Abstract
Background
Various studies have evaluated the significance of phosphohistone-H3 (PHH3) expression in cancer patients, but controversy over its reliability remains. We conducted a meta-analysis to summarize the prognostic relevance of PHH3 expression in cancer patients.
Patients and methods
Nineteen studies, including 4803 patients, were identified by searching PubMed, Web of Science, Embase, and Cochrane Library. The correlation of PHH3 expression level with overall survival (OS), disease-free survival, and recurrence-free survival was analyzed.
Results
Overall, the results suggest that high expression of PHH3 can predict a poor OS (HR=2.66, 95% CI=1.74–4.08, P<0.001), disease-free survival (HR=3.40, 95% CI=1.47–7.87, P=0.004), and recurrence-free survival (HR=2.80, 95% CI=1.61–4.85, P<0.001) in cancer patients. The subgroup analysis showed that highly expressed PHH3 was significantly related to breast cancer (HR=5.66, 95% CI=2.72–11.78, P<0.001) and urogenital tumors (HR=3.01, 95% CI=1.78–5.09, P<0.001). Furthermore, no significant difference was found between Asian (HR=1.98, 95% CI=1.08–3.63, P=0.026) and Caucasian populations (HR=3.01, 95% CI=1.87–4.85, P<0.001) regarding OS and PHH3 expression.
Conclusion
This meta-analysis indicates that high expression of PHH3 may serve as a biomarker for poor prognosis in patients with cancer.
Introduction
Cancer is still considered to be a major challenge for modern medicine and a major public health problem worldwide.Citation1 Tumor-specific markers can be useful for monitoring and evaluating tumor progression and therapeutic efficacy. However, there is still a shortage of highly sensitive and specific markers for assessing cancer progression and prognosis.Citation2 Therefore, it is necessary for decision-making concerning clinical therapy to figure out an effective pretreatment parameter that can be used to evaluate survival probability and prognosis of cancer patients.
Phospho-histone-H3 (PHH3) is a core histone protein, which in its phosphorylated state forms the principal constituents of eukaryotic chromatin, with histone H3 being phosphorylated at serine (Ser) 10 or Ser28 as well as its phosphorylation of Ser10 being strongly correlated with the late G2 to M-phase transition in mammalian mitotic cells.Citation3 On the basis of previous research, a few cell line- and animal model-based researches have displayed an increase in phosphorylation of histone H3 at Ser10 (H3S10ph), the only histone marker that is involved in carcinogenesis and cellular transformation.Citation4–Citation7 Also, earlier in vitro studies have suggested that a higher level of H3S10ph alone directly participated in cellular transformation.Citation5 PHH3 has long been accepted as a cellular proliferation marker in a large variety of cancers, and it was revealed to be of prognostic value in several recent studies.Citation8–Citation10 For instance, PHH3 expression is elevated in gliomas tissues, and patients with high PHH3 expression have a shorter overall survival (OS) compared with patients with lower expression levels of PHH3.Citation8 Elevated PHH3 expression is also detected in prostate carcinoma tissues and is strongly correlated with an advanced clinical stage.Citation9 In breast cancer, increased PHH3 expression is correlated with an lymph vessel invasion.Citation10 However, the association between elevated PHH3 expression and prognosis has not been identified in patients with gastrointestinal stromal tumors.Citation11 Therefore, we conducted this meta-analysis to summarize the global findings from analyses of PHH3 expression as a predictive indicator of clinical results in cancer patients.
Patients and methods
Search strategy
We performed a network search using PubMed, Web of Science, Embase, and Cochrane Library for original articles that analyzed the prognostic value of PHH3 in various cancers, with the last search update on February 1, 2018. The search strategy included the following keywords combined with “phospho-histone-H3:” “PHH3”, “neoplasm”, “cancer”, “prognostic”, and “prognosis”. Furthermore, additional eligible studies were collected by a manual search for relevant research from reference lists.
Selection criteria
The studies were considered eligible if they met all the following criteria: 1) studies that evaluated the relationship of PHH3 expression in cancer patients with detailed information about OS, disease-free survival (DFS), or recurrence-free survival (RFS); 2) HRs and 95% CIs, raw data, or survival curves were reported in the article; and 3) the articles were written in English or Chinese. Articles were excluded based on the following criteria: 1) meeting abstract, review or laboratory articles, as well as case studies or letters; 2) articles that described the survival outcome of other indicators; 3) duplicate publications; and 4) animal studies.
Quality assessment
To control the quality of the research, the Newcastle–Ottawa scale, ranging from 0 to 9 stars,Citation12 was used to assess the quality of the eligible papers. Studies with more than six stars were considered high quality. Two authors (Qian Hao and Yujiao Deng) performed the assessment independently, and any disagreement was resolved by discussion.
Data extraction
Two independent reviewers (Qian Hao and Cong Dai) extracted the details of included studies using a standardized form, and any disagreements were resolved through discussion with a third reviewer (Zhijun Dai). The following information was recorded: the first author’s name, year of publication, number of patients, patient source, tumor types, PHH3 assessment method, prognostic outcomes, analytical method, and HR with the corresponding 95% CI.
Statistical analysis
Statistical analyses were conducted using STATA version C14.0 (Stata Corporation, College Station, TX, USA). The HRs with their 95% CIs were used for evaluating the strength of the correlations between PHH3 and the survival outcomes. We evaluated the study heterogeneity (ICitation2) by chi-squared test. All statistical tests were two-sided, and the significance level was set at 0.05. The fixed effects model was used when no obvious heterogeneity was observed among the included studies (ICitation2<50%), otherwise the random effects model was selected to perform the meta-analysis. Subgroup analyses were conducted to investigate the potential factors of heterogeneity. Publication bias was estimated by Begg’s funnel plot and Egger’s test. Sensitivity analysis was performed by sequential omission of each study.
Results
Characteristics of included studies
Based on the study design, we initially searched a total of 410 articles. After duplicates were removed, 216 articles remained. Hundred and seventy-four articles qualified for review after we screened titles and abstracts. Subsequently, 122 studies were assessed by screening the full text, of which 103 articles were excluded (Table S1). Finally, 19 studies, published between 2011 and 2017, were included in this meta-analysis as shown in . The Newcastle–Ottawa scores of these studies ranged between 6 and 8. The total number of patients included were 4803, ranging between 27 and 637 patients per study. The main characteristics of the 19 articles were extracted and are summarized in . The patients were from People’s Republic of China,Citation8,Citation13,Citation14 Korea,Citation15 Norway,Citation10,Citation16–Citation18 India,Citation19 Germany,Citation9,Citation20 the United Kingdom,Citation21,Citation22 the United States of America,Citation11,Citation23 New Zealand,Citation24 Sweden,Citation25 Australia,Citation26 and Italy.Citation27 The types of carcinoma included glioma, neuroblastoma, gastric cancer, gastrointestinal stromal tumor, Merkel cell carcinoma, cutaneous melanoma, uterine smooth muscle tumor, endometrial cancer, adrenocortical carcinoma, prostate cancer, and breast cancer. Immunohistochemical staining was used to determine the level of PHH3 expression.
Table 1 Main characteristics of all studies included in the meta-analysis
Association of PHH3 expression with OS in human cancer
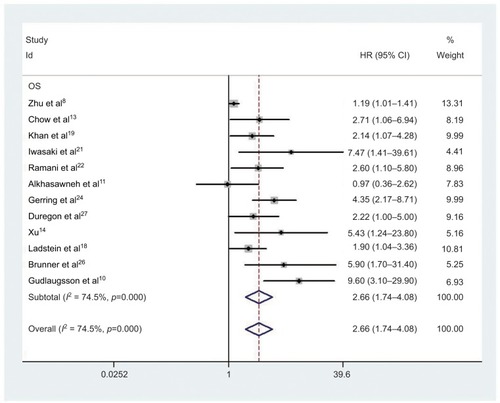
Twelve studies comprising 1259 cancer patients were included in the survival analysis. The pooled HR was 2.66 (95% CI=1.74–4.08, P<0.001; and ). High expression of PHH3 was significantly related to poor OS of cancer patients. Due to the emergence of clear heterogeneity in this study (ICitation2 =74.5%, P<0.001; ), a subgroup analysis was conducted to explore the source of heterogeneity further. The subgroups included tumor types, ethnicity, and HR estimate (). High PHH3 expression was significantly associated with reduced OS in breast cancer (HR=5.66, 95% CI=2.72–11.78, P<0.001) and urogenital tumors (HR=3.01, 95% CI=1.78–5.09, P<0.001), but not with nervous system tumors (HR=1.58, 95% CI=0.76–3.28, P=0.226), digestive system tumors (HR=1.57, 95% CI=0.73–3.35, P=0.247), or cutaneous tumors (HR=2.99, 95% CI=0.85–10.56, P=0.089). There was clear heterogeneity in nervous system tumors (ICitation2=69.4%, P=0.071) and digestive system tumors (ICitation2=56.6%, P=0.129), while no obvious heterogeneity was found in other tumor types. Subgroup analysis showed that the correlation between OS and PHH3 expression did not differ between ethnicities (Asian: HR=1.98, 95% CI=1.08–3.63, P=0.026; Caucasian: HR=3.01, 95% CI=1.87–4.85, P<0.001) and HR estimates (multivariate analysis: HR=3.17, 95% CI=1.66–6.04; univariate analysis: HR=1.93, 95% CI=1.31–2.82).
Table 2 Main meta-analysis results
Association of PHH3 expression with DFS and RFS
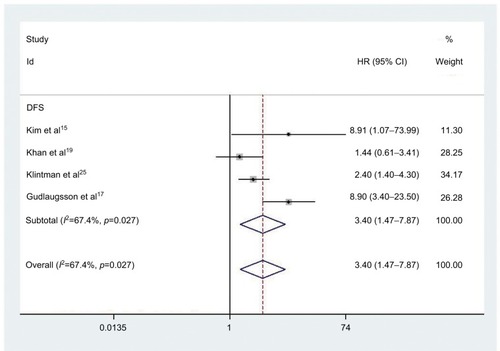
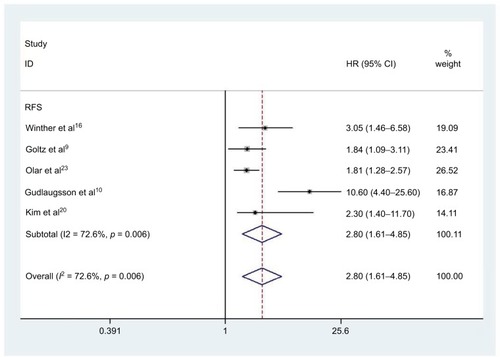
Four studies comprising 777 patients were included in determining the relationship between elevated PHH3 expression and DFS. Correspondingly, five studies involving 1496 patients were included for determining the association between PHH3 overexpression and RFS. The pooled outcome indicated that high expression levels of PHH3 might imply poor DFS (HR=3.40; 95% CI=1.47–7.87, P=0.004; and ) and RFS (HR=2.80; 95% CI=1.61–4.85, P<0.001; and ). Furthermore, significant heterogeneity was noted among the studies (DFS: ICitation2 =67.4%, P=0.027; RFS: ICitation2 =72.6%, P=0.006). However, we did not conduct subgroup analyses to determine sources of heterogeneity because of the small number of studies.
Sensitivity analyses and publication bias
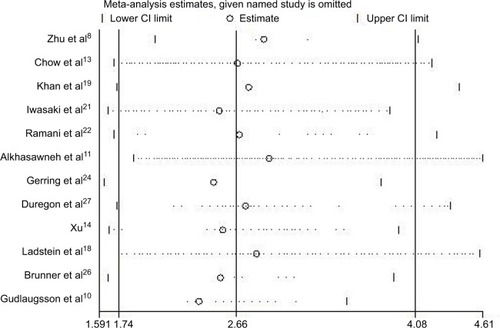
In order to determine whether an individual study had a significant influence on pooled HR and to confirm the stability and reliability of HR estimates, all the studies were sequentially removed. We found that the pooled HRs were not significantly influenced by any single study (). Both the Begg’s and Egger’s tests were performed to assess if any publication bias existed in the included studies. The funnel plot () was asymmetrical. Begg’s test (P=0.193) revealed no publication bias among the 12 eligible studies, while Egger’s test (P=0.002) showed a clear bias.
Discussion
PHH3, a chief protein component of nucleosomes in eukaryotic cells, is a less extensively studied proliferation factor. The clear and contrast-rich staining of PHH3 is easily assessed, with high inter-observer reproducibility, making it the subject of intense research in recent years.Citation28,Citation29 PHH3 has been found to be an independent prognostic factor in meningioma, cutaneous melanoma, and gastric cancer.Citation18,Citation20,Citation30,Citation31 We conducted this meta-analysis to mitigate sample size problems of individual studies and enhance their statistical power. Our study is the first meta-analysis to summarize research on PHH3 and its prognostic role in various types of cancer.
In this meta-analysis, we analyzed 19 studies including 4803 patients, with OS data from 12 studies, DFS data from 4 studies, and RFS data from 5 studies, which revealed that a high expression of PHH3 was correlated with poor OS, DFS, and RFS and significantly associated with a reduced OS in patients with breast cancer and urogenital tumors. Thus, PHH3 expression may be considered as a potential prognostic factor for cancer patients. It is well known that Ki-67 is commonly used to assess cell proliferation. However, according to Takahashi et al and Brenner et al, PHH3 expression was an independent factor for poor prognosis and an improved mitotic marker in gastric cancer and endometrial tissues compared to Ki-67.Citation31,Citation32 Likewise, some previous studies have noted that PHH3 is an excellent predictability marker of survival in long- and short-term follow-up.Citation15,Citation24,Citation25 Therefore, we collected and analyzed the results of several studies and found that PHH3 is a novel, useful proliferation marker that can be used as a prognostic indicator in astrocytoma, melanoma, prostate carcinoma, pituitary adenoma, and breast cancer.Citation25,Citation28,Citation29,Citation33–Citation36 PHH3 may also be a prognostic marker in various other types of cancer. To further analyze the role of PHH3 in different cancers, a subgroup analysis was performed. PHH3 expression was found to be statistically associated with breast, gynecological, cutaneous, and urogenital tumors.
Through the subgroup analysis, we found that the heterogeneity of our study originated from tumors of the nervous and digestive systems. Additionally, PHH3 doesn’t have standard testing methods and it’s also not routinely utilized. Its practical utility is limited so far, different phosphorylation sites (Ser10 and Ser28) and measurement units were used in these studies in different labs, detection assays, antibodies, and thresholds for positivity, given that it may cause significant discrepancies and biases in our studies. Furthermore, we conducted subgroup analyses of study region and analysis method; however, no significant differences were detected. It is important to note that there was a publication bias, suggesting that large-scale investigations are required. An inadequate number of included studies analyzed the OS, DFS, and RFS rates, with controversial conclusions. Overall, our meta-analysis supports the conclusion that PHH3 may be a predictive biomarker of OS, DFS, and RFS. Thus, further research is necessary to evaluate the relationship between overexpression of PHH3 and outcomes in human cancers.
Moreover, we found that some studies have identified a close correlation between PHH3 and mitotic cell condensation.Citation37,Citation38 Immunohistochemical staining of PHH3 has suggested that modification occurs almost exclusively in actively proliferating cells during M-phase, not during apoptosis.Citation39 From a clinical standpoint, PHH3 immunostaining has been validated in pulmonary melanoma, neuroendocrine carcinomas, and pancreatic cancer. Several previous studies have measured PHH3 levels among different types of cancers. Studies suggested that PHH3 index increased with higher grade of tumor, including cancers of breast, ovarian, melanoma, and meningioma.Citation20,Citation29,Citation40–Citation43 Therefore, PHH3 staining not only has prognostic value but also supports tumor grading by facilitating mitotic counting. PHH3 staining can be easily identified and can minimize inter-observer and inter-laboratory technical variations. In addition, one study suggested that the PHH3 index is a more sensitive measure of mitotic activity in Merkel cell carcinoma than the hematoxylin and eosin (H&E)-stained mitotic count index. Thus, it is believed to be a useful complementary diagnostic tool for standard H&E mitotic counts. Another study confirms that PHH3 was a better proliferation marker than Ki-67 due to its high reproductivity of immunohistochemistry, accuracy, and consistency among raters in breast cancer.Citation15 Finally, the “true” negative resection margin in gastric cancer has reportedly been determined using the distance-dependent association of PHH3 with clinical parameters. Therefore, PHH3 could be helpful in limiting the extent of resection and preventing post-surgical locoregional recurrence of the disease.Citation19
Generally, PHH3 expression levels are helpful in predicting the prognosis of different cancers, and PHH3 staining can be a useful complementary tool to routine methods in grading and determining “true” negative resection margins.
Conclusion
In summary, we found that increased expression of PHH3 indicated poor survival outcomes in patients with cancer, and therefore, PHH3 is a potential novel prognostic indicator in cancer patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81471670) and the Key Research and Development Plan, Shaanxi Province, China (2017ZDXM-SF-066).
Author contributions
Qian Hao and Cong Dai designed the study. Qian Hao, Yujiao Deng, and Peng Xu wrote the manuscript. Tian Tian, Shuai Lin, Meng Wang, and Kang Liu extracted the relevant studies and data. Dingli Song, Ying Wu, and Yan Guo analyzed the data. All authors contributed toward data analysis, drafting, revision of and final approval of the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- PritzkerKPPredictive and prognostic cancer biomarkers revisitedExpert Rev Mol Diagn201515897197426134385
- FukushimaSTerasakiMSakataKSensitivity and usefulness of anti-phosphohistone-H3 antibody immunostaining for counting mitotic figures in meningioma casesBrain Tumor Pathol2009262515719856215
- ChadeeDNHendzelMJTylipskiCPIncreased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblastsJ Biol Chem199927435249142492010455166
- ChoiHSChoiBYChoYYPhosphorylation of histone H3 at serine 10 is indispensable for neoplastic cell transformationCancer Res200565135818582715994958
- IbukiYToyookaTZhaoXYoshidaICigarette sidestream smoke induces histone H3 phosphorylation via JNK and PI3K/Akt pathways, leading to the expression of proto-oncogenesCarcinogenesis20143561228123724398671
- LiBHuangGZhangXIncreased phosphorylation of histone H3 at serine 10 is involved in Epstein-Barr virus latent membrane protein-1-induced carcinogenesis of nasopharyngeal carcinomaBMC Cancer20131312423496845
- ZhuPZhangCBYangPPhosphohistone H3 (pHH3) is a prognostic and epithelial to mesenchymal transition marker in diffuse gliomasOncotarget2016729450054501427323851
- GoltzDMontaniMBraunMPrognostic relevance of proliferation markers (Ki-67, PHH3) within the cross-relation of ERG translocation and androgen receptor expression in prostate cancerPathology201547762963626517642
- GudlaugssonESkalandIUndersrudEJanssenEASoilandHBaakJPD2-40/p63 defined lymph vessel invasion has additional prognostic value in highly proliferating operable node negative breast cancer patientsMod Pathol201124450251121317878
- AlkhasawnehAReithJDToroTZInterobserver variability of mitotic index and utility of PHH3 for risk stratification in gastrointestinal stromal tumorsAm J Clin Pathol2015143338539225696796
- StangACritical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- ChowKLTseKYCheungCLThe mitosis-specific marker phosphohistone-H3 (PHH3) is an independent prognosticator in uterine smooth muscle tumours: an outcome-based studyHistopathology201770574675527864989
- XuHExpression of EN2, PHH3 in Prostate Cancer and Prognostic Significance [master’s thesis]TaiyuanShanxi Medical University2014
- KimJYJeongHSChungTThe value of phosphohistone H3 as a proliferation marker for evaluating invasive breast cancers: a comparative study with Ki67Oncotarget2017839650646507629029412
- WintherTLArnliMBSalvesenØTorpSHPhosphohistone-H3 proliferation index is superior to mitotic index and MIB-1 expression as a predictor of recurrence in human meningiomasAm J Clin Pathol2016146451052027686177
- GudlaugssonEKlosJSkalandIPrognostic comparison of the proliferation markers (mitotic activity index, phosphohistone H3, Ki67), steroid receptors, HER2, high molecular weight cytokeratins and classical prognostic factors in T(1)(-)(2)N(0)M(0) breast cancerPol J Pathol20136411823625593
- LadsteinRGBachmannIMStraumeOAkslenLAPrognostic importance of the mitotic marker phosphohistone H3 in cutaneous nodular melanomaJ Invest Dermatol201213241247125222297638
- KhanSAAmnekarRKhadeBp38-MAPK/MSK1-mediated overexpression of histone H3 serine 10 phosphorylation defines distance-dependent prognostic value of negative resection margin in gastric cancerClin Epigenetics201688827588146
- KimYJKetterRSteudelWIFeidenWPrognostic significance of the mitotic index using the mitosis marker anti-phosphohistone H3 in meningiomasAm J Clin Pathol2007128111812517580279
- IwasakiTMatsushitaMNonakaDPhosphohistone-H3 (PHH3) is prognostic relevant in Merkel cell carcinomas but Merkel cell polyomavirus is a more powerful prognostic factor than AJCC clinical stage, PHH3, Ki-67 or mitotic indicesPathol Int201565840440925982855
- RamaniPTaylorSMillerESowa-AvugrahEMayMTHigh phosphohistone H3 expression correlates with adverse clinical, biological, and pathological factors in neuroblastomasJ Histochem Cytochem201563639740725711230
- OlarAWaniKMSulmanEPMitotic index is an independent predictor of recurrence-free survival in meningiomaBrain Pathol201525326627525040885
- GerringZPearsonJFMorrinHRRobinsonBAHarrisGCWalkerLCPhosphohistone H3 outperforms Ki67 as a marker of outcome for breast cancer patientsHistopathology201567453854725728258
- KlintmanMStrandCAhlinCThe prognostic value of mitotic activity index (MAI), phosphohistone H3 (PPH3), cyclin B1, cyclin A, and Ki67, alone and in combinations, in node-negative premenopausal breast cancerPLoS One2013812e8190224324728
- BrunnerARissPHeinzeGBrustmannHpHH3 and survivin are co-expressed in high-risk endometrial cancer and are prognostic relevantBr J Cancer20121071849022644303
- DuregonEMolinaroLVolanteMComparative diagnostic and prognostic performances of the hematoxylin-eosin and phospho-histone H3 mitotic count and Ki-67 index in adrenocortical carcinomaMod Pathol20142791246125424434900
- SkalandIJanssenEAGudlaugssonEHui Ru GuoLBaakJPThe prognostic value of the proliferation marker phosphohistone H3 (PPH3) in luminal, basal-like and triple negative phenotype invasive lymph node-negative breast cancerCell Oncol200931426127119633363
- SkalandIJanssenEAGudlaugssonEPhosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients <55 years of ageMod Pathol200720121307131517917671
- TakeiHBhattacharjeeMBRiveraADancerYPowellSZNew immunohistochemical markers in the evaluation of central nervous system tumors: a review of 7 selected adult and pediatric brain tumorsArch Pathol Lab Med2007131223424117284108
- TakahashiHMuraiYTsuneyamaKOverexpression of phosphorylated histone H3 is an indicator of poor prognosis in gastric adenocarcinoma patientsAppl Immunohistochem Mol Morphol200614329630216932020
- BrennerRMSlaydenODRodgersWHImmunocytochemical assessment of mitotic activity with an antibody to phosphorylated histone H3 in the macaque and human endometriumHum Reprod20031861185119312773444
- SambrookJRussellDWDephosphorylation of plasmid DNA.CSH Protoc200620061
- AliHRDawsonSJBlowsFMProvenzanoEPharoahPDCaldasCAurora kinase A outperforms Ki67 as a prognostic marker in ER-positive breast cancerBr J Cancer2012106111798180622538974
- JonsdottirKAssmusJSlewaAPrognostic value of gene signatures and proliferation in lymphnode-negative breast cancerPLoS One201493e9064224599057
- NowakMSvenssonMACarlssonJPrognostic significance of phospho-histone H3 in prostate carcinomaWorld J Urol201432370370723887713
- GotoHTomonoYAjiroKIdentification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensationJ Biol Chem199927436255432554910464286
- JuanGTraganosFDarzynkiewiczZHistone H3 phosphorylation in human monocytes and during HL-60 cell differentiationExp Cell Res199924612122209882530
- SunAZhouWLuncefordJStrackPDauffenbachLMKerfootCALevel of phosphohistone H3 among various types of human cancersBMJ Open201225e001071
- BossardCJarryAColombeixCPhosphohistone H3 labelling for histoprognostic grading of breast adenocarcinomas and computer-assisted determination of mitotic indexJ Clin Pathol200659770671016461563
- RibaltaTMcCutcheonIEAldapeKDBrunerJMFullerGNThe mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteriaAm J Surg Pathol200428111532153615489659
- SkalandIJanssenEAGudlaugssonEValidating the prognostic value of proliferation measured by phosphohistone H3 (PPH3) in invasive lymph node-negative breast cancer patients <71 years of ageBreast Cancer Res Treat20091141394518373192
- ScottISHeathTMMorrisLSA novel immunohistochemical method for estimating cell cycle phase distribution in ovarian serous neoplasms: implications for the histopathological assessment of paraffin-embedded specimensBr J Cancer20049081583159015083189

![Figure 6 Funnel plots of publication bias for all of the included studies reported with OS.Abbreviations: Iog[HR], logarithm hazard ratio; OS, overall survival; s.e., standard error.](/cms/asset/c7ea3677-f2cc-4160-a912-bf146e814f58/dcmr_a_12185577_f0006_b.jpg)