Abstract
Objectives
The Cervista® high-risk human papillomavirus (HR-HPV) test was evaluated as a primary screening method for cervical cancer in women aged ≥21 years and was compared with different screening and triage combinations.
Materials and methods
A nested case–control study within the Fujian provincial Cervical Lesion Screening Cohorts was used to evaluate the Cervista test as the primary cervical screening method in a hospital-based population. Strategy 1 primarily screened using a cytology screen with HR-HPV testing used for triage. Strategy 2 primarily screened using cytology and HR-HPV co-testing. Strategy 3 primarily screened using HR-HPV testing and triaged HPV-positive women based on cytology. Strategy 4 primarily screened using HR-HPV testing and referred A9 pool HPV-positive women to colposcopy directly, whereas non-A9 HPV-positive women were triaged using cytology.
Results
There were 10,183 women included in this study; 16.49% (1677/10,183) were HR-HPV-positive, 9.52% had abnormal cytology, and 9907 women were normal during followup. A total of 276 women were diagnosed with cervical intraepithelial neoplasia 2 or worse (CIN2+), 197 with CIN3 or worse (CIN3+), and 70 with cervical cancer. Moreover, 10.15% (20/197) women who were CIN3+ were identified as cytology-negative, while 8.63% (17/197) were HR-HPV negative (P>0.05). The cumulative risk rate for HPV–/cytology– was 0.836 (95% CI, 0.424–1.648) in CIN3+ cases. Strategy 4 yielded the highest sensitivity for CIN2+ or CIN3+ and the lowest positive predictive value for CIN2+ or CIN3+ among the four screening strategies.
Conclusion
The Cervista HR-HPV test can provide a reliable and sensitive clinical reference for the cervical cancer primary screen.
Introduction
Cervical cancer is thus far the most common human papillomavirus (HPV)-related cancer;Citation1 of the 600,000 invasive cancer cases caused by HPV in 2012, invasive cervical cancer accounted for >500,000 of the cases, which resulted in approximately 266,000 deaths.Citation2 Persistent infection with high-risk human papillomavirus (HR-HPV) is strongly associated with pre-invasive lower genital tract disease and invasive cancer.Citation3 One of the major preventive strategies involves the detection of treatable precancers and early cancers using HPV assays. Cervical cytology screening programs, which are still the most widely used tests, have substantially reduced the incidence of and mortality due to cervical cancer, particularly in countries with a wide-screening coverage.Citation4,Citation5 However, cytology screening has a lower sensitivity in terms of detecting pre-invasive and invasive cervical lesions than does HPV testing, and the sensitivity of cytology varies according to the laboratory and the expertise of the technologists, as well as the medical infrastructure of a particular region.Citation6,Citation7 Moreover, cytology has lower reassurance against prevalent and especially incident pre-cancers and requires shorter intervals between screens to achieve good sensitivity rates.Citation7,Citation8 Primary HPV screening has been compared with cytology in several large, randomized clinical trials: HPV testing has been allowed for the earlier detection of precancers and reduced the incidence of cancer during the follow-up.Citation9 Accordingly, intervals between screens can be extended. In addition, the reassurance provided by co-testing over HPV testing alone is limited.Citation10 Primary HPV screening algorithms have been approved in the US, Netherlands, and Australia and are planned in Italy (2018).Citation11,Citation12
China accounts for approximately one-fifth of the world’s population, and its cervical cancer burden has a substantial effect on the global estimates of the current and future burden of the disease.Citation13 An estimate suggested that the number of new cervical cancer cases in China was 100,000 every year, accounting for 29% of the world’s total.Citation14 However, only 21% of women reported ever undergoing a Papanicolaou (Pap) test in China. The increasing trend in the incidence and mortality due to cervical cancer may be related to inadequate screening, increasing prevalence of HPV infection, and the lack of an HPV vaccine.Citation15 Since the HPV vaccine was only recently introduced in China, it will take some time until it has been implemented across China;Citation16 therefore, screening alone serves as the major prevention strategy during this period. In 2009, China’s government launched the National Cervical Cancer Screening Program in rural areas. It was the first time that the Chinese government had proposed to gradually widen access to cervical cancer screening services in rural areas, and it represented a step toward the nationwide provision of cervical cancer screening.Citation14 Although the coverage of cervical cancer screening in China is low,Citation17,Citation18 a more sensitive primary HPV testing method was required in clinical practice.
The US Food and Drug Administration (FDA) has approved four kinds of HPV tests: Hybrid Capture® 2 (HC2) HPV DNA (Qiagen, Hilden, Germany), Cervista® (Hologic, Massachusetts, USA), APTIMA® (Hologic, Massachusetts, USA), and the cobas® HPV test (Roche Diagnostics, Indianapolis, USA). Thus far, the FDA has recommended the cobas HPV test alone for HPV testing for primary screening. This led to speculation that primary cervical cancer screening via HPV testing is a promising screening strategy that needs further validation, especially in Chinese women. A negative HC2,Citation19 GP5+/6+ polymerase chain reaction-enzyme immunoassay (PCR-EIA),Citation19,Citation20 or Cobas HPV testCitation21,Citation22 indicates a low risk of developing cervical precancer or cancer in follow-up data of HPV-based primary screen. The Cervista HPV HR test was the second HR-HPV assay approved by the FDA in 2009 and is a qualitative test that uses three separate oligonucleotide mixtures (A5/A6 pool [HPV 51, 56, and 66], A7 pool [18, 39, 45, 59, and 68], and A9 pool [HPV 16, 31, 33, 35, 52, and 58]) depending on the correlation among 14 types of HR-HPV DNA gene sequences used to detect HR-HPV types.Citation23 The Cervista test detects 14 HR-HPV types: HPV66 and the same 13 HR-HPV types as detected by HC2 testing, and several comparative studies have shown its similar sensitivity and specificity to those of the HC2 test.Citation23 However, the utility of the Cervista HPV test as a primary HPV screening method and the clinical performance of different cervical cancer screening strategies, particularly in the Chinese population, remains unclear. This study was designed to provide evidence of the efficiency of a cervical cancer screening strategy using the Cervista HPV test for the detection of cervical intraepithelial neoplasia grade 2 or more severe (CIN2+) and CIN3 or more severe (CIN3+) in a Chinese hospital-based population.
Materials and methods
Study population
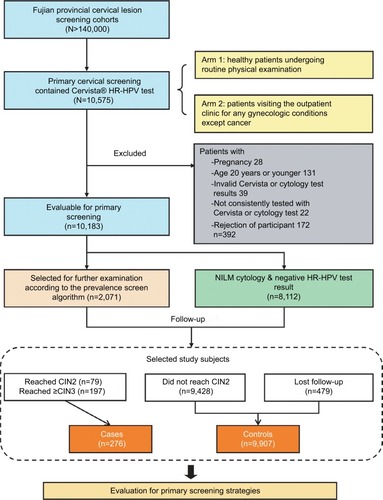
We conducted a nested case–control study within the Fujian provincial Cervical Lesion Screening Cohorts (FCLSCs), a cervical screening cohort study involving a hospital-based and community-based population in Fujian Provincial Maternity and Children’s Health Hospital. This study is an opportunistic screening of a hospital-based population. Women, who underwent primary cervical screening, including the Cervista HR-HPV test, were initially included from 2012 to 2016. The population eligible for this cohort study involved two arms, one consisting of healthy patients undergoing routine physical examinations, and another consisting of patients visiting the outpatient clinic for any gynecologic conditions except cancer. All cases assigned to this nested case–control study which fulfilled the following criteria were included as cases or controls: 1) sexually active nonpregnant women aged ≥21 years; 2) valid Cervista HPV testing and cytology results; 3) consistently tested with Cervista or cytology in the follow-up phase; and 4) willingness to participate in this study. Cases included women with a confirmed diagnosis of CIN2 or worse. Controls included women with none of the above diagnoses during the follow-up. All individuals in this study provided written informed consent. The study protocol was approved by the Hospital Ethics Committee of Fujian Provincial Maternity and Children’s Hospital, Affiliated Hospital of Fujian Medical University. The study flowchart is shown in .
Cervical specimen collection
Cervical cells were collected using plastic brushes from the cervical canal of all participants and placed into 20-mL vials of PreservCyt® solution (Hologic, USA) for cytology or HPV DNA testing. The samples for cytology and HPV assays were stored at 4°C and then sent to laboratory for testing.
Liquid-based cytology
ThinPrep slides were blinded and evaluated, independent from the results of the other assays, by two experienced cytopathologists. If the diagnoses were different, the cervical samples were reviewed again, and a consensus diagnosis was obtained. The results were evaluated using the 2001 Bethesda system. Samples were classified as negative for intraepithelial lesion and malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells, not possible to exclude high-grade squamous intraepithelial lesion (ASC-H), high-grade squamous intraepithelial lesion (HSIL), squamous cervical cancer (SCC), atypical glandular cells (AGC), and adenocarcinoma in situ (AIS).Citation24
Cervista HR-HPV assay
The Cervista HR-HPV assay used magnetic beads for DNA purification and Invader® chemistry for signal amplification, to qualitatively detect the specific nucleic acid sequences of
14 HR-HPV types simultaneously in three different pools (A5/A6 pool: 51, 56, and 66; A7 pool: 18, 39, 45, 59, and 68; A9 pool: 16, 31, 33, 35, 52, and 58).Citation25 All detection procedures and interpretation of the HPV results were conducted in accordance with the manufacturer’s instructions.Citation26 The human histone 2 gene (HIST2H2BE) served as an internal control for cellular DNA present in the sample. A positive result indicated that at least one of high-risk types is present in the groups.
Colposcopy and histology
Women who were HPV-positive and/or had an abnormal cytological result (with a grade higher than ASC-US) were referred for colposcopy and punch biopsy within 12 weeks of the initial visit. Women with a punch biopsy diagnosis greater than HSIL underwent a loop electrosurgical excision procedure cone biopsy or conization using a cold knife. Specimens were fixed in 10% formalin and routinely processed for paraffin embedding. Subsequently, 4-μm-thick histological sections were cut and stained with hematoxylin and eosin using the standard method. Cervical biopsy specimens were then histologically examined and classified according to the CIN system.Citation27 The primary round was included from the first visit to complete colposcopy with biopsy or endocervical curettage (ECC) if necessary. All women who underwent colposcopy in the primary round and who did not have CIN2+ were eligible for the follow-up round. For women with negative results for cytology and HPV tests at primary round, as the disease status, it was assumed that no new disease would be observed until further histology was received.
Screening procedures
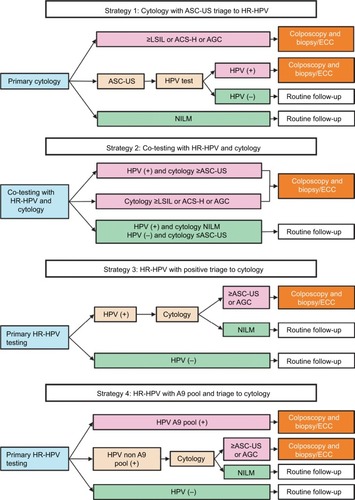
This study evaluated the performance of four screening strategies using the dataset created by the study. A flowchart of the strategies conducted is shown in . The three strategies currently used for cervical cancer screening (primary cytology test, co-testing, and primary HR-HPV test) and another strategy were designed to evaluate the performance of the Cervista HR-HPV test. Strategy 1 comprised cytology with HPV testing performed only for ASC-US. Strategy 2 comprised co-testing women with both cytology and HR-HPV testing, after which HPV-positive women with negative cytology were retested with both tests in a routine follow-up per year and underwent ECC if either test yielded abnormal results. Strategy 3 comprised primary screening of women with HPV testing alone, triage of HPV-positive women with cytology, and referring those with cytology ≥ASC-US/AGC for colposcopy/ECC. Strategy 4 comprised screening women using HPV testing, referring those infected with A9 pool for colposcopy/ECC, triage for those without infection with A9 pool with cytology, and referring those with cytology ≥ASC-US/AGC for colposcopy/ECC.
Figure 2 Flowchart of the four screening strategies for cervical cancer.
Abbreviations: ASC-US, atypical squamous cells of undetermined significance; HR-HPV, high-risk human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, not possible to exclude high-grade squamous intraepithelial lesion; AGC, atypical glandular cells; ECC, endocervical curettage; NILM, negative for intraepithelial lesion and malignancy; HPV, human papillomavirus.
Statistical analysis
The performance characteristics of the screening strategies were evaluated by calculating the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood rate (PLR), negative likelihood rate (NLR), and Youden index according to the standard definitions for CIN2+ or CIN3+. The 95% CIs were calculated using exact binomial CIs. The number of colposcopies needed to detect one case was equivalent to the number colposcopies per case, identified by end point. The χ2 test, Fisher’s exact test, and a cumulative risk analysis were performed. Results for women with missing disease status in primary or follow-up round were imputed that the characteristics of women with valid biopsy results are not significantly different from those with missing disease status. All data analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided, and a P-value <0.05 was considered to indicate statistical significance.
Results
Clinical characteristics of the studied population
A total of 10,575 cases of primary cervical screening used the Cervista HR-HPV test in FCLSCs; of these, 10,183 cases fulfilled the criteria. The median follow-up time was 2.5 years (1–5 years). During the course of follow-up, 2071 women were selected for further examination according to the prevalence screen algorithm; 1592 had available cervical histology results. A flow chart describing the selection of the study cohort with inclusion/exclusion criteria can be found in . The total HR-HPV positivity rate using the Cervista HR test was 16.49%. The frequencies of A5/A6, A7, and A9 pools were 4.44%, 4.45%, and 10.30%, respectively. Moreover, 9.52% of women had abnormal cytology: ASC-US, 4.21%; AGC, 1.10%; LSIL, 1.91%; ASC-H, 0.19%, HSIL, 0.92%, and cervical cancer, 0.56%. Among women who had available histology results, a total of 276 were diagnosed with CIN2+, 197 with CIN3+, and 70 with cervical carcinoma ().
Table 1 Results of the HR-HPV testing and cytology for cervical disease
Cumulative risk for CIN2+, CIN3+, or cancer
Herein, 20 of 197 (10.15%) cases of CIN3+ identified during the study occurred in women with negative baseline cytology. In contrast, 17 (8.63%) occurred in women who tested negative for HR-HPV during the study (P>0.05). The cumulative risk rate was 0.836 (HPV–/cytology–, 95% CI: 0.424–1.648) in CIN3+ cases. Similar results were observed using a CIN2+ end point, and the cumulative risk rate was 0.701 (HPV–/cytology–, 95% CI: 0.403–1.221). Moreover, 68 of 70 (97.14%) women with cervical cancers were HPV-positive, and 66 of 70 (94.28%) had ≥ASC-US cytology. The cumulative risk rate was 0.485 (95% CI: 0.086–2.740) for HPV negative and cytology negative results.
Identification of cervical disease among the four screening strategies
Of the four screening strategies that were evaluated, strategy 4 had the highest sensitivity (89.85%; 95% CI: 85.63–94.07) for the detection of CIN3+ (). In comparison, the sensitivities of strategy 1 and strategy 2 were 88.32% (95% CI: 83.84–92.81) and that of strategy 3 (HR-HPV primary) was 81.73% (95% CI: 76.33–87.12). Strategy 3 had the highest specificity for CIN3+ (95.84%; 95% CI: 95.45–96.24), and strategy 4 had the lowest specificity (89.55%; 95% CI: 88.95–90.15). Strategy 1 and strategy 2 had an intermediate specificity that was between those of the other two strategies. The Youden’s index for strategy 1 and 2 for detecting CIN3+ was higher than that of the other strategies (82.99% vs. 77.57% or 79.39%). Similar results were observed using CIN2+ as the end point. The PPV and NPV, as well as PLR and NLR are shown in . In our study, strategy 1 had a similar sensitivity, specificity, PPV, NPV, PLR, and NLR as that of strategy 2 to detect CIN2+ or CIN3+, but strategy 1 required fewer tests than strategy 2 to detect CIN2+ or CIN3+.
Table 2 Detection of cervical disease using different screening strategies for HR-HPV
Strategy 4 detected a higher number of CIN3+ cases in the primary round than any of the other three strategies (). For example, strategy 4 detected 9.94% more cases of CIN3+ in the primary round than strategy 3. However, strategy 4 resulted in an increase in the number of colposcopies in the primary round and an increase in the number of colposcopies per case of CIN3+ in the primary round (5.42). Comparable results were seen using a CIN2+ end point.
Table 3 Detection of cervical disease using different screening strategies and the number of coloposcopies that each strategy requires
Discussion
Cervical cancer is the second most common gynecological malignancy in women in developing countries, where approximately 85% of cervical cancer cases culminate in death; it is preventable because of its long precancerous lesion status.Citation6 However, limited sensitivity and a correspondingly limited NPV, limits the use of cytology testing, although cytology testing has greatly reduced the incidence and the mortality of cervical cancer in developed countries when implemented as a primary screening test. Persistent HR-HPV infection plays an important role in the development of cervical cancer. Therefore, research on the value of the HPV-based screening has been performed in many institutions.Citation7 The HC2,Citation19 GP5+/6+ PCR-EIA,Citation19,Citation20 and Cobas HPV testsCitation21,Citation22 are considered fully clinically and epidemiologically viable HPV assays, validated following the Meijer validation protocol.Citation28 The Cervista HPV HR test was the second HR-HPV assay approved by the FDA in 2009 and introduced into China by the China Food and Drug Administration (CFDA) in 2011, with a similar sensitivity and specificity to those of the HC2 test.Citation23 However, the clinical performance of Cervista as a primary HPV screening method, especially in a Chinese hospital-based population, has remained uncertain.
FCLSCs are cervical screening cohorts involving a hospital-based and community-based population since 2008 in Fujian Provincial Maternity and Children’s Health Hospital, a local cervical screen center. The clinical performance characteristics of PCR-reverse dot blot (PCR-RDB) assays among 10,442 women in FCLSCs were evaluated previously; the results suggested that PCR-RDB can provide a reliable and sensitive clinical reference for cervical cancer screening.Citation29 In the current study, patient data were derived from that for FCLSCs using the Cervista test since 2012; moreover, the population eligible for this cohort study involved two arms, one consisting of healthy patients undergoing routine physical examinations, and another consisting of patients visiting the outpatient clinic for any gynecologic conditions except cancer. The total infection rate of HR-HPV tested using the Cervista HR-HPV test was estimated at 16.49%, which is in accordance to our previous reportCitation29 and other epidemiological studies regarding HPV in China.Citation30 We also found that 10.15% women with CIN3+ were cytology-negative. In contrast, 8.63% women with CIN3+ were HR-HPV negative (P>0.05). The cumulative risk rate was 0.836 (HPV–/cytology–, 95% CI: 0.424–1.648) in CIN3+ cases, implying that this hospital-based population was representative for evaluating the strategy regarding cervical cancer screening.
Currently, there are three main strategies for HPV testing: as a triage for ASC-US cytology, testing all women with both HPV and cervical cytology (co-testing) results, and primary HPV testing with cytology or colposcopy triage.Citation31–Citation33 In our study, these three main strategies were used to evaluate the Cervista test. We found that the sensitivity of strategy 1 (cytology) and strategy 2 (co-testing) was 88.32% (95% CI: 83.84–92.81) and that of the strategy 3 (HR-HPV primary) was 81.73% (95% CI: 76.33–87.12). The results were similar to those of a previous study.Citation23 Therefore, these data suggest that Cervista HR-HPV testing was robust for HPV screening in Chinese women.Citation22
The most obvious triage approaches are cytology and HPV16/18 genotyping, both of which have been discussed widely in the literature.Citation7 In our previous study, HPV-16 was the most prevalent genotype in the HR-HPV-positive women, followed by, from highest to lowest, HPV-52, -58, -18, -53, -33, -51, -56, -59, -68, -31, -66, -39, -35, and -45.Citation29 The data implied that the A9 pool (HPV-16, -31, -33, -35, -52, and -58) involved approximately 77% HPV positive genotypes and were the major HPV group in CIN2+ cases, compared to the A7 pool (HPV-18, -39, -45, and -59) at 14% and the A5/A6 pool (HPV-51, -56, and -66) at 6%. Based on the A9 pool, which is a major species in Fujian, we also evaluated HR-HPV primary screening for A9 pool as a triage approach to explore the Cervista test role in a primary screen. Strategy 4 (HR-HPV primary screening for A9 pool) had the highest sensitivity at 89.85% for detecting CIN3+. These results are similar to those of the ATHENA study, wherein the HPV primary screening had the highest sensitivity and identified more CIN3+ compared to that of the cytology or hybrid strategy. Furthermore, strategy 4 required the most colposcopies to detect one case of high-grade CIN. Moreover, strategies 1, 2, and 3 had fewer losses to follow-up at the primary round, in which the lifetime risk of cervical cancer was reduced by 25%–35%.Citation34 In general, the Cervista HR-HPV test can provide a reliable and sensitive clinical reference for a cervical cancer primary screen.
An estimate suggests that the number of new cervical cancer cases in China accounted for 29% of the new cases of cervical cancer in the world.Citation14 In China, a hospital-based cervical screen is currently the main strategy for cervical cancer prevention. Nevertheless, the coverage of cervical cancer screening in China is still much lower than that in developed countries.Citation17,Citation18 Therefore, a more sensitive primary HPV testing method is required in clinical practice. Since 2011 when Cervista as approved by the CFDA, many hospitals initiated use of the technology in clinical practice and explored how applications of the Cervista test would be meaningful and effective in Chinese women.
Based on the above analysis, strategy 4, which used the Cervista HPV HR test as an initial screening method and referred those infected with A9 pool for colposcopy, triage for those without infection with A9 pool, and referring those with cytology ≥ASC-US for colposcopy could be a suitable option for cervical cancer screening in Chinese women.
Acknowledgments
The authors would like to acknowledge the contribution of Tingting Lin and Lili Chen for kindly helping to collect data and Meimei Huang and Weiyi Huang for advice in preparation of figures. This work was supported by the Fujian Provincial Key Projects in Science and Technology (grant no. 2015YZ0002-1) and by Fujian Provincial Healthy Innovation Project (grant no. 2009-CXB-33).
Disclosure
The authors report no conflicts of interest in this work.
References
- PlummerMde MartelCVignatJFerlayJBrayFFranceschiSGlobal burden of cancers attributable to infections in 2012: a synthetic analysisLancet Glob Health201649e609e61627470177
- GlobocanCervical Cancer estimated incidence, mortality and prevalence worldwide in 2012 Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxAccessed July 20, 2017
- de SanjoséSDiazMCastellsaguéXCliffordGBruniLMuñozNBoschFXWorldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysisLancet Infect Dis20077745345917597569
- VaccarellaSFranceschiSEngholmGLonnbergSKhanSBrayF50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidenceBr J Cancer2014111596596924992581
- VaccarellaSLortet-TieulentJPlummerMFranceschiSBrayFWorldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factorsEur J Cancer201349153262327323751569
- GoodmanAHPV testing as a screen for cervical cancerBMJ2015350h237226126623
- ArbynMRoncoGAnttilaAEvidence regarding human papillomavirus testing in secondary prevention of cervical cancerVaccine201230Suppl 5F88F9923199969
- SchiffmanMDoorbarJWentzensenNCarcinogenic human papillomavirus infectionNat Rev Dis Primers201621608627905473
- RoncoGDillnerJElfströmKEfficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trialsLancet2014383991652453224192252
- GageJCSchiffmanMKatkiHAReassurance against future risk of precancer and cancer conferred by a negative human papillomavirus testJ Natl Cancer Inst20141068dju15325038467
- SchiffmanMDoorbarJWentzensenNCarcinogenic human papillomavirus infectionNat Rev Dis Primers201621608627905473
- HuhWAultKChelmowDUse of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidanceGynecol Oncol2015136217818225579107
- DiJRutherfordSChuCReview of the cervical cancer burden and population-based cervical cancer screening in ChinaAsian Pac J Cancer Prev201516177401740726625735
- Women’s health in rural ChinaLancet20093749687358
- WangRGuoXLWismanGBNationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in ChinaBMC Infect Dis20151525726142044
- JiangXTangHChenTEpidemiology of gynecologic cancers in ChinaJ Gynecol Oncol2018291e729185265
- WangBHeMChaoACervical cancer screening among adult women in China, 2010Oncologist201520662763425956407
- ZhaoFHuSYZhangSWChenWQQiaoYLCervical cancer mortality in 2004–2005 and changes during last 30 years in ChinaZhonghua Yu Fang Yi Xue Za Zhi201044540841220654229
- DillnerJReboljMBirembautPLong term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort studyBMJ2008337a175418852164
- ElfströmKMSmelovVJohanssonALLong term duration of protective effect for HPV negative women: follow-up of primary HPV screening randomised controlled trialBMJ2014348g13024435414
- ArbynMSnijdersPJMeijerCJWhich high-risk HPV assays fulfil criteria for use in primary cervical cancer screening?Clin Microbiol Infect201521981782625936581
- WrightTCStolerMHBehrensCMSharmaAZhangGWrightTLPrimary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening testGynecol Oncol2015136218919725579108
- BoersAWangRSlagter-MenkemaLClinical validation of the Cervista HPV HR test according to the international guidelines for human papillomavirus test requirements for cervical cancer screeningJ Clin Microbiol201452124391439325297324
- SolomonDDaveyDKurmanRThe 2001 Bethesda System: terminology for reporting results of cervical cytologyJAMA2002287162114211911966386
- DaySPHudsonAMastAAnalytical performance of the Investigational Use Only Cervista HPV HR test as determined by a multi-center studyJ Clin Virol200945Suppl 1S63S7219651371
- CervistaTM HPV HR. Available from: https://www.hologic.com/sites/default/files/package-insert/15-3100_105_01.pdfAccessed July 20, 2017
- WaxmanAGChelmowDDarraghTMLawsonHMoscickiABRevised terminology for cervical histopathology and its implications for management of high-grade squamous intraepithelial lesions of the cervixObstet Gynecol201212061465147123168774
- MeijerCBerkhofJCastlePEGuidelines for human papilloma-virus DNA test requirements for primary cervical cancer screening in women of 30 years and olderInt J Cancer2009124351652018973271
- SunPSongYRuanGClinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population studyJ Gynecol Oncol2017285e5028657218
- PanQJHuSYGuoHQLiquid-based cytology and human papillomavirus testing: a pooled analysis using the data from 13 population-based cervical cancer screening studies from ChinaGynecol Oncol2014133217217924631450
- ASCUS-LSIL Traige Study (ALTS) GroupResults of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significanceAm J Obstet Gynecol200318861383139212824967
- SaslowDSolomonDLawsonHWAmerican Cancer SocietyAmerican Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancerJ Low Genit Tract Dis201216317520422418039
- HuhWKAultKAChelmowDUse of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidanceGynecol Oncol2015136217818225579107
- GoldieSJGaffikinLGoldhaber-FiebertJDCost-effectiveness of cervical-cancer screening in five developing countriesN Engl J Med2005353202158216816291985