Abstract
Prostate cancer (PCa) is the second most common cancer in men worldwide. When the disease becomes metastatic, limited treatment strategies exist, and metastatic disease prognoses are difficult to predict. Recently, evidence has emerged, which indicates that small RNAs are detectable in patient fluids, and exosomal small RNA ectopic expression is correlated with the development, progression, and metastasis of human PCa; however, the role of small RNAs in PCa is only partially understood. In this review, we discuss the research status regarding circulating exosomal small RNAs and applications using these small RNAs in PCa particularly looking at metastatic disease. Exosomal small RNAs could be used as potential biomarkers for the early diagnosis, micrometastasis detection, and prognosis of PCa.
Keywords:
Introduction
Prostate cancer (PCa) is the second most commonly diagnosed cancer and the fifth leading cause of cancer death in men worldwide.Citation1 PCa is a complex disease, with multiple risk factors involving age, ethnicity, heredity, diets, and hormone levels.
PCa occurs as a localized disease in the early stages, at which time the treatment options include prostatectomy or radiotherapy. If the disease progresses as a recurrence or metastasis, androgen deprivation therapy (ADT) becomes the standard treatment. Unfortunately, patients with recurrent or metastatic PCa will inevitably develop castration-resistant PCa (CRPC), an end-stage metastatic disease, after a period of hormone responsiveness. The standard first-line CRPC treatment is docetaxel, but several newer systemic anticancer therapies that have been shown to improve overall survival are available.Citation2–Citation4 However, most cancer deaths are due to metastatic disease and are resistant to these therapies. Until now, the mechanisms that lead to metastases remain incompletely understood.
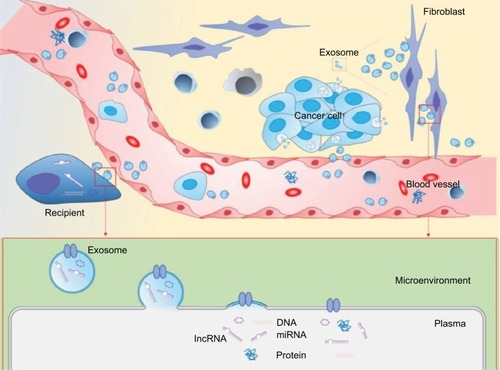
Cancer metastasis is a complex process. When tumor cells become motile and invasive during the epithelial–mesenchymal transition (EMT) period, motile cancer cells can enter the blood, where the cells become trapped in capillary beds distant from the primary tumor site. Circulating tumor cells enter secondary metastatic sites by extravasation into unfamiliar microenvironments. After converting to epithelial phenotypes, tumor cells will proliferate and form macrometastases at the second site. Behind this coherent process, the adaptation between tumor cells and the microenvironments plays a crucial role in metastasis regulation. Initially, tumor cells must overcome the extracellular matrix (ECM) barrier, which requires disruption of endothelial cell phenotypes for intravasation and extravasation. Although cancer cells survive in circulation, they cannot complete distant metastatic spread without adaptive modulation.Citation5 The “metastatic niche model” presented below is based on the “Seed and Soil” hypothesis, which suggests that a suitable conducive microenvironment must evolve for tumor cells to engraft and proliferate at secondary sites.Citation6–Citation8 This model supports the notion that metastatic cells have preferred terminal organs, such as PCa cells that have a higher risk of bone metastasis.Citation9,Citation10 From the moment that metastasis occurs, cancer and stromal cells secrete molecules that adapt the environment for metastatic spread. Aside from conventional factors such as cytokines and chemokines, noncoding RNAs (ncRNAs) delivered by exosomes have been recently found to play an important role in communications between tumor cells and stromal cells in the tumor microenvironment ().Citation11–Citation13 In this review, we summarize the roles of exosomal ncRNAs in PCa metastases. For comprehensive information about ncRNA-mediated disease, a global network can be found at the mammalian ncRNA-disease repository (www.rna-society.org/mndr/). Besides, miR2Disease (http://www.mir2disease.org/) is a good resource for retrieving miRNA-associated diseases.
Exosomes and exosomal ncRNAs
Exosomes are 40–100 nm vesicles that contain various molecules, including RNA, proteins, and cytokines, which are derived from several different cell types and are shed into extracellular spaces and body fluids.Citation14 Different from microvesicles that are directly shed from cell membranes, exosomes are released when multivesicular bodies (MVBs) fuse with cell membranes. MVBs, containing intraluminal vesicles, are generated by the inward budding of clathrin-coated domains in the plasma membrane.Citation15,Citation16 Exosomes facilitate cell–cell communications and RNA transfer between cells and have been found to be useful in cell signaling studies and can impact biological processes in recipient cells.Citation17–Citation20
ncRNAs represent about 98% of all transcriptional outputs in human bodies and are classified by size, small ncRNAs (<200 bp) and long ncRNAs (>200 bp).Citation21 ncRNAs regulate gene expression using several different mechanisms such as RNA interference, cosuppression, transgene silencing, imprinting, methylation, and possibly position-effect variegation, and transvection, although no proteins are encoded.Citation22 ncRNAs are divided into several categories: microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), small nucleolar RNAs, long ncRNAs (lncRNAs), and several other types. The most widely described ncRNAs are miRNAs and lncRNAs.Citation23 miRNAs are single-stranded RNAs and are ~22 nucleotides in length, which play important roles in regulating gene expression. miRNAs are estimated to regulate translation in more than 60% of the protein-coding genes. As is commonly known, the way in which messenger RNAs (mRNAs) are silenced depends on the degree of complementarity between the miRNAs and the 3′-untranslated region (3′-URT) of the target mRNA. When perfect base-paring homology exists between the miRNA and the mRNA, the miRNA will cleave the mRNA with the help of Argonaute. Imperfect binding usually occurs that results in target mRNA regulation by suppressed protein translation.Citation24
lncRNAs are RNA molecules more than 200 nucleotides in length. With the development of RNA-sequencing techniques, the discovery of novel RNA species including lncRNAs has rapidly expanded. Although many fewer studies have been performed using lncRNAs compared with that of miRNAs, the significance of lncRNA performance in cancer therapy has gained more attention recently. lncRNAs control transcription, translation, and protein function at multiple levels. Possible lncRNA mechanisms are 1) influencing transcription of protein-coding gene upstream promoter regions to interfere with downstream gene expression, 2) inhibiting RNA polymerase II or mediating chromatin remodeling and histone modifications to affect downstream gene expression, 3) disturbing mRNA splicing by complementary binding with protein-coding pre-mRNAs, 4) producing endogenous siRNAs under control of Dicer, 5) combining with specific proteins to modulate corresponding protein activities, 6) being components of nucleic acid–protein complexes, 7) binding to the specific proteins and changing cellular localization of the proteins, and 8) serving as precursors of small RNAs.Citation25–Citation27 Because of their functional diversity, ncRNAs are involved in the regulation of a variety of biological processes, especially those of cancer progression and metastasis.
Recently, the discovery of stable miRNAs in bodily fluids introduced new insights into ncRNAs and miRNAs that can lead to new diagnostic approaches using less invasive assays.Citation28 In addition to being packed into exosomes or microvesicles, circulating miRNAs are also combined delivered with high-density lipoproteinsCitation29 or AGO2 proteinsCitation30 to maintain stability. Gallo et al reported that most detectable circulating miRNAs in serum and saliva were concentrated within exosomes,Citation31 which suggests that exosomes and exosomal ncRNAs are the primary forms of cellular RNA-based communication. Huang et al performed RNA sequencing to annotate the exosomal RNA species from the plasma of 23 CRPC patients following the guideline previously reported.Citation32 Among the mapped reads, mature miRNAs were the most common and represented 41.72% of reads, followed by piR-NAs at 20.92%, lncRNAs at 20.19%, and mRNAs at 6.52%. Collectively, six other RNA species constituted 10.64% of the mappable sequences.Citation33 During cancer metastases, exosomes can act as delivery vehicles of circulating ncRNAs and transport them from primary to metastatic cancer sitesCitation11,Citation34,Citation35 and mediate adaptations between cancer and stromal cells. This mechanism is similar to that of primary cancer cell motility, which is modulated by EMT processes or establishment of favorable environments at possible metastatic sites to aid in neoplastic cell survival.Citation36
Differently expressed circulating miRNAs in PCa patients
Four miRNAs altered in the serum of transgenic adenocarcinoma of mouse prostate mice – miR-141, miR-298, miR-346, and miR-375 – also showed concordant changes in men with metastatic castration-resistant PCa (mCRPC).Citation37 Yaman Agaoglu et al found that the differences in miRNA plasma levels between the healthy controls and the patients were highly significant for the miR-21 (P<0.001) and -221 (P<0.001) but not for the miR-141(P=0.23).Citation38 An analysis of 742 miRNAs using plasma-derived circulating microvesicles (cMVs) from 78 PCa patients and 28 healthy individuals identified differentially expressed miRNAs that included upregulation of miR-107, -130b, -141, -2110, -301a, -326, -331-3p, -432, -484, -574-3p, and -625 and downregulation of miR-181a-2. In urine samples, miR-107 and miR-574-3p concentrations were also higher in patients compared with controls.Citation39 These clinical results suggested that circulating miRNAs can assist in the detection of PCa. Moreover, some miRNAs have been identified that are associated with PCa progression, staging, and outcomes, and these miRNAs could be more useful in clinical practice.
Metastasis-associated circulating miRNAs in PCa patients
The first report to evaluate the utility of circulating miRNAs to distinguish PCa patients with metastasis from those with advanced, but localized disease revealed that miRNA-21, -221, and -141 plasma levels were significantly higher in the metastatic cohort.Citation38 Circulating miRNAs were also reported to be significantly upregulated in the serum of metastatic PCa patients, and miR-375 was the most prominent and the only miRNA that showed greater serum concentrations in PCa patients with distant metastases compared with that of PCa patients with localized PCa. This study also confirmed that circulating miR-375 and miR-141 correlated with the risk of disease progression.Citation40 An analysis using plasma samples from 16 PCa patients with metastatic disease and 55 PCa patients with localized disease found 16 miRNAs that were differently expressed in cMVs. Among these 16 miRNAs, 15 had significantly greater concentrations and one (miR-572) had significantly lower concentrations in PCa patients with metastatic disease compared with those of PCa patients with localized disease. In a subsequent study, cMVs and exosomes were separated in the serum of 47 PCa patients with metastatic disease and 72 PCa patients in remission. This study showed that miR-375 and miR-141 were significantly increased in both the cMVs and the exosomes.Citation39 Another study demonstrated that miR-375, -141, and -378 expression was significantly upregulated and miR-409-3p expression was significantly downregulated in the serum of CRPC patients compared with patients who had a low risk of developing CRPC.Citation41 Overall, over 30 differentially expressed circulating miRNAs have been identified. A summary of differentially circulating ncRNAs found in PCa patients with metastatic disease compared with those with localized disease is presented in . Only miR-375 and miR-141 have higher repetition rates. Some deviations in the compiled data could be due to the different study designs, cohort selections, treatment strategies, methods of sample collection, and the sensitivity and specificity of platforms used.Citation42
Table 1 ncRNAs proven to be differently expressed in the circulation of prostate cancer patients with metastatic disease compared with those with localized disease
illustrates miRNA and lncRNA targets that regulate the development of PCa metastases and that are involved in signaling pathways. Overall, the mechanisms behind how miRNAs function in PCa metastases have not been substantially studied; however, a few preclinical studies have been reported. miR-375 has been shown to have multiple functions in several different types of cancer. In most cancer cases, miR-375 plays a role in tumor suppression of several cancer types, such as head and neck squamous cell carcinoma,Citation43,Citation44 esophageal cancer,Citation45 gastric cancer,Citation46 pancreatic cancer,Citation47 and hepatocellular carcinoma.Citation48,Citation49 However, some studies implicate miR-375 upregulation in the propagation of PCa.Citation50,Citation51 miR-375 might play a dual role in prostate carcinogenesis.Citation52 The reason for the antagonistic roles of miR-375 in different study toward PCa could be attributed to PCa heterogeneity. Circulating miR-375 expression is considered a prognostic biomarker for PCa.Citation33,Citation53 Higher miRNA-375 expression in circulation is the most confirmed miRNA that is related to metastatic PCa.Citation37,Citation40,Citation41,Citation50,Citation54,Citation55 However, scant information regarding the mechanism of circulating miR-375 in regulating metastatic spread of PCa has been discovered. A couple of studies showed that miR-375 promotes cell growth by targeting Sec23A.Citation56,Citation57 This was validated by a recent study, which demonstrated that miR-375 exerts its oncogenic effects by targeting CBX7 and thus regulates critical cancer pathways such as the EMT and Wnt/β-catenin signaling pathways.Citation58 More details on these processes remain to be determined.
Table 2 Proven targets and signal pathways of ncRNAs in prostate cancer patients with metastatic disease
miR-200 family including miR-200a, -200b, miR-200c, -141, and -429 was found to be associated with several cancer types. Members of this family directly target the metastasis-promoting protein, WAVE3, to inhibit PCa cell invasion.Citation59 These miRNAs also target the E-cadherin transcriptional repressors, ZEB1 and ZEB2, and were identified as determining factors in the development of cancer cell epithelial phenotypes.Citation60–Citation64 Moreover, miR-200b negatively regulates vascular endothelial growth factor (VEGF) signaling by targeting the VEGF and the VEGF receptor and could have therapeutic potential as an angiogenic inhibitor.Citation65 However, the miR-200 family has been associated with Sec23-mediated inhibition of metastasis-suppressing proteinsCitation66 and adhesion improvements at distant sites to promote EMT and increased colonization.Citation67 Therefore, the miR-200 family was found to have different roles in various stages of metastasis.Citation36
miR-141, a special member of the miR-200 family, has been reported to be a tumor suppressor in several malignancies, such as gastric cancer,Citation68,Citation69 pancreatic cancer,Citation70 breast cancer,Citation71,Citation72 renal cell carcinoma,Citation73,Citation74 and hepatocellular carcinoma.Citation75 Recently, circulating miR-141 was positively upregulated in the blood of metastatic PCa patients.Citation37–Citation39,Citation41,Citation76,Citation77 miR-141 targeted the small heterodimer partner protein to modulate androgen receptor (AR)-regulated transcriptional activity in AR-responsive LNCaP cells,Citation78 and AR signaling was closely related to PCa formation,Citation79 progression,Citation80 and metastasis.Citation81 miR-141 inhibits cell migration and invasion by targeting transforming growth factor (TGF)-β2Citation82 and insulin receptor substrate 2.Citation83 miR-141 was also reported to regulate the transcriptional coactivator, PDZ-binding motif (TAZ), a transcription cofactor that plays pivotal roles in the EMT.Citation84
miR-21 is one of the most widely studied metastasis-related miRNAs, which have been shown to be at significantly higher levels in the blood of PCa patients with metastases compared with those who had localized or advanced disease.Citation38 This molecule was also shown to promote cancer metastasis by regulating several aspects of the metastatic cascade. miR-21 promotes tumor cell invasion by targeting the tumor suppressor PDCD4, an inhibitor of the urokinase receptor, prometastatic factor.Citation85 miR-21 also modulates the expression of matrix metalloproteinases (MMPs) by directly targeting the MMP inhibitors, such as PTEN,Citation86 RECK,Citation87 and TIMP3Citation88 to degrade the ECM and promote cancer metastasis. Exosomal miR-21 can also regulate tumor microenvironments by binding to Toll-like receptors (TLRs) in immune cells, which triggers a TLR-mediated prometastatic inflammatory response that can ultimately lead to tumor growth and metastasis.Citation89
miR-126 was also found to be upregulated in the plasma of metastatic PCa patients,Citation55 and miR-126* regulates protein translation and invasion of PCa LNCaP cells.Citation90 Moreover, miR-126 has a suppressor role in cancer cell invasion through direct repression of a disintegrin and metalloprotease 9 (ADAM9).Citation91 Another study identified that when miR-126 partnered with another miR-126, the combination repressed mesenchymal stem cell and monocyte recruitment into the tumor stroma, which effectively inhibited tumor cell invasion and metastasis.Citation92
miR-152 upregulation in plasma samples from mCRPC patients was confirmed compared with those that had localized PCa.Citation55 miR-152 could also control PCa cell migration and invasive potential by directly targeting TGFα.Citation93 In other types of cancer, miR-152 was shown to inhibit non-small-cell lung cancer cell metastasis by targeting neuropilin-1.Citation94 Elevated levels of circulating miR-221 were detected in PCa patients with metastases.Citation38 miR-221 promotes the EMT by targeting PTEN in extrahepatic cholangiocarcinoma, which forms a positive feedback loop with the β-catenin/c-Jun signaling pathwayCitation95 and whose inhibition led to reduced cell migration in PCa cells that was partly mediated by Sirtuin 1 (SIRT1) activation.Citation96 miR-221 also promoted cancer invasion and angiogenesis by targeting TIMP2,Citation97 TIMP3, and PTEN.Citation98 miR-205 and miR-409-3p were downregulated in the circulation of metastatic PCa patients, and it was confirmed that miR-409-3p regulated the EMT process.Citation61,Citation99,Citation100 In summary, circulating miRNAs are present and have diverse roles throughout the entire metastatic process of PCa. In one stage of metastasis, multiple miRNAs have roles in regulating the process.
Metastasis-related circulating lncRNA in PCa
As seen in , there are many fewer reports about circulating lncRNAs than miRNAs. For many years, studies mainly focused on differential lncRNA expression in PCa tissues. Recently, significant differential expression of circulating lncRNAs in metastatic PCa compared with localized PCa has been disclosed. Prostate cancer-associated noncoding RNA transcript 18 (PCAT18) was detected in PCa patient plasma, and its expression was shown to be increased as PCa progresses from localized to metastatic disease, which suggests that PCAT18 is a potential biomarker for metastatic PCa. PCAT18 expression was significantly associated with AR signaling, and PCAT18 silencing was shown to inhibit PCa cell proliferation, migration, and invasion: however, the molecular mechanisms behind its actions remain unclear.Citation101 A few other lncRNAs were reported to be involved with PCa metastatic disease, but the differential expression in the blood of PCa patients with metastatic disease compared with PCa patients with localized disease remains uncovered. The prostate cancer antigen 3 lncRNA (PCA3; also referred to as DD3) was initially found to be significantly overexpressed in more than 95% of primary and metastatic PCa tissue sections.Citation102 PCA3 can be detected in urine and used as a noninvasive method for PCa diagnoses.Citation103,Citation104 Moreover, higher PCA3 scores were associated with greater tumor aggressiveness.Citation105
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is the most studied metastatic-associated lncRNA and has been shown to be overexpressed during PCa progression.Citation106 In early studies, MALAT1 transcript fragments were higher in the plasma of PCa patients compared with that of non-PCa patients.Citation107 MALAT1 is associated with EMT; loss of MALAT1 results in reduced LPHN2 and ABCA1 levels, which are important factors of EMT.Citation108 MALAT1 knockdown in PCa cell lines abrogates cell growth, migration, and invasion and induces G0/G1 cell cycle arrest.Citation109 HOX transcript antisense RNA (HOTAIR) expression increases in PCa cells during growth and invasion.Citation110 HOTAIR is also connected with both EMT and ECM remodeling. Therefore, the regulation of MMP expression partially mediates HOTAIR functions in cancer metastasis. Moreover, HOTAIR could affect EMT processes by modulating specific EMT-related genes.Citation111–Citation113 A recent study demonstrated that lncRNA CCAT2 knockdown stimulated EMT by abrogating N-cadherin and vimentin expression and by intensifying E-cadherin expression in PCa.Citation114
A multi-institutional high-throughput analysis of PCa tissue samples from 1,008 patients confirmed the prognostic value of measuring lncRNA, SChLAP1 (second chromosome locus associated with prostate-1) levels to detect metastatic progression.Citation115 SChLAP1 impairs the SNF5-mediated regulation of gene expression and genomic binding to coordinate cancer cell invasion.Citation116 A study recently identified the lncRNA, Linc00963, which regulates the epidermal growth factor receptor (EGFR) signaling pathway to promote PCa cell growth, migration, and invasion.Citation117
Ongoing PCa clinical trials
With the accumulating evidence from preclinical studies, clinical trials have recently been launched. The Medical College of Wisconsin (Milwaukee, Wisconsin) sponsored a study in June 2017 that is recruiting PCa patients (estimated to 60), with aims to identify exosomal miRNAs that can predict responses to ADT. Patients who were intermediate to high risk at diagnosis, those who were treated but now have recurrent disease, and those with metastatic disease are recruited to the study. Blood samples will be collected at the time of pretreatment, 3 months’ posttreatment, and during disease progression. Exosomal RNA markers that predict responses to ADT will be validated.
Another observational study in 2016 sponsored by the Assuta Medical Center (Israel) and enrolling as many as 120 PCa patients is aiming to find correlations among circulating miRNAs associated with PCa metastases to bones and lymph nodes using PET imaging.
In another study sponsored by the Hospital of Ghent University (Belgium), blood from PCa patients with lymph node metastases will be collected to examine the role of circulating miRNAs as a biomarker to diagnose and predict relapse-free survival rates. The sample size is expected to be 330. None of the clinical trials mentioned have posted results, which we eagerly await.
Conclusion and prospects
The identification of circulating ncRNA provides novel biomarkers and therapeutic targets for mCRPC. Several molecules have been proven to serve as predictors of PCa metastases. Exosomes protect the stability of circulating ncRNAs in body fluids and can be easily quantified. Exosomal ncRNA expression is related to cancer progression and the microenvironments during cancer metastasis. It is well accepted that the communication between cancer cells and the extracellular environments begin in the early stage of metastasis before the cancer cells move to their colonized sites. Therefore, more studies have shown that circulating ncRNAs are associated with the PCa stages.Citation101,Citation118,Citation119 The results of these studies suggest that circulating ncRNAs could be promising as biomarkers to predict cancer metastases.
Further understanding of ncRNAs has provided novel insights into PCa therapeutics. In the last few years, ncRNA-based cancer therapeutics inspire the interest of researchers.Citation120–Citation122 However, the methods needed to deliver miRNA mimics or inhibitors to target cells steadily and effectively remain a crucial obstacle. Exosomes have been studied as vehicle transporting molecules that can deliver genetic materials to target sites with high efficiency.Citation123,Citation124 Ohno et al performed a study using exosomes to deliver let-7a to EGFR-expressing breast cancer tissues.Citation125 Exosomal ncRNAs for clinical use require more research to resolve problems, such as enhancing exosome production and reducing immunogenicity. Emerging evidence suggests that exosomal ncRNA could eventually play an essential role in metastatic PCa treatment.
In summary, abundant research on exosomal miRNAs is present, while much less evidence exists that determines if lncRNAs may play an essential role in PCa metastases. With new technologies evolving at a rapid pace, lncRNA studies will overcome limitations and more promising lncRNAs will be discovered. However, in the field of ncRNAs, many challenges and opportunities still exist.
Acknowledgments
This study was jointly supported by the National Natural Science Foundation of China (Grant No. 81572528), the Haiyan Foundation of Harbin Medical University Cancer Hospital, Outstanding Youth Fund of the Heilongjiang Province (Grant No. JC2018024), and the Heilongjiang Innovation Ability Promoting Program for Scientific Research Institutions (Grant No. YC2016D002) to XH.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- HathawayARBakerMKSonpavdeGEmerging agents for the therapy of advanced prostate cancerFuture Oncol201511202775278726367474
- FaleiroILeãoRBinnieAde MelloRAMaiaATCastelo-BrancoPEpigenetic therapy in urologic cancers: an update on clinical trialsOncotarget201787124841250028036257
- MacfarlaneRJChiKNResearch in castration-resistant prostate cancer: what does the future hold?Curr Oncol201017Suppl 2S808620882138
- TorpyJLynmCGlassRMNon-coding RNAs in cancer brain metastasisFront Biosci20168187202
- PsailaBLydenDThe metastatic niche: adapting the foreign soilNat Rev Cancer20099428529319308068
- PagetSThe distribution of secondary growths in cancer of the breast. 1889Cancer Metastasis Rev198982981012673568
- Costa-SilvaBAielloNMOceanAJPancreatic cancer exosomes initiate pre-metastatic niche formation in the liverNat Cell Biol201517681682625985394
- BubendorfLSchöpferAWagnerUMetastatic patterns of prostate cancer: an autopsy study of 1,589 patientsHum Pathol200031557858310836297
- SunYXSchneiderAJungYSkeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivoJ Bone Miner Res200520231832915647826
- ValadiHEkströmKBossiosASjöstrandMLeeJJLötvallJOExosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cellsNat Cell Biol20079665465917486113
- JiangCLiXZhaoHLiuHLong non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasisMol Cancer20161516227686732
- AhadiABrennanSKennedyPJHutvagnerGTranNLong non-coding RNAs harboring miRNA seed regions are enriched in prostate cancer exosomesSci Rep201662492227102850
- ZhangJLiSLiLExosome and exosomal microRNA: trafficking, sorting, and functionGenomics Proteomics Bioinformatics2015131172425724326
- Sato-KuwabaraYMeloSASoaresFACalinGAThe fusion of two worlds: non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications (Review)Int J Oncol2015461172725338714
- HeijnenHFSchielAEFijnheerRGeuzeHJSixmaJJActivated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granulesBlood199994113791379910572093
- OstenfeldMSJeppesenDKLaurbergJRCellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic propertiesCancer Res201474205758577125261234
- OhshimaKInoueKFujiwaraALet-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell linePLoS One2010510e1324720949044
- MontecalvoALarreginaATShufeskyWJMechanism of transfer of functional microRNAs between mouse dendritic cells via exosomesBlood2012119375676622031862
- LiuYXiangXZhuangXContribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cellsAm J Pathol201017652490249920348242
- GibbEABrownCJLamWLThe functional role of long non-coding RNA in human carcinomasMol Cancer2011103821489289
- MattickJSNon-coding RNAs: the architects of eukaryotic complexityEMBO Rep200121198699111713189
- EstellerMNon-coding RNAs in human diseaseNat Rev Genet2011121286187422094949
- DoenchJGSharpPASpecificity of microRNA target selection in translational repressionGenes Dev200418550451115014042
- BatistaPJChangHYLong noncoding RNAs: cellular address codes in development and diseaseCell201315261298130723498938
- WiluszJESunwooHSpectorDLLong noncoding RNAs: functional surprises from the RNA worldGenes Dev200923131494150419571179
- PontingCPOliverPLReikWEvolution and functions of long non-coding RNAsCell2009136462964119239885
- ChenXBaYMaLCharacterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseasesCell Res20081810997100618766170
- VickersKCPalmisanoBTShoucriBMShamburekRDRemaleyATMicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteinsNat Cell Biol201113442343321423178
- ArroyoJDChevilletJRKrohEMArgonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasmaProc Natl Acad Sci U S A2011108125003500821383194
- GalloATandonMAlevizosIIlleiGGThe majority of microRNAs detectable in serum and saliva is concentrated in exosomesPLoS One201273e3067922427800
- HuangXYuanTTschannenMCharacterization of human plasma-derived exosomal RNAs by deep sequencingBMC Genomics20131431923663360
- HuangXYuanTLiangMExosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancerEur Urol2015671334125129854
- AlhasanAHPatelPCChoiCHMirkinCAExosome encased spherical nucleic acid gold nanoparticle conjugates as potent microRNA regulation agentsSmall201410118619224106176
- MinhTNPhLGuoCmiR-200–containing extracellular vesicles promote breast cancer cell metastasisJ Clin Invest2014124125109512825401471
- AlečkovićMKangYRegulation of cancer metastasis by cell-free miRNAsBiochim Biophys Acta201518551244225450578
- SelthLATownleySGillisJLDiscovery of circulating microR-NAs associated with human prostate cancer using a mouse model of diseaseInt J Cancer2012131365266122052531
- Yaman AgaogluFKovancilarMDizdarYInvestigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancerTumour Biol201132358358821274675
- BryantRJPawlowskiTCattoJWChanges in circulating microRNA levels associated with prostate cancerBr J Cancer2012106476877422240788
- BraseJCJohannesMSchlommTCirculating miRNAs are correlated with tumor progression in prostate cancerInt J Cancer2011128360861620473869
- NguyenHCXieWYangMExpression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancerProstate201373434635422887127
- JossonSChungLWGururajanMmicroRNAs and prostate cancerAdv Exp Med Biol201588910511826658999
- KinoshitaTNohataNYoshinoHTumor suppressive microRNA-375 regulates lactate dehydrogenase B in maxillary sinus squamous cell carcinomaInt J Oncol201240118519321922130
- HarrisTJimenezLKawachiNLow-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomasAm J Pathol2012180391792822234174
- KongKLKwongDLChanTHMicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptorGut2012611334221813472
- TsukamotoYNakadaCNoguchiTMicroRNA-375 is down-regulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zetaCancer Res20107062339234920215506
- BhattiILeeAJamesVKnockdown of microRNA-21 inhibits proliferation and increases cell death by targeting programmed cell death 4 (PDCD4) in pancreatic ductal adenocarcinomaJ Gastrointest Surg201115119920821088996
- LiuAMPoonRTLukJMMicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor propertiesBiochem Biophys Res Commun2010394362362720226166
- HeXXChangYMengFYMicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivoOncogene201231283357336922056881
- ChengHHMitchellPSKrohEMCirculating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxiaPLoS One201387e6923923935962
- NguyenHCXieWYangMExpression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancerProstate201373434635422887127
- Costa-PinheiroPRamalho-CarvalhoJVieiraFQMicroRNA-375 plays a dual role in prostate carcinogenesisClin Epigenetics201574225977730
- KachakovaDMitkovaAPopovECombinations of serum prostate-specific antigen and plasma expression levels of let-7c, miR-30c, miR-141, and miR-375 as potential better diagnostic biomarkers for prostate cancerDNA Cell Biol201534318920025521481
- HoriNNaritaMYamashitaAChanges in the expression of IL-6-mediated microRNAs in the dorsal root ganglion under neuropathic pain in miceSynapse201670831732426990296
- WatahikiAMacfarlaneRJGleaveMEPlasma miRNAs as biomarkers to identify patients with castration-resistant metastatic prostate cancerInt J Mol Sci20131447757777023574937
- SzczyrbaJNolteEWachSDownregulation of Sec23A protein by miRNA-375 in prostate carcinomaMol Cancer Res20119679180021593139
- WangYLiebermanRPanJmiR-375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1Mol Cancer20161517027832783
- PicklJMTichyDKuryshevVYAgo-RIP-Seq identifies Polycomb repressive complex I member CBX7 as a major target of miR-375 in prostate cancer progressionOncotarget2016737595895960327449098
- Sossey-AlaouiKBialkowskaKPlowEFThe miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasionJ Biol Chem200928448330193302919801681
- ParkSMGaurABLengyelEPeterMEThe miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2Genes Dev200822789490718381893
- GregoryPABertAGPatersonELThe miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1Nat Cell Biol200810559360118376396
- HurteauGJCarlsonJASpivackSDBrockGJOverexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherinCancer Res200767177972797617804704
- BurkUSchubertJWellnerUA reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cellsEMBO Rep20089658258918483486
- KorpalMLeeESHuGKangYThe miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2J Biol Chem200828322149101491418411277
- ChoiYCYoonSJeongYYoonJBaekKRegulation of vascular endothelial growth factor signaling by miR-200bMol Cells2011321778221544626
- KorpalMEllBJBuffaFMDirect targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonizationNat Med20111791101110821822286
- DykxhoornDMWuYXieHmiR-200 enhances mouse breast cancer cell colonization to form distant metastasesPLoS One200949e718119787069
- ZhouXWangYShanBThe downregulation of miR-200c/141 promotes ZEB1/2 expression and gastric cancer progressionMed Oncol201532142825502084
- duYXuYDingLDown-regulation of miR-141 in gastric cancer and its involvement in cell growthJ Gastroenterol200944655656119363643
- ZhuZMXuYFSuQJYfXQjSPrognostic significance of microRNA-141 expression and its tumor suppressor function in human pancreatic ductal adenocarcinomaMol Cell Biochem20143881–2394924242138
- XuFHeHHuangWDecreased expression of microRNA-200 family in human breast cancer is associated with lymph node metastasisClin Transl Oncol201618328328826201425
- O’DayELalAMicroRNAs and their target gene networks in breast cancerBreast Cancer Res201012220120346098
- YuXYZhangZLiuJZhanBKongCZMicroRNA-141 is down-regulated in human renal cell carcinoma and regulates cell survival by targeting CDC25BOnco Targets Ther2013634935423596351
- NakadaCMatsuuraKTsukamotoYGenome-wide microRNA expression profiling in renal cell carcinoma: significant down-regulation of miR-141 and miR-200cJ Pathol2008216441842718925646
- XueJNiuYFHuangJmiR-141 suppresses the growth and metastasis of HCC cells by targeting E2F3Tumour Biol20143512121031210725142234
- LiZMaYYWangJExosomal microRNA-141 is upregulated in the serum of prostate cancer patientsOnco Targets Ther2016913914826770063
- ZhangHLQinXJCaoDLAn elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesionsAsian J Androl201315223123523377530
- XiaoJGongAYEischeidANmiR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner proteinProstate201272141514152222314666
- CuligZBartschGAndrogen axis in prostate cancerJ Cell Biochem200699237338116598769
- LiHXieNChenRUGT2B17 expedites progression of castration-resistant prostate cancers by promoting ligand-independent AR signalingCancer Res201676226701671127659047
- HuSLiLYehSInfiltrating T cells promote prostate cancer metastasis via modulation of FGF11→miRNA-541→androgen receptor (AR)→MMP9 signalingMol Oncol201591445725135278
- PengTZhangSLiWFuSLuanYZuoLMicroRNA-141 inhibits glioma cells growth and metastasis by targeting TGF-β2Am J Transl Res2016883513352127648141
- DongSMengXXueSYanZRenPLiuJMicroRNA-141 inhibits thyroid cancer cell growth and metastasis by targeting insulin receptor substrate 2Am J Transl Res2016831471148127186273
- ZuoQFZhangRLiBSMicroRNA-141 inhibits tumor growth and metastasis in gastric cancer by directly targeting transcriptional co-activator with PDZ-binding motif, TAZCell Death Dis20156e162325633292
- AsanganiIARasheedSANikolovaDAMicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancerOncogene200827152128213617968323
- MengFHensonRWehbe-JanekHGhoshalKJacobSTPatelTMicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancerGastroenterology2007133264765817681183
- ReisSTPontes-JuniorJAntunesAAmiR-21 may acts as an oncomir by targeting RECK, a matrix metalloproteinase regulator, in prostate cancerBMC Urol2012121422642976
- GabrielyGWurdingerTKesariSMicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulatorsMol Cell Biol200828175369538018591254
- FabbriaMMicroRNAs bind to Toll like receptors to induce prometastatic inflammatory response201210931E21102116
- MusiyenkoABitkoVBarikSEctopic expression of miR-126*, an intronic product of the vascular endothelial EGF-like 7 gene, regulates prostein translation and invasiveness of prostate cancer LNCaP cellsJ Mol Med200886331332218193184
- WangCZYuanPLiYmiR-126 regulated breast cancer cell invasion by targeting ADAM9Int J Clin Exp Pathol2015866547655326261534
- ZhangYYangPSunTmiR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasisNat Cell Biol201315328429423396050
- ZhuCLiJDingQmiR-152 controls migration and invasive potential by targeting TGFα in prostate cancer cell linesProstate201373101082108923460133
- ZhangYJLiuXCduJZhangYJmiR-152 regulates metastases of non-small cell lung cancer cells by targeting neuropilin-1Int J Clin Exp Pathol2015811142351424026823738
- LiJYaoLLiGmiR-221 promotes epithelial-mesenchymal transition through targeting PTEN and forms a positive feedback loop with β-catenin/c-Jun signaling pathway in extra-hepatic cholangiocarcinomaPLoS One20151010e014116826501139
- YangXYangYGanRDown-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1PLoS One201496e9883324892674
- YangFWangWZhouCmiR-221/222 promote human glioma cell invasion and angiogenesis by targeting TIMP2Tumour Biol20153653763377325731730
- GarofaloMdi LevaGRomanoGmiR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulationCancer Cell200916649850919962668
- TucciPAgostiniMGrespiFLoss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancerProc Natl Acad Sci U S A201210938153121531722949650
- JossonSGururajanMHuPmiR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancerClin Cancer Res201420174636464624963047
- CreaFWatahikiAQuagliataLIdentification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancerOncotarget20145376477424519926
- HesselsDKlein GunnewiekJMTvan OortIvanOIDD3PCA3-based molecular urine analysis for the diagnosis of prostate cancerEur Urol200344181612814669
- XuY-HXueW-JYingX-LJiangJ-HYhXProstate cancer antigen 3 as a biomarker in the urine for prostate cancer diagnosis: a meta-analysisJ Cancer Res Ther2014107218221
- HuBYangHYangHDiagnostic value of urine prostate cancer antigen 3 test using a cutoff value of 35 μg/L in patients with prostate cancerTumor Biol201435985738580
- MerolaRTomaoLAntenucciAPCA3 in prostate cancer and tumor aggressiveness detection on 407 high-risk patients: a National Cancer Institute experienceJ Exp Clin Cancer Res2015341525651917
- WangDDingLWangLlncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancerOncotarget2015638410454105526516927
- RenSWangFShenJLong non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancerEur J Cancer201349132949295923726266
- GutschnerTHämmerleMEissmannMThe noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cellsCancer Res20137331180118923243023
- RenSLiuYXuWLong noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancerJ Urol201319062278228723845456
- ZhangAZhaoJCKimJLncRNA HOTAIR enhances the androgen-receptor-mediated transcriptional program and drives castration-resistant prostate cancerCell Rep201513120922126411689
- XuZYYuQMDuYAKnockdown of long non- coding RNA HOTAIR suppresses tumor invasion and reverses epithelial- mesenchymal transition in gastric cancerInt J Biol Sci20139658759723847441
- WuSZhengCChenSOverexpression of long non-coding RNA HOTAIR predicts a poor prognosis in patients with acute myeloid leukemiaOncol Lett20151042410241426622861
- WuYZhangLZhangLLong non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinomaInt J Oncol20154662586259425901533
- ZhengJZhaoSHeXThe up-regulation of long non-coding RNA CCAT2 indicates a poor prognosis for prostate cancer and promotes metastasis by affecting epithelial-mesenchymal transitionBiochem Biophys Res Commun2016480450851427558961
- PrensnerJRZhaoSErhoNRNA biomarkers associated with metastatic progression in prostate cancer: a multi-institutional high-throughput analysis of SChLAP1Lancet Oncol201415131469148025456366
- MehraRShiYUdagerAMA novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinically localized prostate cancerNeoplasia201416121121112725499224
- WangLHanSJinGLinc00963: a novel, long non-coding RNA involved in the transition of prostate cancer from androgen-dependence to androgen-independenceInt J Oncol20144462041204924691949
- AlhasanAHScottAWWuJJJjWCirculating microRNA signature for the diagnosis of very high-risk prostate cancerProc Natl Acad Sci U S A201611338106551066027601638
- KellyBDMillerNSweeneyKJA circulating microRNA signature as a biomarker for prostate cancer in a high risk groupJ Clin Med2015471369137926239681
- van RooijEKauppinenSDevelopment of microRNA therapeutics is coming of ageEMBO Mol Med20146785186424935956
- WenDDanquahMChaudharyAKMahatoRISmall molecules targeting microRNA for cancer therapy: promises and obstaclesJ Control Release201521923724726256260
- MouravievVLeeBPatelVClinical prospects of long noncoding RNAs as novel biomarkers and therapeutic targets in prostate cancerProstate Cancer Prostatic Dis2016191142026503110
- Alvarez-ErvitiLSeowYYinHBettsCLakhalSWoodMJDelivery of siRNA to the mouse brain by systemic injection of targeted exosomesNat Biotechnol201129434134521423189
- ZhangDLeeHZhuZMinhasJKJinYEnrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivoAm J Physiol Lung Cell Mol Physiol20173121L110L12127881406
- OhnoSTakanashiMSudoKSystemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cellsMol Ther201321118519123032975