Abstract
Background
Radiotherapy (RT) concurrent with cisplatin (CDDP) is the standard regimen used for treatment of locally advanced cervical carcinoma. In this meta-analysis, we compared the weekly and triweekly single CDDP concomitant chemoradiation regimens for treatment of cervical cancer with respect to compliance, recurrence, survival, and acute adverse effects.
Materials and methods
A systematic search for relevant studies was conducted in PubMed, Cochrane Library, EMBASE, and Medline databases. Fixed- or random-effects model was used for pooled analysis. The end points were overall survival, recurrence, compliance, and acute adverse effect reported as odds ratios (ORs) and 95% CIs.
Results
Six randomized trials and two retrospective studies qualified the inclusion criteria. The regimen of triweekly CDDP alone concurrent with RT showed better compliance (OR, 0.49; 95% CI, 0.29–0.83; P=0.009). No significant difference was observed between the 2 arms with respect to recurrence, survival, and acute adverse effects (all P>0.05). However, triweekly CDDP regimen was associated with significantly lower incidence of local recurrence (OR, 1.83; 95% CI: 1.12–3.01; P=0.02), while weekly CDDP regimen was associated with a lower risk of leucopenia (OR, 0.30; 95% CI: 0.10–0.92; P=0.03).
Conclusion
Triweekly single platinum chemotherapy plus concurrent RT was superior to weekly CDDP regimen with respect to local recurrence and treatment compliance in patients with locally advanced cervical carcinoma.
Introduction
Cervical cancer (CC) is the fourth most commonly diagnosed cancer and the fourth leading cause of cancer deaths among women worldwide. An estimated 90% of deaths from CC occur in the developing countries.Citation1 The application of human papillomavirus vaccine and advances in screening technology have contributed to the great achievements in prevention and treatment of CC and premalignant disease; however, the worldwide survival and prognosis of this malignancy is still very poor, especially for locally advanced cervical carcinoma (LACC). Based on favorable outcomes in 5 randomized clinical trials (RCTs), cisplatin (CDDP)-based chemoradiotherapy is strongly recommended for patients with LACC who require radiotherapy (RT).Citation2–Citation6 Furthermore, weekly CDDP regimen concurrent with radiation is widely accepted due to better compliance and low toxicity, compared with triweekly CDDP plus 5-fluorouracil (5-FU) regimen among these 5 studies. In a study by Lanciano et al,Citation7 outcomes in the 5-FU treatment arm were not superior to those in the weekly CDDP arm. Therefore, various chemotherapeutic combinations based on CDDP should be tried for concurrent chemoradiationtherapy (CCRT) in patients with CC. Moreover, comparing the alternative CDDP dose and dosing schedules in chemotherapy concurrent with RT is necessary.
In a meta-analysis by Petrelli et al, single CDDP and CDDP-based doublet chemotherapy (including 5-FU, hydroxyurea, cyclophosphamide, paclitaxel, and docetaxel) combined with RT were compared in the treatment of patients with CC. They found that platinum-based doublet chemotherapy plus concurrent RT increased the overall survival (OS) and progression-free survival (PFS) compared with RT plus single platin. Therefore, platinum-based combination therapy plus RT should be preferred over single platin plus RT for CC. In addition, regimen of CDDP plus 5-FU is recommended by leading guidelines among the various polychemotherapies.Citation8 However, 2 of the previous 8 clinical trials adopted weekly CDDP, while 5 trials used triweekly CDDP, and the other 2 trials employed 4-weekly agent as the chemotherapeutic combinations. The optimal chemotherapy regimen based on CDDP is yet to be established.
Hu et al conducted a meta-analysis to evaluate the efficacy of weekly and triweekly CDDP with RT for treatment of CC. They found that weekly CDDP was associated with a lower risk of hematological toxicity compared with the triweekly CDDP with CCRT. However, the 2 regimens were comparable with respect to PFS and OS (P>0.05),Citation9 which is similar to the results of another meta-analysis conducted by Chen et al.Citation10 Nevertheless, most regimens of triweekly CDDP were combined with other chemotherapeutic drugs (such as tirapazamine, 5-FU, hydroyurea, paclitaxel) in both the meta-analyses. Use of these drugs may have confounded the analysis and affected the validity of the results. Therefore, the real difference between the 2 regimens may still be unknown to us.
Several recent studies have compared the 2 different single CDDP schedules with respect to survival and incidence of adverse events. Ryu et al found triweekly CDDP CCRT had better 5-year OS and higher relatively completion rate of scheduled chemotherapy cycles, but less severe neutropenia compared with the conventional weekly CDDP regimen.Citation11 Conversely, outcomes of weekly CDDP regimen for CCRT were found to be better with respect to hematological toxicity in a study conducted by Kinjyo et al in 2017.Citation12 No definitive conclusions were drawn from these studies. Therefore, 6 randomized trials and 2 retrospective studies were included in our meta-analysis to explore the difference between the different CDDP-alone regimens for patients with LACC.
Materials and methods
Search strategy
The databases of PubMed, Cochrane Library, EMBASE databases, and Medline were searched by using the following key words, (Cisplatin or Platinum or cis-Platinum or Platinol or Platidiam or CDDP), (Uterine Cervical Neoplasms or Cervical Neoplasms or Cervix Neoplasms or Uterine Cervix Cancers or Cervix Cancers or Cervical Cancers), (Triweekly or Every 3 weeks or 3 weeks), (Per week or Every week or Weekly or Once a week), and (chemoradiotherapy or chemoradiation or radiochemotherapy or chemotherapy or RT, or radiation or electromagnetic radiation). Only those studies published from 1990 to December 29, 2017 in English were considered. References of the included studies and related citations was also checked manually for potentially relevant studies. Two independent investigators evaluated each study. A consensus should be reached by discussion or the third investigator to resolve the disagreements created between the 2 reviewers.
Inclusion and exclusion criteria
Studies were included in the analysis if: 1) they were randomized controlled trials or retrospective studies that compared triweekly single CDDP plus RT plus vs weekly single CDDP plus RT; 2) no evidence of para-aortic lymph node or distant metastasis on pretreatment imaging (stages I–IVA); and 3) the long-term OS and recurrence rate, including local and distance, were assessed as outcomes to measure the effect of the treatment. If studies were duplicates, the study with the most up-to-date results was included. Studies were excluded if patients had previous histories of chemotherapy or RT, or other factors seriously affecting the survival and treatment processes.
We used the revised Jadad scale to evaluate the quality of the randomized controlled trials included in the primary outcome analysis. An article of high quality scored 4–7 points. The Nottingham Ottawa Scale (NOS) was used to assess observational studies. On the basis of NOS criteria, studies are scored between 0 and 9 stars. Six stars or greater was considered to be sufficiently higher-quality studies.Citation13
Statistical analysis
OS and recurrence rate, including the locoregional relapse rate and rate of distant metastasis were the primary end points, and appliance, acute adverse were secondary end points. RevMan 5.1 software (Cochrane Collaboration’s Information Management System) was used to conduct this meta-analysis. Variables among studies with minimal heterogeneity were assessed by fixed-effect model/Mantel–Haenszel method, otherwise, random-effects model/DerSimonian–Laird method was used when calculating the odds ratios (ORs) and 95% CIs in the specific event. Funnel plots and Harbord tests were used to examine potential publication bias in the meta-analysis.
Results
Study selection and characteristics
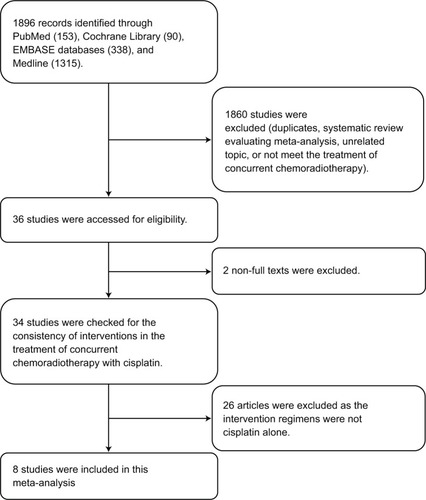
The search initially yielded a total of 1896 citations. A total of 8 trialsCitation14–Citation19 were included in this review after exclusion of studies that did not qualify the inclusion criteria, duplicate publications, review articles, and meta-analyses. Two trials were not included due to lack of availability of relevant data. The study selection criteria for this meta-analysis are illustrated in .
Among the 8 publications considered in this analysis, there were 6 prospective randomized trials and 2 retrospective case series. The 8 studies with a combined sample size of 934 patients were conducted in the USA, Japan, India, Korea, and Romania and were published between 2007 and 2017. All the patients recruited in these studies were newly diagnosed as LACC and received primary radical CCRT. Out of the 934 patients, 436 patients received weekly CDDP-based chemotherapy concurrent with RT, while 498 patients received triweekly regimen. For the retrospective studies, the NOS grades were 6–7 stars (out of a maximum possible score of 9 stars). For all the 6 randomized studies, the overall quality described by Jadad scores was 3 out of 5. shows the detailed analysis of the studies.
Table 1 Characteristics of the included trials
Primary end points: 5-year OS and recurrence rate
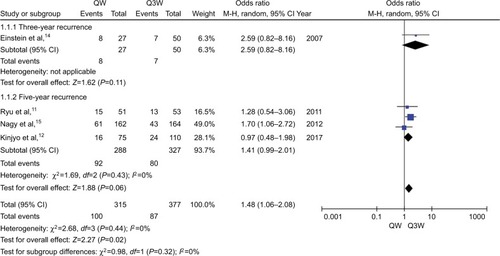
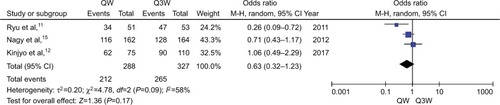
The meta-analysis of 5-year OS (n=3 studies) was affected by a relatively high heterogeneity among the trials (ICitation2=58%). Therefore, the random-effects model was chosen for pooled analysis. The Harbord test showed lack of significant heterogeneity among the trials (P>0.05). The analysis revealed no statistically significant difference between the triweekly and weekly regimens of CDDP-based chemotherapy plus RT with respect to 5-year OS (OR, 0.63; 95% CI: 0.32–1.23; P=0.17; ).
Figure 2 Meta-analysis evaluating 5-year OS of weekly single cisplatin or triweekly cisplatin alone combined with radiotherapy.
Abbreviations: OS, overall survival; QW, weekly; Q3W, triweekly.
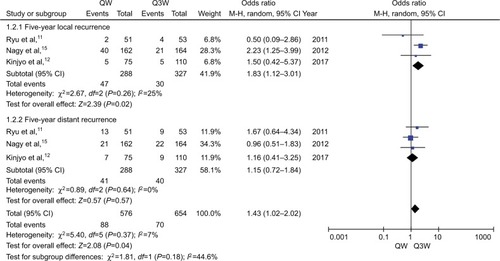
For the meta-analysis of recurrence rate (n=4 studies), no significant heterogeneity was observed among the trials. Therefore, the fixed-effects model was chosen for pooled analysis. The study by Einstein et al showed no significant difference between the 2 regimens of CCRT with respect to 3-year recurrence (P=0.11).Citation14 Analysis of data from the other 3 studies also showed no significant difference with respect to 5-year recurrence (OR, 1.41; 95% CI: 0.99–2.01; P=0.06; ). We performed subgroup analysis in terms of 5-year recurrence and found that triweekly CDDP plus RT was associated with a 43% reduced risk of 5-year local recurrence compared with that with weekly CDDP-based CT plus RT (OR, 1.83; 95% CI: 1.12–3.01; P=0.02; ). However, no significant difference was observed between the 2 regimens of CCRT with respect to 5-year distant recurrence (OR, 1.15; 95% CI: 0.72–1.84; P=0.57; ).
Secondary end points: compliance and acute adverse events
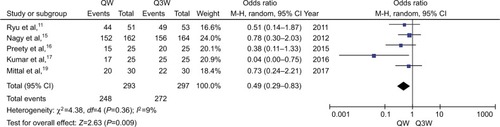
Not completing the total cycles of chemotherapy or missing any dose of CDDP or delaying radiation period longer than a certain period of time, varieties existed in different studies, was defined as patients with bad compliance. The meta-analysis of compliance (n=5 studies) determined that triweekly CDDP plus RT was associated with a 14% increased risk of compliance compared with weekly CDDP-based CT plus RT (OR, 0.49; 95% CI: 0.29–0.83; P=0.009; ). Early or late toxicities of RT and chemotherapy might be the main influences.
Figure 5 Meta-analysis evaluating the compliance of weekly single cisplatin or triweekly cisplatin alone combined with radiotherapy.
Abbreviations: QW, weekly; Q3W, triweekly.
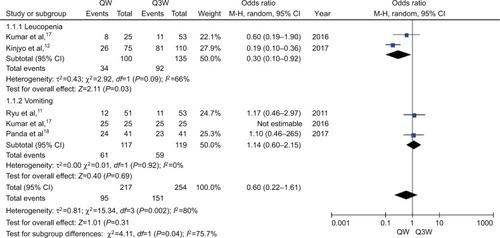
We only chose 2 and 3 trials for the meta-analysis of leu-copenia and vomiting, respectively, for assessment of acute adverse events because of differences in evaluation methods and underreporting of data in the included publications. Tri-weekly CDDP plus RT was associated with a 100% increased risk of leucopenia compared with weekly CDDP-based CT plus RT (OR, 0.30; 95% CI: 0.10–0.92; P=0.03; ). No significant difference was observed between the 2 regimens of CDDP-based CCRT with respect to incidence of vomiting (OR, 1.14; 95% CI: 0.60–2.15; P=0.69; ).
Risk of bias
The hardboard tests for all the indices did not show any evidence of publication bias (all P>0.05) (details can be seen in Supplementary materials of Harbord tests [–]).
Discussion
Weekly CDDP treatment regimen was recommended by National Comprehensive Cancer Network guidelines, based on the results of 5 randomized trials 2–6 conducted during the 1990s. However, in an RCT by Ryu et al triweekly single CDDP chemotherapy concurrent with RT was associated with better 5-year survival and lower incidence of hematological toxicity compared with the conventional weekly CDDP in patients with LACC. A meta-analysis in 2014 showed better outcomes in patients with LACC treated with CDDP-based plus another drug combined with RT. However, we found that 4 out of the 8 articles included in this meta-analysis used triweekly CDDP in combination with another drug in the experiment group. Only 2 of the 8 trials used weekly CDDP regimen as part of doublet chemotherapy. Therefore, whether the triweekly CDDP regimen in the CCRT itself contributed to the good outcomes is questionable. Two meta-analyses compared concurrent weekly CDDP vs triweekly CDDP in combination with RT for treatment of CC. Both these meta-analyses suggested the superiority of weekly CDDP regimen based only on the lower incidence of hematological toxicity.Citation9,Citation10 Nevertheless, other drugs were also used in most of the triweekly regimens in addition to CDDP, which may have influenced the outcomes. Therefore, we compared the efficacy and side effects between weekly and triweekly CDDP-alone regimen.
In this meta-analysis, we found that triweekly CDDP (20 mg/m2 for 5 days or 75 mg/m2) alone combined with RT was associated with a lower rate of local recurrence and better compliance compared with weekly CDDP (40 mg/m2) plus RT in patients with LACC. The incidence of hematological toxicity was higher in the triweekly CDDP arm, which is similar to the findings of a previous meta-analysis. Besides, the 5-year OS was relatively better in the triweekly CDDP arm (P=0.06). In our meta-analysis, we found a higher treatment completion rate among patients treated with triweekly CDDP regimen. Our findings with respect to treatment compliance are consistent with that of Einstein et al;Citation14 however, triweekly CDDP regimen was largely used for hospitalized patients with poor general physical condition, while outpatients with good physical condition always received weekly CDDP regimen. Moreover, as inpatients tend to receive prophylactic medications, we excluded the data from this study from the meta-analysis of compliance and adverse events. We still observed a better treatment completion rate and the higher incidence of hematological toxicity in the triweekly CDDP arm. Two factors may explain the lower local recurrence and relatively better 5-year survival. First, the higher the peak concentration of CDDP, the better are the outcomes to a certain degree. Although the dose–response slope of CDDP is not steep, the response of tumor to CDDP has been shown to increase with increase in the peak concentration of CDDP up to 100 mg/m2.Citation20,Citation21 The improved or sustained high peak blood levels of CDDP may be more effective not only in enhancing the synergy of chemoradiation but also in eliminating micrometastases, with the resultant decrease in local failure, and eventual survival benefit. Another factor may be the synergistic effect of high peak concentrations of CDDP with brachytherapy during the last few cycles of chemotherapy. Whether CDDP acts as a radiosensitizer during brachytherapy needs further study.
One criticism of our analysis could emanate from our inclusion of 1 trial that included some patients who received surgery after CCRT. In the study by Nagy, 5-year local relapse-free survival in the triweekly arm (87%) was significantly superior than that in the weekly CDDP arm (77%) (P<0.01). No statistically significant differences were observed with respect to OS (QW vs Q3W; 72% vs 78%; P=0.14) and disease-free survival (DFS) (QW vs Q3W; 69% vs 73%; P=0.09). However, patients who underwent surgery had better OS, DFS, and local relapse-free survival. Surgery may improve outcomes of CCRT for LACC; however, it is worth noting that only patients who showed good response received surgery. Few studies have analyzed the role of surgery in advanced stages at present. Another point of contention is that we did not make a further subgroup to analyze the difference between the completion rates for chemotherapy and RT. Pelvic radiation therapy in patients with CC may cause acute radiation enteritis, radio-cystitis, and radiodermatitis. Severe diarrhea and urinary tract symptoms may also delay the treatment process.
Our study does have some limitations. First, both prospective and retrospective studies were included in the meta-analysis. More favorable characteristics and end points were chosen for analysis. In addition, the diversity of methods used to assess treatment outcomes, such as acute adverse events led to less data inclusion. Therefore, we only selected leucopenia and vomiting as the parameters for assessment of side effects of CCRT. Additionally, some of the included studies were conducted in developing countries with limited medical facilities and trained personnel. Differences with respect to dose and duration of RT may also have influenced our results. However, almost 90% of deaths from CC occur in the developing world; India alone accounts for about 25% of the total cases.Citation1 Finally, only published literature was included in this meta-analysis, and lack of individual patient data prevented us from adjusting for the confounding influence of disease- and patient-related variables on the treatment effect.
To our knowledge, this is the first systematic review that compares the efficiency and adverse events associated with single agent triweekly CDDP plus RT and weekly CDDP alone plus concurrent RT in patients with LACC. We found that the lower rate of local relapse and the tendency for better OS probably occurs at the cost of more side effects. We recommend triweekly single CDDP regimen over weekly CDDP-alone regimen for CCRT in patients with LACC.
Acknowledgments
This study was sponsored partly by Research and Development Key Program of Jiangsu Province (Grant BE2015645) and Grant Z201413 from Scientific Research Project of Health Department of Jiangsu Province and Suzhou Key Medical Center Program (Grant szzx201506).
Supplementary materials
Compliance
Metabias qwevent qwtotal q3wevent q3wtotal, Harbord graph.
Note: data input format tcases tnoncases ccases cnoncases assumed. Odds ratios assumed as effect estimate of interest ().
Five-year OS
Metabias qwevent qwtotal q3wevent q3wtotal, Harbord graph.
Note: data input format tcases tnoncases ccases cnoncases assumed. Odds ratios assumed as effect estimate of interest ().
Five-year recurrence
Metabias qwevent qwtotal q3wevent q3wtotal, Harbord graph.
Note: data input format tcases tnoncases ccases cnoncases assumed. Odds ratios assumed as effect estimate of interest ().
Subgroup-local
Metabias qwevent qwtotal q3wevent q3wtotal, Harbord graph.
Note: data input format tcases tnoncases ccases cnoncases assumed. Odds ratios assumed as effect estimate of interest ().
Subgroup-distance
Metabias qwevent qwtotal q3wevent q3wtotal, Harbord graph.
Note: data input format tcases tnoncases ccases cnoncases assumed. Odds ratios assumed as effect estimate of interest ().
Vomiting
Metabias qwevent qwtotal q3wevent q3wtotal, Harbord graph.
Note: data input format tcases tnoncases ccases cnoncases assumed. Odds ratios assumed as effect estimate of interest ().
Table S1 Harbord’s modified test for small-study effects
Table S2 Harbord’s modified test for small-study effects
Table S3 Harbord’s modified test for small-study effects
Table S4 Harbord’s modified test for small-study effects
Table S5 Harbord’s modified test for small-study effects
Table S6 Harbord’s modified test for small-study effects
Disclosure
The authors report no conflicts of interest in this work.
References
- FitzmauriceCDickerDPainAThe global burden of cancer 2013JAMA Oncol20151450526181261
- LinJCisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinomaN Engl J Med1999340151154116110202166
- MorrisMEifelPJLuJPelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancerN Engl J Med1999340151137114310202164
- PetersWStockRMonkBConcurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervixJ Clin Oncol20001881606161310764420
- MalfetanoJConcurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancerN Engl J Med199934015114410202165
- WhitneyCWSauseWBundyBNRandomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group studyJ Clin Oncol19991751339134810334517
- LancianoRCalkinsABundyBNRandomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a Gynecologic Oncology Group studyJ Clin Oncol200523338289829516230678
- PetrelliFDeSARaspagliesiFLorussoDBarniSRadiotherapy with concurrent cisplatin-based doublet or weekly cisplatin for cervical cancer: a systematic review and meta-analysisGynecol Oncol2014134116617124793000
- HuYCaiZQSuXYConcurrent weekly cisplatin versus triweekly cisplatin with radiotherapy in the treatment of cervical cancer: a meta-analysis resultAsian Pac J Cancer Prev20121394301430423167332
- ChenXZouHLiHWeekly versus triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of cervical cancer: a meta-analysisInt J Gynecol Cancer2016272344349
- RyuSYLeeWMKimKRandomized clinical trial of weekly vs. triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of locally advanced cervical cancerInt J Radiat Oncol Biol Phys2011814577581
- KinjyoYNagaiYToitaTConcurrent weekly cisplatin versus triweekly cisplatin with radiotherapy for locally advanced squamous cell carcinoma of the cervix: a retrospective analysis from a single institutionBr J Radiol20179010762017024128707541
- WellsGASheaBJO’ConnellDThe Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysisAppl Eng Agric2014186727734
- EinsteinMHNovetskyAPGargMSurvival and toxicity differences between 5-day and weekly cisplatin in patients with locally advanced cervical cancerCancer201010914853
- NagyVMOrdeanuCCozaOAlinCRTrailaATodorNRandomized phase 3 trial comparing 2 cisplatin dose schedules in 326 patients with locally advanced squamous cell cervical carcinoma: long-term follow-upInt J Gynecol Cancer20122291538154423070071
- PreetyJFareedKAmitAComparative study of weekly versus three weekly cisplatin in advanced cases of carcinoma cervix alone with radiotherapyJournal of Evolution of Medical and Dental Sciences20154881531315320
- KumarPSharmaKSinghDPWeekly verses tri-weekly concurrent cisplatin with radiotherapy in the treatment of cervical cancer: a study comparing efficacy and toxicitySRMS Journal of Medical Sciences2017126672
- PandaNBagSSamantaraySRandomised clinical trial of weekly vs. triweekly cisplatin based chemotherapy concurrent with radiotherapy in the treatment of locally advanced cervical cancerInt J Med Clin Res20175042006020064
- MittalSChauhanAKaurPComparing weekly versus three weekly schedules of cisplatinum concomitant with radical radiotherapy in locally advanced carcinoma uterine cervixGynecology Obstetrics201771
- BonomiPBlessingJAStehmanFBRandomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group StudyJ Clin Oncol19853810793894589
- ThigpenTShingletonHHomesleyHcis-Dichlorodiammineplatinum (II) in the treatment of gynecologic malignancies: phase II trials by the Gynecologic Oncology GroupCancer Treat Rep1979639–1015491555498154