical enhancement patterns of HCC were not depicted, the diagnosis of HCC depended on histopathological analyses by tumor biopsy. Exclusion criteria were as follows: 1) presence of massive ascites or subcutaneous edema; 2) undergoing initial treatment other than TACE or TAI; and 3) achievement of complete response (CR) by additional radical treatment with surgical resection or local ablation therapies such as radiofrequency ablation following transcatheter intra-arterial therapies.
This retrospective study was approved by the ethics committee of Niigata University School of Medicine and carried out in accordance with the 1975 Helsinki Declaration (approval number 2442). Because of the anonymous nature of the data, the requirement for additional informed consent to participate in this study was waived.
Treatment procedure
TACE and/or TAI were performed according to the clinical practice guidelines for HCC of the Japan Society of Hepatology.Citation6 Briefly, TACE or TAI is recommended for patients with multiple tumors and liver damage of Child–Pugh class A or B. The TAI procedure consists of injecting IA-Call® (cisplatin) (Nippon Kayaku, Tokyo, Japan), Miripla® (miriplatin) (Dainippon Sumitomo Pharma, Osaka, Japan), or an emulsion of Farmorubicin® (epirubicin) (Pfizer Inc., New York, NY, USA) in lipiodol into hepatic arteries including tumor nourishing arteries. TACE includes subsequent embolization of the feeding arteries with Gelpart® (gelatin sponge particles) (Nihonkayaku, Tokyo, Japan) after TAI. TAI, which was devoid of embolization, was implemented when multiple tumors were extensively distributed in bilateral lobes of the liver, the arterial anatomy precluded a super selective injection, or significant arteriovenous fistulas or tumor thrombi in the main trunk of portal vein existed.
Body composition quantification
The body composition variables were quantified by the acquisition of a CT scan slice image at the third lumbar vertebra (L3) level undertaken prior to treatment for the purpose of the clinical workup for HCC. The skeletal muscle area was measured using sliceOmatic software (version 5.0; Tomovision, Montreal, QC, Canada; ), and the adipose tissue area was determined using Ziostation2 (version 2.1; Ziosoft, Tokyo, Japan; ). As previously described, thresholds of tissue Hounsfield units (HU) for delineation of the regions were adopted as follows: −29 to +150 HU for skeletal muscle, −150 to −50 HU for VAT, and −190 to −30 HU for SAT.Citation19,Citation20 These cross-sectional areas (cm2) were normalized by the height squared (m2) to determine the skeletal muscle index (SMI), VAT index (VATI), and SAT index (SATI). The visceral to subcutaneous adipose tissue area ratio (VSR) was also calculated. The cutoff values for the classification of SMI into low-SMI or high-SMI groups were defined as SMI<42 cm2/m2 for men and <38 cm2/m2 for women according to the Japanese Society of Hepatology guideline for sarcopenia in liver disease.Citation21 The cutoff values of the VATI, SATI, and VSR were set for each sex using optimal stratification to find the most significant p-value by means of a log-rank test as previously described.Citation22,Citation23
Clinical data
The following clinical data were retrospectively collected from medical records and used for analysis: age; sex; BMI; hepatitis virus infection, including hepatitis B virus (HBV), hepatitis C virus (HCV), or non-B non-C (NBNC); serum alanine transaminase (ALT) level; serum total bilirubin level; serum albumin level; platelet count; Child–Pugh classification; serum alpha-fetoprotein (AFP) level; tumor node metastasis (TNM) stage;Citation24 maximal tumor size; number of tumors classified into solitary or multiple; branched-chain amino acid (BCAA) supplementation; and treatment response of transcatheter intra-arterial therapies. The treatment response was assessed according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST), and classified as follows: CR, partial response (PR), stable disease (SD), and progressive disease (PD).Citation25
Overall survival (OS) was calculated based on the days between the date of CT examination prior to treatment and the date of death or December 2015 for surviving patients.
Statistical analyses
Continuous variables were expressed as median (interquartile range), and categorical variables were expressed as numbers of patients. The Mann–Whitney U-test was used to compare differences in median values. Either Fisher’s exact test or the chi-squared test was used to compare differences in categorical variables between groups. The correlations between each body composition index and BMI were analyzed using Spear-man’s correlation analysis by sex. OS rates were calculated using the Kaplan–Meier method and compared between groups using a log-rank test. Univariate and multivariate analyses with the Cox proportional hazards model were used to analyze prognostic variables for OS, expressed as HR and 95% CI. All variables were dichotomized, and significant variables in univariate analysis were included in multivariate models. All statistical analyses were conducted using SPSS Statistics version 21 (IBM Corporation, Armonk, NY, USA). All tests of significance were two-sided and p<0.05 was considered statistically significant.
Results
Baseline demographic and clinical characteristics
Baseline demographic and clinical characteristics of the patients are shown in . A total of 100 patients, including 69 male and 31 female patients, were enrolled in this study. HCC was diagnosed according to histopathological findings in only 4 patients and imaging features in the other 96 patients. Sixty-five patients died during the median observation period of 746 days. The median age of all patients was 71 years, and female patients were significantly older than male patients in the cohort. The SMI and VSR were significantly higher in male than in female patients, whereas the BMI, VATI, and SATI did not significantly differ between men and women. Seventy-two patients were classified into Child–Pugh class A, 28 into class B, and 0 into class C. Fifty-nine patients had advanced HCC which was classified into TNM stage III and IV. Fifty-three patients were treated with the combination of TACE and TAI, and the treatment response of transcatheter intra-arterial therapies was PD in more than half of patients. No difference was observed in TNM stages and treatment response between men and women.
Table 1 Baseline demographic and clinical characteristics
Correlation analyses of body composition indexes and BMI
The correlations between each body composition index and BMI were analyzed (). In men, the BMI was significantly correlated with the SMI, VATI, and SATI, but not with the VSR. The SATI showed a significantly negative correlation with the VSR. In contrast, in women there was no significant correlation between the BMI and the VSR or SMI. The correlation between the SATI and the VSR was also not significant in women.
Table 2 Spearman’s correlation analysis among body composition indexes
Survival analysis
Sex-specific cutoff values for the VATI, SATI, and VSR determined by optimal stratification were as follows: VATI<44.0 cm2/m2 for men and <35.0 cm2/m2 for women, SATI<40.0 cm2/m2 for men and <30.0 cm2/m2 for women, and VSR<1.08 for men and <0.86 for women. The patients were classified by the cutoff values into low- or high-index groups for each body composition () and incorporated into the survival analysis.
Table 3 Sex-specific cutoff values of body composition indexes
The results of univariate and multivariate analyses of the Cox proportional hazards model are shown in . Of the body composition indexes, only the SATI was significantly associated with OS in univariate analysis. In addition to the SATI, the serum albumin level, Child–Pugh classification, serum AFP level, TNM stage, maximal tumor size, and mRECIST assessment were also statistically significant predictors for OS in univariate analysis. Of these variables, the SATI (low- vs. high-SATI; HR, 2.065; 95% CI, 1.187–3.593; p=0.010), serum albumin level (<3.5 vs. ≥3.5 g/dL; HR, 2.007; 95% CI, 1.037–3.886; p=0.039), serum AFP level (<20 vs. ≥20 ng/mL; HR, 0.311; 95% CI, 0.179–0.540; p<0.001), and mRECIST assessment (CR+PR+SD vs. PD; HR, 0.392; 95% CI, 0.221–0.696; p=0.001) were indicated as independent prognostic factors for OS in multivariate analysis.
Table 4 Univariate and multivariate analysis of clinical characteristics for overall survival using the Cox proportional hazards model
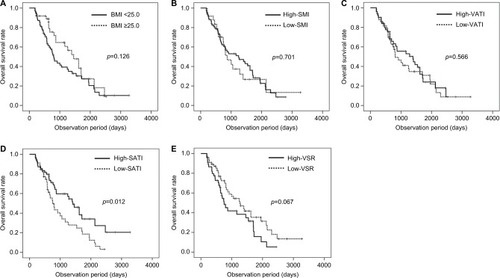
Kaplan–Meier survival curves of the patients stratified by BMI and body composition indexes are shown in . The log-rank test showed a significant difference in OS stratified only by the SATI (p=0.012; ), but not by BMI (p=0.126; ), SMI (p=0.701; ), and VATI (p=0.566; ). Although not statistically significant, the OS of the high-VSR group tended to be lower than that of the low-VSR group (p=0.067; ).
Figure 2 Overall survival rate stratified by body mass index (BMI, A), skeletal muscle index (SMI, B), visceral adipose tissue index (VATI, C), subcutaneous adipose tissue index (SATI, D), and visceral to subcutaneous adipose tissue area ratio (VSR, E).
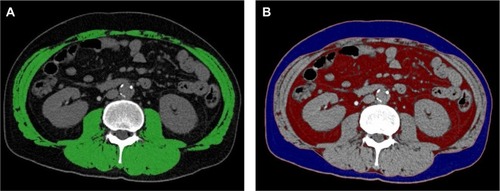
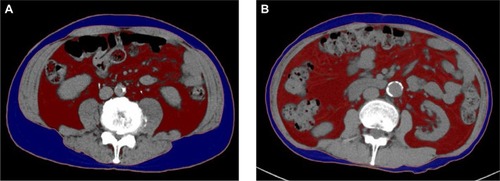
Cross-sectional areas of SAT and VAT measured on CT images in typical cases with high and low SATI are demonstrated in . The CT image of patient number 1 with high SATI is shown in . The patient was a 68-year-old man and had an HBV infection, TNM stage III, Child–Pugh class B, SATI of 59.5, and VATI of 56.0. The patient was alive for 1800 days at the observation end time. Patient number 2 was a 73-year-old man with low SATI (). The patient had an HCV infection, TNM stage II, Child–Pugh class A, SATI of 19.5, and VATI of 57.3. The patient died at 751 days.
Comparison of demographic and clinical characteristics between low- and high-SATI groups
The comparison of baseline demographic and clinical characteristics between patients in the low- and high-SATI groups is shown in . The low-SATI group consisted of 38 men and 7 women, which was a significantly lower proportion of women than in the high-SATI group. The BMI, SMI, and VATI were significantly higher in the high-SATI group; however, the VSR was not. Regarding etiology, NBNC were also significantly more frequent in the high-SATI group. No other variables, including laboratory data, tumor-specific factors, or course of treatment, differed significantly between the two groups.
Table 5 Comparison of demographic and clinical characteristics between low- and high-SATI groups
Discussion
In this study, we retrospectively quantified the volume of skeletal muscle, VAT, and SAT using cross-sectional CT images and investigated the association with the survival outcome of HCC patients treated with transcatheter intra-arterial therapies. It was revealed that a low SATI was an independent prognostic factor of poor OS in these patients, whereas no other body composition index affected the OS.
In cancer patients, adipose tissue lipolysis is augmented, whereas adipogenesis is weakened with cancer progression.Citation26–Citation30 One of the major biological functions of white adipose tissue is energy storage, and it can protect cancer patients against increased energy exhaustion induced by the cachectic state.Citation31–Citation33 In previous studies, SAT has been reported to be beneficial for lipid and glucose metabolism.Citation34,Citation35 Thus, these functions of SAT may contribute to the improvement of outcomes of patients with advanced HCC in this study.
Whether VAT accumulation is beneficial or harmful for the survival of HCC patients is currently controversial.Citation14–Citation17 In contrast, a high VSR was revealed as an adverse prognostic factor for various malignancies, including HCC, in previous studies.Citation12,Citation36–Citation38 Contrary to the favorable effects of SAT, the detrimental effects of VAT have frequently been observed in cancer patients. Although VAT is also a component of white adipose tissues, it has functions distinct from SAT.Citation34 VAT is a metabolically active endocrine organ, and its excess accumulation induces the alteration of expression levels of various adipokines, such as interleukin-6, tumor necrosis factor, and leptin, leading to carcinogenesis and tumor progression.Citation39–Citation41 Furthermore, many studies have demonstrated that low skeletal muscle volume was also associated with poor clinical outcomes in HCC patients.Citation11,Citation12,Citation42–Citation46
Contrary to the significantly detrimental impacts of high VAT volume, high VSR, and low skeletal muscle volume reported previously, other than the SATI, the body composition indexes examined in this study were not significant prognosticators. We have considered that this discrepancy may be attributed to the more advanced tumor stages and less curative treatment undergone in most of our patients compared to subjects in previous studies, because, in general, tumor progression and treatment methods affect prognosis more profoundly than body composition changes in cancer patients.
To our knowledge, this is the first study that demonstrated the association between high SAT volume and improved survival outcomes of HCC patients treated with transcatheter intra-arterial therapies. The results of our study are consistent with those of previous studies on subjects with prostate cancer and multiple myeloma.Citation47,Citation48 The study that investigated multiple myeloma additionally showed that fluorodeoxyglucose (FDG) uptake as assessed by positron emission tomography CT (PET/CT) was significantly increased in patients with lower SAT volumes.Citation48 This finding suggests that a more active tumor metabolism indicated by a higher FDG uptake may lead to hypercatabolism, which is responsible for a decrease in SAT volume. Although we were unable to assess FDG uptake because PET/CT is not performed as the routine workup for HCC, a similar mechanism may also be at work in our patients.
Several limitations of this study should be acknowledged. First, this was a single-institution study with a relatively small number of patients which was not sufficient to determine optimal cutoff values of the SATI to predict the outcomes of HCC patients. Besides, there were differences in sex and etiology between low- and high-SATI groups which may become confounding factors of the prognosis. Further study including a larger number of patients is needed to confirm our results. Second, the design of this study was retrospective, and we were unable to reveal the mechanism underlying the relationship between lower SAT volume and worse survival. A comprehensive investigation, including a biological analysis of SAT in patients with HCC, is required.
Conclusion
This study found that high SAT volume is associated with better survival outcomes in HCC patients treated with transcatheter intra-arterial therapies. Further investigation to elucidate the mechanisms regulating SAT volume in cancer patients may lead to an improvement in clinical outcomes through early therapeutic interventions.
Acknowledgments
We would like to express our deep sorrow over Dr Satoshi Yamagiwa’s passing, and sincere gratitude for his great contribution to this study.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin2015658710825651787
- LlovetJMRealMIMontañaXArterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trialLancet20023591734173912049862
- TakayasuKAriiSIkaiIProspective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patientsGastroenterology200613146146916890600
- OkusakaTKasugaiHShioyamaYTransarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: a randomized phase III trialJ Hepatol2009511030103619864035
- BruixJShermanMAmerican Association for the Study of Liver DiseasesManagement of hepatocellular carcinoma: an updateHepatology2011531020102221374666
- KokudoNHasegawaKAkahaneMEvidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines)Hepatol Res201545
- OgasawaraSChibaTOokaYA prognostic score for patients with intermediate-stage hepatocellular carcinoma treated with transarterial chemoembolizationPLoS One201510e012524425919025
- BarmanPMSharmaPKrishnamurthyVPredictors of mortality in patients with hepatocellular carcinoma undergoing transarterial chemoembolizationDig Dis Sci2014592821282524973040
- HuHTKimJHLeeLSChemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohortJ Vasc Interv Radiol20112291792321571545
- IkedaMMaedaSAshiharaHNagahamaHTanakaMSasakiYTranscatheter arterial infusion chemotherapy with cisplatin-lipiodol suspension in patients with hepatocellular carcinomaJ Gastroenterol201045606719655081
- IritaniSImaiKTakaiKSkeletal muscle depletion is an independent prognostic factor for hepatocellular carcinomaJ Gastroenterol20155032333224817668
- FujiwaraNNakagawaHKudoYSarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinomaJ Hepatol20156313114025724366
- CalleEERodriguezCWalker-ThurmondKThunMJOverweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adultsN Engl J Med20033481625163812711737
- OhkiTTateishiRShiinaSVisceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASHGut20095883984419174415
- ItohSShirabeKMatsumotoYEffect of body composition on outcomes after hepatic resection for hepatocellular carcinomaAnn Surg Oncol2014213063306824719020
- HigashiTHayashiHKaidaTPrognostic impact of visceral fat amount and branched-chain amino acids (BCAA) in hepatocellular carcinomaAnn Surg Oncol201522S1041S104726305023
- NaultJCPigneurFNelsonACVisceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitorsDig Liver Dis20154786987626211871
- European Association For The Study Of The LiverEuropean Organisation For Research And Treatment Of CancerEASL-EORTC clinical practice guidelines: management of hepatocellular carcinomaJ Hepatol20125690894322424438
- MitsiopoulosNBaumgartnerRNHeymsfieldSBLyonsWGallagherDRossRCadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomographyJ Appl Physiol (1985)1998851151229655763
- MillerKDJonesEYanovskiJAShankarRFeuersteinIFalloonJVisceral abdominal-fat accumulation associated with use of indinavirLancet19983518718759525365
- NishikawaHShirakiMHiramatsuAMoriyaKHinoKNishiguchiSJapan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteriaHepatol Res20164695196327481650
- PradoCMLieffersJRMcCargarLJPrevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based studyLancet Oncol2008962963518539529
- van VledderMGLevolgerSAyezNVerhoefCTranTCIjzermansJNBody composition and outcome in patients undergoing resection of colorectal liver metastasesBr J Surg20129955055722246799
- Liver Cancer Study Group of JapanGeneral Rules for the Clinical and Pathological Study of Primary Liver Cancer3rd English edTokyoKanehara20102627
- LencioniRLlovetJMModified RECIST (mRECIST) assessment for hepatocellular carcinomaSemin Liver Dis201030526020175033
- TsoliMSwarbrickMMRobertsonGRLipolytic and thermogenic depletion of adipose tissue in cancer cachexiaSemin Cell Dev Biol201654688126529279
- BatistaMLJrNevesRXPeresSBHeterogeneous time-dependent response of adipose tissue during the development of cancer cachexiaJ Endocrinol201221536337323033362
- DahlmanIMejhertNLinderKAdipose tissue pathways involved in weight loss of cancer cachexiaBr J Cancer20101021541154820407445
- BingCRussellSBecketEAdipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing miceBr J Cancer2006951028103717047651
- Zuijdgeest-van LeeuwenSDvan den BergJWWattimenaJLLipolysis and lipid oxidation in weight-losing cancer patients and healthy subjectsMetabolism20004993193610910006
- MurphyRAWilkeMSPerrineMLoss of adipose tissue and plasma phospholipids: relationship to survival in advanced cancer patientsClin Nutr20102948248719959263
- CamusVLanicHKrautJPrognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapyEur J Haematol201493918
- CooperABSlackRFogelmanDCharacterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancerAnn Surg Oncol2015222416242325519927
- IbrahimMMSubcutaneous and visceral adipose tissue: structural and functional differencesObes Rev201011111819656312
- TranTTYamamotoYGestaSKahnCRBeneficial effects of subcutaneous fat transplantation on metabolismCell Metab2008741042018460332
- WuWLiuXChaftariPAssociation of body composition with outcome of docetaxel chemotherapy in metastatic prostate cancer: a retrospective reviewPLoS One201510e012204725822612
- GrignolVPSmithADShlapakDZhangXDel CampoSMCarsonWEIncreased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapySurg Oncol20152435335826690825
- OkamuraAWatanabeMMineSClinical impact of abdominal fat distribution on prognosis after esophagectomy for esophageal squamous cell carcinomaAnn Surg Oncol2016231387139426668084
- CabiaBAndradeSCarreiraMCCasanuevaFFCrujeirasABA role for novel adipose tissue-secreted factors in obesity-related carcinogenesisObes Rev20161736137626914773
- ParkEJLeeJHYuGYDietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expressionCell201014019720820141834
- SharmaDWangJFuPPAdiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesisHepatology2010521713172220941777
- HarimotoNShirabeKYamashitaYISarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinomaBr J Surg20131001523153024037576
- LevolgerSvan VledderMGMuslemRSarcopenia impairs survival in patients with potentially curable hepatocellular carcinomaJ Surg Oncol201511220821326266324
- KamachiSMizutaTOtsukaTSarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatmentHepatol Res20164620120826223826
- YabusakiNFujiiTYamadaSAdverse impact of low skeletal muscle index on the prognosis of hepatocellular carcinoma after hepatic resectionInt J Surg20163013614227154615
- HarimotoNYoshizumiTShimokawaMSarcopenia is a poor prognostic factor following hepatic resection in patients 70 years of age and older with hepatocellular carcinomaHepatol Res2016461247125526880049
- AntounSBayarAIleanaEHigh subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy settingEur J Cancer2015512570257726278649
- TakeokaYSakatokuKMiuraAPrognostic effect of low subcutaneous adipose tissue on survival outcome in patients with multiple myelomaClin Lymphoma Myeloma Leuk20161643444127263047