Abstract
Purpose
Research has revealed that some patients who develop resistance to the first taxane treatment exhibit a moderate response to the second taxane treatment (incomplete cross-resistance between paclitaxel and docetaxel). However, which patients are most likely to respond to the second treatment remains unclear. The aim of this study was to determine the predictive factors for the efficacy of the second taxane treatment in patients resistant to the first.
Patients and methods
We enrolled patients treated with paclitaxel and docetaxel (n=31) in this study. Using univariate and multivariate analyses, we determined the predictive factors for the efficacy of the second taxane treatment. Then, we assigned patients to one of the three groups: 1) those with a partial response (PR) to the first taxane treatment who subsequently became refractory (PR group); 2) those whose response was stable disease (SD) and subsequently became refractory (SD group); and 3) those whose response was the progression of the disease with the first taxane treatment (progression disease [PD] group). Furthermore, the response rates were assessed for each group. All statistical analyses were performed using JMP 11.
Results
Responses to the first taxane treatment considerably correlated with the efficacy of the second treatment in patients with a PR to the first taxane treatment (P=0.0061, univariate analysis; P=0.0056, multivariate analysis). In addition, response rates to the second taxane treatment in the PR, SD, and PD groups were 33.3%, 0%, and 0%, respectively.
Conclusion
The response to the first taxane treatment was a predictive factor for the efficacy of the second taxane treatment in patients with a PR to the first. Thus, the second treatment is highly recommended for patients who exhibit tumor shrinkage (a PR) by the first treatment.
Introduction
Taxanes (paclitaxel and docetaxel) are cytotoxic anticancer agents used to treat various types of cancers. In patients with advanced-stage cancer, therapeutic options regarding treatment with anticancer drugs are limited.Citation1–Citation3 Although various anticancer drugs, including taxanes, are known to be effective for various types of cancers,Citation4–Citation8 a few options for anticancer drug treatment are available for patients with advanced cancer. Hence, it is important to try all the available anticancer drugs with these patients.
Previously, several retrospective studies have reported an incomplete cross-resistance between paclitaxel and docetaxel in various cancer types.Citation9–Citation12 These studies reported the moderate efficacy of the second taxane treatment in some patients who became refractory to the first taxane treatment. Based on these findings, patients refractory to the first taxane treatment have often been treated with the second taxane treatment. However, little research has been conducted on the predictive factors for the efficacy of the second taxane treatment in patients refractory to the first taxane.
The aim of this study was to determine the predictive factors for the efficacy of the second taxane treatment in patients with various types of cancers who developed resistance to the first taxane treatment.
Patients and methods
Patients
We retrospectively reviewed the medical records of patients between 2005 and 2017 (n=2,233) with a histopathological diagnosis of squamous cell carcinoma of the head and neck or esophagus or adenocarcinoma of the stomach or breast at the Department of Medical Oncology, Tohoku University Hospital (Sendai, Japan). In this study, we enrolled all patients successively treated with paclitaxel and docetaxel (either paclitaxel–docetaxel or docetaxel–paclitaxel; n=31). In contrast, we excluded patients treated with anticancer drugs other than the two taxanes.
Chemotherapy regimens
Patients with head and neck cancer and esophageal cancer received recurrent docetaxel (70 mg/m2) infusions every 3 weeks or recurrent paclitaxel (100 mg/m2) once weekly for 6 weeks followed by 1 week rest. Patients with gastric cancer received recurrent docetaxel (60 mg/m2) infusions every 3 weeks or recurrent paclitaxel (80 mg/m2) once weekly for 3 weeks followed by 1 week rest. Patients with breast cancer received recurrent paclitaxel (80 mg/m2) once weekly for 3 weeks followed by 1 week rest. When the tumor progression or adverse event (allergy or interstitial pneumonia) was observed in patients, the first taxane was changed to the second one in this study.
Evaluation and statistical analysis
We assigned patients to one of the three groups. Patients in the first group exhibited only a partial response (PR) to the first taxane treatment (PR group; defined as a ≧30% reduction in the diameter of measurable lesions on computed tomography [CT]) and subsequently became refractory to the treatment. The second group comprised patients whose response to the first taxane treatment was stable disease (SD; SD group; defined as a <30% reduction and a <20% increase in the diameter of measurable lesions as shown on CT). Finally, the third group comprised patients whose response was disease progression (progression disease [PD] group; defined as a ≧20% increase in the diameter of measurable lesions as shown on CT). Finally, the third group comprised patients whose response was disease progression (progression disease [PD] group; defined as a ≧20% increase in the diameter of measurable lesions as shown on CT). Notably, the responses were assessed using the Response Criteria in Solid Tumor, version 1.0.Citation13 We combined the rate of the complete response (CR; or all signs of cancer disappearing with the taxane treatment) and PR and used as the response rate. In addition, the rates of CR, PR, and SD were combined and used as the disease control rate. Then, all toxicities were reviewed from the medical records and evaluated as per the Common Terminology Criteria for Adverse Events, version 4.0.Citation14 Furthermore, we performed a univariate analysis, multivariate analysis, and Pearson’s chi-squared test using JMP 11, version 13.1 (SAS Institute Inc., Cary, NC, USA). We considered P=0.05 as the statistical significance.
Ethical statement
This study protocol was approved by the ethics committee of Tohoku University Hospital.
The ethics committee of Tohoku University Hospital has permitted to conduct retrospective studies without consent statements by patients (opt-out system).
All data in the current study had no personal identifiers and were kept confidential.
Results
Patients’ characteristics
We identified 31 patients who were successively treated with paclitaxel or docetaxel during their treatment. summarizes the patients’ characteristics. The primary cancer sites were the esophagus (n=14), stomach (n=12), head and neck (n=4), and breast (n=1). While 19 patients were first treated with paclitaxel, followed by docetaxel, 12 were first treated with docetaxel, followed by paclitaxel. In addition, 28 patients received, at least, one anticancer drug other than the taxanes.
Table 1 Patients’ characteristics
Predictive factors for the efficacy of the second taxane treatment
We conducted univariate and multivariate analyses to determine the predictive factors for the efficacy of the second taxane treatment in patients who became refractory to the first taxane and assess a correlation between both (). We established statistically significant correlations between the tumor shrinkage with the first taxane treatment and the tumor shrinkage with the second taxane treatment (P=0.0061 and P=0.0056, respectively). In addition, we analyzed the other three factors, the time interval between the first taxane treatment and the second, histology, and the order of the taxane treatment that did not markedly correlate with the efficacy of the second taxane treatment.
Table 2 Univariate and multivariate analyses for the relationship between the tumor response by the first taxane treatment and the tumor response by the second taxane treatment in patients who were resistant to the first taxane treatment
Efficacy of the second taxane treatment
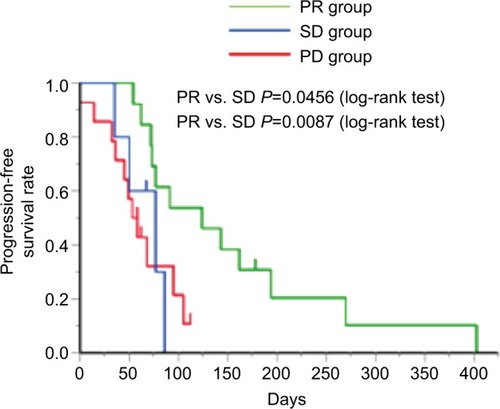
Next, we assessed the efficacy of the second taxane treatment in patients who were resistant to the first. We assigned patients to either the PR group (n=12), SD group (n=5), or PD group (n=14) and assessed the differences in the response rates in each group (). Response rates to the second taxane treatment in the PR, SD, and PD groups were 33.3%, 0%, and 0%, respectively (). In addition, disease control rates with the second taxane treatment in the PR, SD, and PD groups were 58.3%, 40.0%, and 14.3%, respectively (). All patients who exhibited tumor shrinkage with the second taxane treatment were included in the PR group (). A large proportion of patients in the SD and PD groups experienced the disease progression with the second taxane treatment. The median progression-free survival time of the PR, SD, and PD groups was 124 days (95% CI: 72–194 days), 77 days (95% CI: 35–86 days), and 55.5 days (95% CI: 36–95 days), respectively (). Based on the results of the log-rank test, the progression-free survival rate of the PR group was significantly higher than that of the SD and PD groups (the PR group vs the SD group, P=0.0456; the PR group vs the PD group, P=0.0087).
Table 3 Characteristics of patients in the first taxane PR, SD, and PD groups
Table 4 Responses to the second taxane treatment in patients resistant to the first taxane treatment
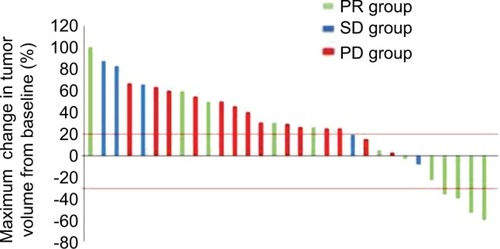
Figure 1 Waterfall plot of the maximum percentage change in tumor volume.
Note: Waterfall plot of the maximum percentage change in tumor volume with the second taxane treatment from baseline in patients in the PR group (green columns), SD group (blue columns), and PD group (red columns).
Abbreviations: PD, progression disease; PR, partial response; SD, stable disease.
Toxicities
presents the toxicities caused by the second taxane treatment in the PR, SD, and PD groups. We observed severe hematological toxicities in approximately 20% of the patients in each group. In addition, we observed a few patients with nonhematological toxicities (anorexia and fatigue) in each group. However, no significant differences were observed in the percentages of both hematological and nonhematological toxicities among the three groups.
Table 5 Toxicities caused by the second taxane treatment in the first taxane PR, SD, and PD groups
Discussion
In this study, we used univariate and multivariate analyses to determine the predictive factors for the efficacy of the second taxane treatment in patients refractory to the first treatment. These findings suggest that tumor shrinkage by the second taxane treatment is anticipated in patients who exhibited tumor shrinkage with the first treatment, even when these tumors become refractory to the first treatment.
In this study, we categorized patients into one of the three groups (PR, SD, and PD groups; ) and analyzed the response rate of each group to the second taxane treatment. The PR group comprised all patients who exhibited tumor shrinkage with the second taxane treatment ( and ). In the PR group, the progression-free survival rate with the second taxane treatment was considerably longer than that of patients in the SD and PD groups (). Prior studies reported that response rates to the second taxane treatment in patients with gastric cancer, esophageal cancer, and breast cancer resistant to the first taxane treatment were 12.8%, 20.0%, and 19.5%, respectively.Citation9–Citation11 In this study, the response rate to the second taxane treatment in the PR group (33.3%) was higher than that reported previously, suggesting that the determined predictive factor could be applied to patients in whom tumors were highly expected to respond to the second taxane treatment when tumors were refractory to the first taxane treatment. Furthermore, no patient exhibited tumor shrinkage with the second taxane treatment in either the SD or the PD group, suggesting that the second taxane treatment comprised only a weak ability to control tumor growth in patients who did not exhibit tumor shrinkage with the first taxane treatment.
Consistent with previous studies, the response rate to the second taxane (paclitaxel) treatment in patients with breast cancer who exhibited tumor shrinkage with the first (docetaxel) treatment (24.1%) was higher than the response rate in patients who did not exhibit tumor shrinkage with the first (docetaxel) treatment (8.3%).Citation11 These results supported the idea that tumor shrinkage with the first taxane treatment could be used as a predictive factor for a response to the second treatment.
Reportedly, a variation in an isotype of β-tubulin in cancer cells seemingly associated with the response to taxanes.Citation15,Citation16 In these studies, the βIII isotype of tubulin exhibited less stability than other isotypes of tubulin. As taxanes affect the stabilized β-tubulin,Citation16 the overexpression of βIII-tubulin could be related to a poor response to paclitaxel or docetaxel. In addition, the overexpression of βIII-tubulin was reported to be a substantial cause of resistance to taxanes.Citation15,Citation16 Perhaps, several patients in this study did not exhibit tumor shrinkage by either paclitaxel or docetaxel because of this βIII-tubulin mechanism.Citation15,Citation16 However, why all four patients who exhibited tumor shrinkage with the second taxane treatment were included in the PR group in this study cannot be explained by such a mechanism. Reportedly, cancer cells treated with taxanes expressed the multidrug resistance (MDR1) gene,Citation17 encoded the plasma membrane P-glycoprotein (ABCB1), which acted as adenosine triphosphate (ATP)-dependent efflux pump for taxanes. This mechanism leads cancer cells to become refractory to taxanes.Citation17 In the four patients who exhibited tumor shrinkage with the second taxane treatment in this study, ABCB1 in their cancer cells might not have had the ability to excrete the second taxane. If this hypothesis is correct, it would clarify why all the patients who exhibited tumor shrinkage with the second taxane treatment were included in the PR group. However, the precise mechanism remains unclear to date. Thus, further studies on cross-resistance between the two taxanes are warranted.
Cabazitaxel is a next-generation taxane that has been approved for metastatic castration-resistant prostate cancer.Citation18 A previous Phase III study reported that the median overall survival time by a cabazitaxel-containing regimen in patients who had progressed during or after a docetaxel-based regimen was 15.1 months, which was markedly longer than that with the control arm (12.7 months).Citation19 Thus, cabazitaxel seems to be modestly effective in patients refractory to docetaxel therapy. Of note, it is intriguing to assess whether cabazitaxel therapy has higher efficacy in patients who exhibited tumor shrinkage by docetaxel therapy than in patients who did not exhibit tumor shrinkage by docetaxel therapy.
This study has some limitations. First, this study enrolled a low number of eligible patients. Second, the study was retrospective in nature. Third, the fact that we did not enroll patients with lung and ovarian cancer, for whom taxane is an approved treatment, is another major limitation. Fourth, the basal condition varied among the three groups. Although the tumor volume of those in the PR group was decreased by the first taxane treatment, the tumor volume of those in the SD and PD groups was not decreased by the first taxane treatment. In addition, tumors in patients in the SD and PD groups might exhibit a low reactivity to taxanes compared with patients in the first taxane PR group. However, all tumors in the PR and SD groups became refractory to the first taxane treatment before the second taxane treatment was started. Thus, the tumors in all three groups were growing when the second taxane treatment was initiated. Hence, it should be emphasized that tumor shrinkages were observed only in patients in the first taxane PR group.
Conclusion
The second taxane treatment after a PR to the first is highly recommended. Nevertheless, further studies are warranted to investigate the efficacy of the second taxane treatment in patients who are refractory to their first taxane treatment.
Author contributions
Hiroo Imai designed the study and wrote the initial draft of the manuscript. Chikashi Ishioka contributed to analysis and interpretation of data and assisted in the preparation of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Chikashi Ishioka has received research funding from the Tokyo Cooperative Oncology Group, received contributions from Chugai Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, AstraZeneca, Bristol-Myers Squibb, Janssen Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo Company, Limited, and Takeda Pharmaceutical, and is a representative of Tohoku Clinical Oncology Research and Education Society, a specified nonprofit corporation. Masanobu Takahashi has received research funding from Ono Pharmaceutical, MSD, and Novartis and also received contributions from Chugai Pharmaceutical, Ono Pharmaceutical, MSD, Bristol-Myers Squibb, Taiho Pharmaceutical, Kyowa Hakko Kirin, and Daiichi Sankyo Company, Limited. The other authors report no conflicts of interest in this work.
References
- ScheithauerWEsophageal cancer: chemotherapy as palliative therapyAnn Oncol200415Suppl 4iv9710015477344
- BeheraMOwonikokoTKKimSConcurrent therapy with taxane versus non-taxane containing regimens in locally advanced squamous cell carcinomas of the head and neck (SCCHN): a systematic reviewOral Oncol201450988889425060589
- TakahariDSecond-line chemotherapy for patients with advanced gastric cancerGastric Cancer201720339540628260227
- HidaTNokiharaHKondoMAlectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trialLancet201739010089293928501140
- KangYKBokuNSatohTNivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trialLancet2017390101112461247128993052
- KiyotaNHasegawaYTakahashiSA randomized, open-label, Phase III clinical trial of nivolumab vs. therapy of investigator’s choice in recurrent squamous cell carcinoma of the head and neck: A subanalysis of Asian patients versus the global population in checkmate 141Oral Oncol20177313814628939066
- SolomonBJMokTKimDWFirst-line crizotinib versus chemotherapy in ALK-positive lung cancerN Engl J Med2014371232167217725470694
- ArnoldDFuchsCSTaberneroJMeta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumabAnn Oncol201728122932294228950290
- AndoTHosokawaAKajiuraSEfficacy of weekly paclitaxel in patients with advanced gastric cancer refractory to docetaxel-based chemotherapyGastric Cancer201215442743222252156
- ImaiHKomineKTakahashiSEfficacy and Safety Assessment of Paclitaxel in Patients with Docetaxel-Resistant Esophageal Squamous Cell CarcinomaChemotherapy201661526226827043795
- YonemoriKKatsumataNUnoHEfficacy of weekly paclitaxel in patients with docetaxel-resistant metastatic breast cancerBreast Cancer Res Treat200589323724115754121
- MarkmanMZanottiKWebsterKPetersonGKulpBBelinsonJPhase 2 trial of single agent docetaxel in platinum and paclitaxel-refractory ovarian cancer, fallopian tube cancer, and primary carcinoma of the peritoneumGynecol Oncol200391357357614675679
- TherassePArbuckSGEisenhauerEANew guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of CanadaJ Natl Cancer Inst200092320521610655437
- TobinaiKKohnoAShimadaYToxicity grading criteria of the Japan Clinical Oncology Group. The Clinical Trial Review Committee of the Japan Clinical Oncology GroupJpn J Clin Oncol19932342502578411739
- NoguchiSPredictive factors for response to docetaxel in human breast cancersCancer Sci200697981382016805818
- KamathKWilsonLCabralFJordanMABetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instabilityJ Biol Chem200528013129021290715695826
- MitsuhashiJTsukaharaSSuzukiRRetroviral integration site analysis and the fate of transduced clones in an MDR1 gene therapy protocol targeting metastatic breast cancerHum Gene Ther2007181089590617907967
- RestelliUCeresoliGLCroceDEconomic burden of the management of metastatic castrate-resistant prostate cancer in Italy: a cost of illness studyCancer Manag Res2017978980029263702
- de BonoJSOudardSOzgurogluMPrednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trialLancet201037697471147115420888992