Abstract
Background and purpose
The relationship between neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR) and the prognostic and clinicopathological significance in patients with esophageal squamous cell carcinoma (ESCC) has been reported by many studies. However, inconsistent results have been presented. The current study aimed to investigate the prognostic and clinicopathological role of NLR, PLR, and LMR in patients with ESCC by meta-analysis.
Methods
Eligible studies were identified in databases and the relationship between NLR/PLR/LMR and the prognosis or clinicopathological features in patients with ESCC was evaluated. OR or HR with 95% CI was calculated to estimate the risk or hazard association.
Result
Twenty-six studies including 8,586 ESCC patients were included for the analysis. We found that high NLR, PLR and low LMR were associated with poor overall survival/cancer-specific survival and event-free survival and malignant phenotypes such as deeper depth of invasion (T), positive lymph node metastasis (N), and advanced TNM stage.
Conclusion
NLR, PLR, and LMR might serve as prognostic markers in patients with ESCC.
Introduction
Esophageal cancer (EC) ranks as the eighth most common cancer and is the sixth most common cause of death from cancer worldwide.Citation1 Esophageal squamous cell carcinoma (ESCC), usually prevalent in Asia, accounts for about 90% of cases of EC.Citation2,Citation3 TNM staging is widely used as predictive models for prognosis in ESCC.Citation4 However, the prognosis is usually very heterogeneous and unpredictable even in ESCC patients with same stage or similar pathologic features. It would be of great value to identify useful complementary biomarkers to stratify ESCC patients with high risk and to improve individualized treatment.
Recent evidence suggested that a systemic inflammatory response is associated with tumor development, apoptosis inhibition, angiogenesis promotion, and damage of DNA, thus resulting in tumor progression and metastasis.Citation5 Various markers such as C-reactive protein (CRP) levels and blood neutrophil, lymphocyte, and platelet counts, either alone or expressed as ratios, might provide prognostic information on various cancers. Neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR) have recently been proposed as easily accessible inflammatory biomarkers.Citation5,Citation6 Although the prognostic role of NLR, PLR, and LMR and the prognosis of ESCC have been reported by many studies, inconsistent results have been presented. Previous meta-analyses have also investigated the prognostic role of NLR and PLR in EC. However, they also focused on one or two parameters (NLR and PLR). The prognostic role of LMR in ESCC has not been evaluated yet. What is more, after the publication of these meta-analyses, some new studies have been published. As a result, a meta-analysis is necessary to comprehensively evaluate the prognostic and clinicopathological significance of NLR, PLR, and LMR in patients with ESCC with updated evidence.
Materials and methods
Literature search
A comprehensive literature search was conducted in the databases of PubMed, EMBASE, and Web of Science up to March 31, 2018. The following terms were used to identify studies: “NLR” (“neutrophil to lymphocyte ratio,” “neutrophil-to-lymphocyte ratio,” “neutrophil–lymphocyte ratio,” “neutrophil/lymphocyte ratio”) OR “PLR” (“platelet lymphocyte ratio,” “platelet–lymphocyte ratio,” “platelet to lymphocyte ratio,” “platelet-to-lymphocyte ratio,” “platelet/lymphocyte ratio”) OR “LMR”(“lymphocyte monocyte ratio,” “lymphocyte–monocyte ratio,” “lymphocyte to monocyte ratio,” “lymphocyte-to-monocyte ratio,” “lymphocyte/monocyte ratio”) AND “ESCC” (“esophageal neoplasm,” “esophageal cancer,” “esophageal carcinoma,” “esophageal squamous cell carcinoma”).
Inclusion and exclusion criteria
The following inclusion criteria were applied to identify eligible studies that: 1) involved pathologically confirmed ESCC; 2) had full texts published in English or Chinese; 3) evaluated the relationship between pretreatment NLR/PLR/LMR and survival outcomes or clinicopathological parameters, where survival outcomes included overall survival/cancer-specific survival (OS/CSS) and event-free survival (EFS), including progression-free survival, disease-free survival, and recurrence-free survival; 4) provided sufficient information to estimate HR or OR and their 95% CIs.
The exclusion criteria included the following: 1) cell line and animal studies, case reports, letters, reviews, or meta-analyses; 2) studies in which necessary data were not provided; 3) studies that reported HRs based on continuous NLR/PLR/LMR without a clear cutoff point; 4) overlapping studies and studies with low quality.
Data extraction
Two investigators (Sun and Zhang) independently reviewed the eligible studies and extracted the following data: surname of the first author, publication year, country, ethnicity, sample size, disease stage, histology type, cutoff value, and the outcomes. All data were then examined by the two investigators independently (Sun and Zhang). Disagreements were resolved by discussion.
Quality assessment
The quality of the methodology of the included studies was assessed by the Newcastle–Ottawa scale (NOS) recommended by the Cochrane Non-Randomized Studies Methods Working Group.Citation7 Studies with five or more stars were defined as high quality studies. Quality assessment was performed by two investigators (Sun and Zhang) independently. Disagreements were resolved by discussion.
Statistical analysis
The relationship between NLR/PLR/LMR and OS/CSS or EFS was measured by the combined HRs and their 95% CIs extracted from each eligible study. The HR and its 95% CI in each eligible study were directly extracted from the report, or indirectly estimated by methods described by Tierney et al.Citation8 The combined HRs were estimated graphically by forest plots. ORs and their 95% CIs were combined to estimate the relationship between NLR/PLR/LMR and clinicopathological parameters. The overall HR/OR and its 95% CI overlap 1 were considered statistically significant and indicated a worse effect for the group with high NLR/PLR/LMR. Heterogeneity between studies was detected by the Q test and the I2 metric (no heterogeneity: I2=0%–25%; moderate heterogeneity: 25%–50%; large heterogeneity: 50%–75%; and extreme heterogeneity: 75%–100%).Citation9 If P≥0.10 in the Q test or I2<50%, the fixed effect model (Mantel–Haenszel method) was used.Citation10 Otherwise, random effect model analysis was conducted.Citation11 Subgroup analysis by different analytical methods (cutoff value, sample size, treatment, and geographic region) was performed in the analysis of OS/CSS or EFS. In addition, publication bias was assessed by the method reported by Begg and Egger.Citation12,Citation13 All P-values were two-tailed and a P-value <0.05 was considered statistically significant. Most of the statistical analyses in this study were conducted using the STATA software (version 11.2; StataCorp LP, College Station, TX, USA).
Results
Literature search and study characteristics
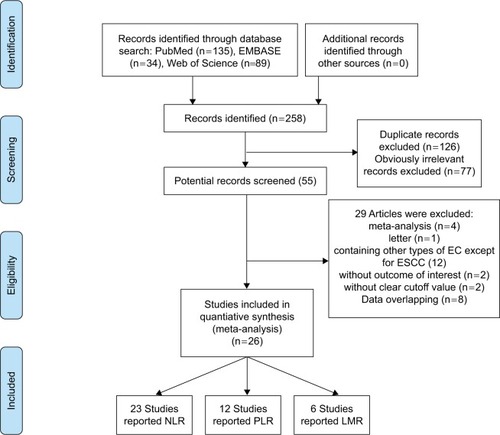
The initial search yielded 258 studies. After removing duplicate articles, 132 studies were left. On screening titles and abstracts, 77 studies were excluded because they obviously did not meet our selection criteria. The remaining 55 studies were further examined. After carefully reading the full articles, 29 studies were excluded: four meta-analysis studies, one letter, 12 studies that included adenocarcinoma or other subtypes of EC, two studies that did not provide outcomes of interest, two studies that did not provide the cutoff value of NLR/PLR/LMR, and eight studies with overlapping data. Finally, 26 studies including 8,586 ESCC patients were available for further analysisCitation14–Citation39 (). Among these, 23 studies reported NLR,Citation14,Citation15,Citation17–Citation23,Citation26–Citation39 12 studies reported PLR,Citation15,Citation18–Citation20,Citation22,Citation26,Citation27,Citation32–Citation34,Citation36,Citation39 and LMR was reported by six studies.Citation16,Citation20,Citation22,Citation24–Citation26 All the included studies were carried out in Asia (15 studies in China, 10 studies in Japan, and only one study in Korea). The sample size of 16 studies was >200,Citation14,Citation15,Citation17,Citation18,Citation20,Citation22,Citation24–Citation26,Citation29–Citation34,Citation37 and the remaining 10 studies involved <200 patients.Citation16,Citation19,Citation21,Citation23,Citation27,Citation28,Citation35,Citation36,Citation38,Citation39 Thirteen studies reported both survival and clinicopathological data,Citation15,Citation16,Citation20,Citation22–Citation26,Citation31–Citation34,Citation37 and the other 13 studies only reported survival data.Citation14,Citation17–Citation19,Citation21,Citation27–Citation30,Citation35,Citation36,Citation38,Citation39 Most studiesCitation15,Citation18–Citation34,Citation37 (n=19) involved surgery-based treatment, sixCitation14,Citation16,Citation17,Citation35,Citation36,Citation38 used chemoradiotherapy (CRT), and one study involved both treatments.Citation39 The blood cell counts used to evaluate NLR/PLR/LMR were obtained before treatment in all the included studies. The cutoff value of NLR in 23 studies ranged from 1.6 to 5; 13 studiesCitation14,Citation15,Citation17,Citation19,Citation21,Citation27,Citation30,Citation31,Citation33,Citation35,Citation36,Citation38,Citation39 had a cutoff value ≥3, and the remaining 10 studiesCitation18,Citation20,Citation22,Citation23,Citation26,Citation28,Citation29,Citation32,Citation34,Citation37 had a cutoff value <3. The cutoff value of PLR ranged from 103 to 244; five studiesCitation15,Citation19,Citation26,Citation33,Citation39 had a cutoff value ≥150, and seven studiesCitation18,Citation20,Citation22,Citation23,Citation27,Citation34,Citation36 had a cutoff value <150. Among the six studies that reported LMR, the cutoff value ranged from 2.57 to 4.02. LMR ≥3.5 was reported by three studies,Citation16,Citation20,Citation22 and the remaining three studiesCitation24–Citation26 reported LMR values <3.5. All included studies were scored high (with six stars or more). The baseline characteristics of the included studies are presented in . Details of blood testing time, treatment methods, and NOS score are presented in .
Table 1 Main characteristics of all studies included in the meta-analysis
The prognostic role of NLR in ESCC
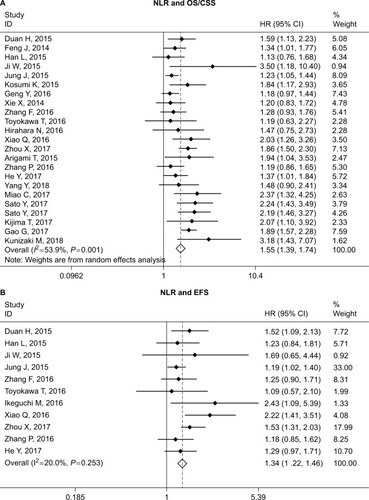
Twenty-two studies reported the relationship between NLR and OS/CSS in ESCC.Citation14,Citation15,Citation17–Citation20,Citation22,Citation23,Citation26–Citation39 The overall analysis suggested that high NLR was associated with worse OS/CSS (HR =1.54, 95% CI =1.39–1.74, P<0.001, I2=53.9%, P=0.001). Eleven studies reported the relationship between NLR and EFS in ESCC.Citation14,Citation15,Citation17–Citation19,Citation21,Citation23,Citation24,Citation26,Citation28,Citation31 Similarly, high NLR was associated with worse EFS (HR =1.34, 95% CI =1.22–1.46, P<0.001, I2=20%, P=0.25) ( and ).
Table 2 Meta-analysis results of NLR, PLR, and LMR and OS/CSS and EFS in patients with ESCC
Figure 2 Forest plot of HR for the association of NLR with OS/CSS (A) and EFS (B) in patients with ESCC.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; EFS, event-free survival; ESCC, esophageal squamous cell carcinoma; NLR, neutrophil to lymphocyte ratio.
The following clinicopathological parameters extracted from studies were collected for analysis: sex,Citation15,Citation18,Citation20,Citation22,Citation23,Citation26,Citation27,Citation31–Citation34,Citation37 tumor location,Citation15,Citation18,Citation20,Citation22,Citation23,Citation26,Citation27,Citation31–Citation34,Citation37 differentiation,Citation15,Citation18,Citation22,Citation26,Citation27,Citation31–Citation34,Citation37 depth of invasion (T),Citation18,Citation20,Citation22,Citation23,Citation26,Citation27,Citation31–Citation34 tumor length,Citation15,Citation26,Citation33 lymph node metastasis,Citation15,Citation18,Citation20,Citation22,Citation23,Citation26,Citation27,Citation31–Citation34 and TNM stage.Citation15,Citation20,Citation22,Citation23,Citation26,Citation27,Citation31,Citation32,Citation34,Citation37 We found high NLR was more common in males than in females (male vs female: OR =1.58, 95% CI =1.19–2.10, P=0.002); high NLR was associated with deeper depth of invasion (T3–T4 vs T1–T2: OR =1.95, 95% CI =1.46–2.60, P<0.001), longer tumor length (≥3 vs <3 cm: OR =2.82, 95% CI =1.67–4.77, P<0.001), positive lymph node metastasis (yes vs no: OR =1.27, 95% CI =1.03–1.57, P=0.026), and advanced stage (III–IV vs I–II: OR =1.47, 95% CI =1.28–1.69, P<0.001). No significant association was found in tumor location and differentiation ().
Table 3 Meta-analysis results of NLR, PLR, and LMR and clinicopathological parameters in patients with ESCC
The prognostic role of PLR in ESCC
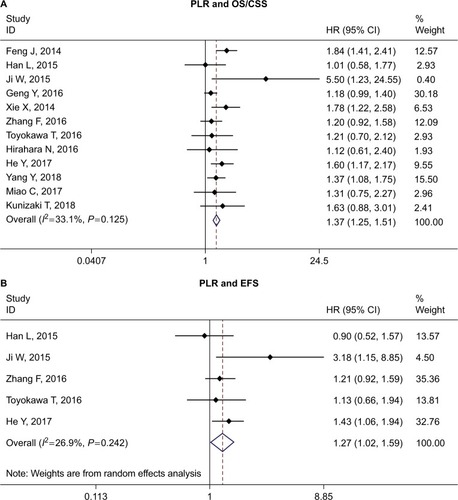
The relationship between PLR and OS/CSS was investigated by 12 studies.Citation15,Citation18–Citation20,Citation22,Citation26,Citation27,Citation32–Citation34,Citation36,Citation39 We found that ESCC patients with high PLR may have worse OS/CSS (HR =1.37, 95% CI =1.25–1.51, P<0.001, I2=33.1%, P=0.125). The pooled analysis of five studiesCitation15,Citation18,Citation19,Citation26,Citation31 also suggested high PLR was associated with worse EFS (HR =1.27, 95% CI =1.07–1.52, P=0.007, I2=26.9%, P=0.242) ( and ).
Figure 3 Forest plot of HR for the association of PLR with OS/CSS (A) and EFS (B) in patients with ESCC.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; EFS, event-free survival; ESCC, esophageal squamous cell carcinoma; PLR, platelet to lymphocyte ratio.
Eight studiesCitation15,Citation18,Citation20,Citation22,Citation26,Citation32–Citation34 reported the relationship between PLR and clinicopathological parameters. We also found that high PLR was associated with depth of invasion (T3–T4 vs T1–T2: OR =1.49, 95% CI =1.26–1.76, P>0.001), tumor length (≥3 vs <3 cm: OR =1.82, 95% CI =1.32–2.49, P<0.001), lymph node metastasis (yes vs no: OR =1.53, 95% CI =1.20–1.96, P=0.001), and stage (III–IV vs I–II: OR =1.32, 95% CI =1.12–1.57, P=0.001). No association was found in sex, tumor location, and differentiation ().
The prognostic role of LMR in ESCC
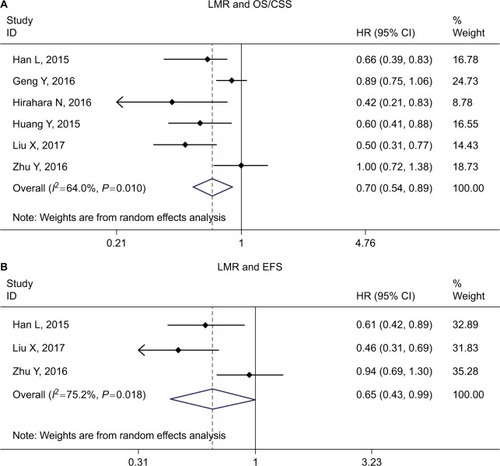
The impact of LMR on OS/CSS was evaluated by six studies,Citation16,Citation20,Citation22,Citation24–Citation26 and three studiesCitation16,Citation24,Citation26 evaluated the relationship between LMR and EFS. The combined HR for OS/CSS and EFS suggested that high LMR might be associated with better survival (HR =0.70, 95% CI =0.54–0.89, P<0.001 for OS/CSS; HR =0.65, 95% CI =0.43–0.99, P<0.001 for EFS, respectively.) ( and ).
Figure 4 Forest plot of HR for the association of LMR with OS/CSS (A) and EFS (B) in patients with ESCC.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; EFS, event-free survival; ESCC, esophageal squamous cell carcinoma; LMR, lymphocyte to monocyte ratio.
The relationship between LMR and clinicopathological parameters was evaluated by six studies.Citation16,Citation20,Citation22,Citation24–Citation26 Low LMR was associated with sex (male vs female: OR =0.61, 95% CI =0.37–1.00, P=0.049), tumor location (upper/middle vs lower: OR =1.23, 95% CI =1.03–1.46, P=0.021), depth of invasion (T3–T4 vs T1–T2: OR =0.58, 95% CI =0.48–0.69, P<0.001), lymph node metastasis (yes vs no: OR =0.68, 95% CI =0.57–0.82, P<0.001), and stage (III–IV vs I–II: OR =0.63, 95% CI =0.50–0.80, P<0.001). No association was found in tumor differentiation and tumor length ().
Subgroup analysis
Subgroup analysis was further conducted according to cutoff value (≥3 vs <3 for NLR, ≥150 vs <150 for PLR, and ≥3.5 vs <3.5 for LMR), sample size (≥200 vs <200), treatment (surgery-based vs CRT only), and geographic region (China vs non-China). The results of the subgroup analyses are presented in .
When the subgroup analysis was performed according to cutoff value, the HRs in studies with cutoff value ≥3 were 1.71 and 1.43 for OS/CSS and EFS, respectively, which were more significant than studies with cutoff value <3 (HRs were 1.41 and 1.26 for OS/CSS and EFS, respectively). This suggested that the higher the NLR, the worse the prognosis of the patient. Also, the heterogeneity in studies with cutoff value ≥3 (I2=40.1% and 0.0% for OS/CSS and EFS, respectively) was lower than studies with cutoff value <3 (I2=57.3% and 39.0% for OS/CSS and EFS, respectively). When the subgroup analysis was performed according to treatment (surgery-based vs CRT only) in OS/CSS, I2 decreased in both subgroups (I2=45.7% and 37.0% for surgery-based and CRT only studies, respectively). Treatment methods might be the source of heterogeneity. Other subgroup analyses suggested a significant association between high NLR and poor OS/CSS and EFS.
In studies exploring the prognostic role of PLR in ESCC, I2 in the overall analysis was 33.1% (P=0.125) for OS/CSS. When the subgroup analysis was performed according to cutoff value, both studies with cutoff value ≥150 and <150 showed significant association. However, the heterogeneity in each group was reduced when compared with the overall analysis (I2=12.4%, P=0.36 for cutoff value ≥150; I2=26.0%, P=0.23 for cutoff value <150). This may suggest that different cutoff values of PLR maybe one of the sources of heterogeneity.
Low LMR was associated with poor OS/CSS in all subgroup analyses. The heterogeneity in studies with sample size ≥200 was moderate (I2=49.5%, P=0.114), and no heterogeneity was observed in studies with sample size <200 (I2=0.0%, P=0.70). As a result, the high heterogeneity (I2=64.0%, P=0.016) in the overall analysis might account for the subgroup analysis by sample size (≥200 and <200).
Publication bias
Begg’s and Egger’s tests were applied for publication bias detection. Only the analysis of the effect of LMR on OS/CSS showed significant publication bias (PBegg =0.02, PEgger =0.05); other comparisons did not show any publication bias ().
Discussion
It is now generally recognized that inflammation response plays a critical role in tumor progression and may influence survival outcomes in patients with cancer. As systematic inflammatory markers, high neutrophil, platelet, and macrophage counts, low lymphocyte, and also high NLR, PLR, and low LMR have been recognized to be associated with unfavorable prognosis in solid tumors. In this meta-analysis, we comprehensively assessed the prognostic role of NLR, PLR, and LMR in ESCC by collecting the data set of 26 studies including 8,586 ESCC patients. We found that high NLR, PLR and low LMR were associated with poor survival and malignant phenotype such as deeper depth of invasion (T), positive lymph node metastasis (N), and advanced TNM stage. This preclinical and clinical research may lay the foundation for NLR, PLR, and LMR to serve as useful prognostic biomarkers and to stratify ESCC patients with high risk.
Previous meta-analyses have investigated the prognostic role of NLR and PLR in EC. Yang et alCitation40 first investigated the relationship between NLR and EC by summarizing six studies involving 1,633 patients with EC. Yodying et al analyzed seven studies including 1,540 EC patients to investigate the prognostic role of NLR and PLR in EC.Citation41 Huang et alCitation42 focused on the relationship between NLR and ESCC. Nine studies with 2,513 patients were included in their study. In a study by Zhao et al,Citation43 16 studies including 6,699 patients were utilized to investigate the prognostic role of PLR in EC patients. All these meta-analyses found that high NLR and PLR were associated with poor survival in EC. Our study showed the following advancements when compared with previous work. Firstly, our study was more comprehensive than earlier work. Prior to this, the prognostic role of NLR was mostly investigated by meta-analyses. One study evaluated both NLR and PLR;Citation41 PLR was investigated by one study.Citation43 The prognostic role of LMR had not been investigated yet. As NLR, PLR, and LMR were the mostly studied inflammatory biomarkers, for the first time we comprehensively investigated the prognostic role of the three inflammatory markers (NLR, PLR, and LMR) in ESCC. We found all these three markers to be associated with tumor progression and prognosis of ESCC patients. Secondly, the sample size in our analysis was larger than any previous meta-analysis. Twenty-six studies including 8,586 ESCC patients were available in our study, which was larger than the study by Yang et alCitation40 (1,633), Yodying et alCitation41 (1,540), Huang et alCitation42 (2,513), and Zhao et alCitation43 (6,699). To some degree, our result was more robust and reliable than previous work. Lastly, as ESCC was a major type of EC (accounted for 90% of EC cases), we only focused on the prognostic role of NLR, PLR, and LMR on ESCC. This would reduce the potential bias induced by histology type. In a nutshell, our meta-analysis is more updated and comprehensive than previous works.
As ESCC is a complicated disease, many clinical variables or biomarkers are associated with the prognosis of ESCC, for example hemoglobin, CRP, squamous cell carcinoma antigen (SCC-Ag), fibrinogen, nutritional parameters, cell-free circulating tumor DNA, and circulating noncoding RNA. NLR, PLR, and LMR were just systemic inflammatory response-related markers that affected the prognosis of ESCC. In some cases, these factors may even contradict each other. For example, all NLR, PLR and LMR are high, how to determine the prognosis of ESCC? As no studies have reported such a case, and available data are insufficient to analyze this situation, we failed to explore the result. However, to predict the prognosis of ESCC more accurately and usefully, we think it is better to combine these useful biomarkers together just as the Glasgow prognostic score, an inflammation-based score which combined albumin and C-reactive protein (CRP).
Some limitations should also be acknowledged.
Firstly, the cutoff value of NLR/PLR/LMR varied in the included studies. The criteria and method used in different institutions to determine the cutoff value were different; we could not propose an appropriate cutoff value by statistical analysis. This may affect the results and induce unavoidable potential heterogeneity and bias. It may limit the usefulness of NLR/PLR/LMR in clinical practice. Therefore, a standard and uniform cutoff value defining NLR/PLR/LMR is needed. Secondly, some factors such as age, sex, smoking history, tumor stage, comorbidities, and treatment method may affect the level of NLR/PLR/LMR,Citation44,Citation45 and also the prognosis in ESCC. However, we could not conduct a stratified analysis to assess the effects of confounding factors on the prognostic role of NLR/PLR/LMR in ESCC patients because of the limited information provided in the original studies. Lastly, publication bias was detected in the comparison of LMR in OS. Apart from this, potential publication biases may exist. Studies that failed to get published because of negative or null results could not be identified in our literature search and thus were not included in this analysis. In addition, some reports that did not provide sufficient data were also excluded from our analyses of the publications.
In conclusion, our analysis suggested high NLR, PLR and low LMR were associated with poor survival and malignant phenotype in ESCC patients. With the limitations, heterogeneities, and bias of meta-analysis, our conclusions in this study need to be interpreted with caution. Future large prospective studies with rigorously designed methodologies are warranted to confirm our results.
Supplementary material
Table S1 Details of blood testing time, treatment methods, and NOS score of included studies
References
- YangYXuHZhouLPlatelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinomaClin Chim Acta201847916016529325800
- KunizakiMTominagaTWakataKClinical significance of the C-reactive protein-to-albumin ratio for the prognosis of patients with esophageal squamous cell carcinomaMol Clin Oncol20188237037429435305
- SatoYGondaKHaradaMIncreased neutrophil-to-lymphocyte ratio is a novel marker for nutrition, inflammation and chemotherapy outcome in patients with locally advanced and metastatic esophageal squamous cell carcinomaBiomed Rep201771798428685065
- KijimaTArigamiTUchikadoYCombined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinomaCancer Sci2017108219319927889946
- GaoGDSunBWangXBWangSMNeutrophil to lymphocyte ratio as prognostic indicator for patients with esophageal squamous cell cancerInt J Biol Markers2017324409414
- MiaoCZhuSPanHCaoXYuanSHuXCombined neutrophil-platelet score and hemoglobin level predict survival in esophageal squamous cell carcinoma patients treated with chemoradiotherapyOncotarget2017850879718797929152134
- ZhouXLLiYQZhuWGNeutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapySci Rep201774258128195186
- HeYFLuoHQWangWPreoperative NLR and PLR in the middle or lower ESCC patients with radical operationEur J Cancer Care2017262
- LiuXLiMZhaoFThe lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapyOnco Targets Ther20171087187728243122
- ZhangPXiMZhaoLComparison of two inflammation-based prognostic scores in patients with thoracic esophageal cancer undergoing chemoradiotherapyInt J Clin Exp Med20169217641771
- ZhangFChenZWangPHuXGaoYHeJCombination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patientsTumour Biol20163779323933126779631
- ToyokawaTKuboNTamuraTThe pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective studyBMC Cancer20161672227599460
- HiraharaNMatsubaraTMizotaYIshibashiSTajimaYPrognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomyBMC Surg20161616627650456
- IkeguchiMKounoYKiharaKEvaluation of prognostic markers for patients with curatively resected thoracic esophageal squamous cell carcinomasMol Clin Oncol20165676777228105355
- GengYShaoYZhuDSystemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysisSci Rep201663948228000729
- XiaoQZhangBDengXThe preoperative neutrophil-to-lymphocyte ratio is a novel immune parameter for the prognosis of esophageal basaloid squamous cell carcinomaPLoS One20161112e016829927959959
- ZhuYLiMBoCPrognostic significance of the lymphocyte-to-monocyte ratio and the tumor-infiltrating lymphocyte to tumor-associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinomaCancer Immunol Immunother201766334335427915370
- HuangYFengJFLow preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinomaOnco Targets Ther2015813714525609981
- HanLHJiaYBSongQXWangJBWangNNChengYFPrognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinomaAsian Pac J Cancer Prev20151662245225025824745
- JiWHJiangYHJiYLLiBMaoWMPrechemotherapy neutrophil : lymphocyte ratio is superior to the platelet : lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapyDis Esophagus201629540341125625421
- JungJParkSYParkSJParkJPrognostic value of the neutrophil-to-lymphocyte ratio for overall and disease-free survival in patients with surgically treated esophageal squamous cell carcinomaTumour Biol20163767149715426662960
- KosumiKBabaYIshimotoTNeutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patientsSurg Today201646440541326036223
- ArigamiTOkumuraHMatsumotoMAnalysis of the Fibrinogen and Neutrophil-Lymphocyte Ratio in Esophageal Squamous Cell Carcinoma: A Promising Blood Marker of Tumor Progression and PrognosisMedicine20159442e170226496280
- DuanHZhangXWangFXPrognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinomaWorld J Gastroenterol201521185591559725987784
- XieXLuoKJHuYWangJYChenJPrognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancerDis Esophagus2016291798525410116
- FengJFHuangYChenQXPreoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinomaWorld J Surg Oncol2014125824641770
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA: A Cancer Journal for Clinicians20156528710825651787
- PennathurAGibsonMKJobeBALuketichJDOesophageal carcinomaLancet2013381986440041223374478
- LinYTotsukaYHeYEpidemiology of esophageal cancer in Japan and ChinaJ Epidemiol201323423324223629646
- RiceTWBlackstoneEHEsophageal cancer staging: past, present, and futureThorac Surg Clin201323446146924199696
- MunnLLCancer and inflammationWiley Interdiscip Rev Syst Biol Med201792e1370
- GuthrieGJCharlesKARoxburghCSHorganPGMcmillanDCClarkeSJThe systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancerCrit Rev Oncol Hematol201388121823023602134
- WellsGASheaBO’ConnellDThe Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses2014 Available from: www.ohrica/programs/clinical_epidemiology/oxford.aspAccessed April 25, 2017
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- DersimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- ZhouXLLiYQZhuWGNeutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapySci Rep201774258128195186
- HeYFLuoHQWangWPreoperative NLR and PLR in the middle or lower ESCC patients with radical operationEur J Cancer Care2017262
- LiuXLiMZhaoFThe lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapyOnco Targets Ther20171087187728243122
- ZhangPXiMZhaoLComparison of two inflammation-based prognostic scores in patients with thoracic esophageal cancer undergoing chemoradiotherapyInt J Clin Exp Med20169217641771
- ZhangFChenZWangPHuXGaoYHeJCombination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patientsTumour Biol20163779323933126779631
- ToyokawaTKuboNTamuraTThe pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective studyBMC Cancer20161672227599460
- HiraharaNMatsubaraTMizotaYIshibashiSTajimaYPrognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomyBMC Surg20161616627650456
- IkeguchiMKounoYKiharaKEvaluation of prognostic markers for patients with curatively resected thoracic esophageal squamous cell carcinomasMol Clin Oncol20165676777228105355
- GengYShaoYZhuDSystemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysisSci Rep201663948228000729
- XiaoQZhangBDengXThe preoperative neutrophil-to-lymphocyte ratio is a novel immune parameter for the prognosis of esophageal basaloid squamous cell carcinomaPLoS One20161112e016829927959959
- ZhuYLiMBoCPrognostic significance of the lymphocyte-to-monocyte ratio and the tumor-infiltrating lymphocyte to tumor-associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinomaCancer Immunol Immunother201766334335427915370
- HuangYFengJFLow preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinomaOnco Targets Ther2015813714525609981
- HanLHJiaYBSongQXWangJBWangNNChengYFPrognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinomaAsian Pac J Cancer Prev20151662245225025824745
- JiWHJiangYHJiYLLiBMaoWMPrechemotherapy neutrophil : lymphocyte ratio is superior to the platelet : lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapyDis Esophagus201629540341125625421
- JungJParkSYParkSJParkJPrognostic value of the neutrophil-to-lymphocyte ratio for overall and disease-free survival in patients with surgically treated esophageal squamous cell carcinomaTumour Biol20163767149715426662960
- KosumiKBabaYIshimotoTNeutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patientsSurg Today201646440541326036223
- ArigamiTOkumuraHMatsumotoMAnalysis of the Fibrinogen and Neutrophil-Lymphocyte Ratio in Esophageal Squamous Cell Carcinoma: A Promising Blood Marker of Tumor Progression and PrognosisMedicine20159442e170226496280
- DuanHZhangXWangFXPrognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinomaWorld J Gastroenterol201521185591559725987784
- XieXLuoKJHuYWangJYChenJPrognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancerDis Esophagus2016291798525410116
- FengJFHuangYChenQXPreoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinomaWorld J Surg Oncol2014125824641770
- YangYXuHZhouLPlatelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinomaClin Chim Acta201847916016529325800
- SatoYGondaKHaradaMIncreased neutrophil-to-lymphocyte ratio is a novel marker for nutrition, inflammation and chemotherapy outcome in patients with locally advanced and metastatic esophageal squamous cell carcinomaBiomed Rep201771798428685065
- MiaoCZhuSPanHCaoXYuanSHuXCombined neutrophil-platelet score and hemoglobin level predict survival in esophageal squamous cell carcinoma patients treated with chemoradiotherapyOncotarget2017850879718797929152134
- GaoGDSunBWangXBWangSMNeutrophil to lymphocyte ratio as prognostic indicator for patients with esophageal squamous cell cancerInt J Biol Markers2017324409414
- KijimaTArigamiTUchikadoYCombined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinomaCancer Sci2017108219319927889946
- KunizakiMTominagaTWakataKClinical significance of the C-reactive protein-to-albumin ratio for the prognosis of patients with esophageal squamous cell carcinomaMol Clin Oncol20188237037429435305
- YangXHuangYFengJFLiuJSPrognostic significance of neutrophil-to- lymphocyte ratio in esophageal cancer: a meta-analysisOnco Targets Ther2015878979425914549
- YodyingHMatsudaAMiyashitaMPrognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysisAnn Surg Oncol201623264665426416715
- HuangYSunYPengPZhuSSunWZhangPPrognostic and clinicopathologic significance of neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma: evidence from a meta-analysisOnco Targets Ther2017101165117228260931
- ZhaoQTZhangXPZhangHDuanGCPrognostic role of platelet to lymphocyte ratio in esophageal cancer: A meta-analysisOncotarget201786711208511209329340113
- GülgünMMethodology, as well as physiological and pathological conditions, can affect analysis of the lymphocyte-to-monocyte ratioRev Port Cardiol201736432328343789
- WangSXiaoTHuCAre preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio prognostic factors for patients with esophageal squamous cell cancer?Dis Esophagus201629670425833202