Abstract
Purpose
This study was designed to explore the expression levels of Galectin-3 (Gal-3) and β-catenin in serous epithelial ovarian cancer (SEOC), the linkage between their expressions, and the clinicopathological features of SEOC patients.
Patients and methods
Seventy-four SEOC patients’ specimens were detected for Gal-3 and β-Catenin expressions using immunohistochemistry, and the association between β-catenin or Gal-3 protein expressions and clinicopathological features, treatment effects, and prognosis were analyzed using SPSS 19.0. Western blot was used to analyze protein expressions of Wnt/β-catenin pathway in ovarian cancer cell lines.
Results
There was a statistically significant positive correlation between Gal-3 and β-catenin expressions in SEOC (r=0.304 and P=0.001). Gal-3 expression was related to the grade (P=0.037), clinical stage (P=0.034), platinum resistance (P=0.030), and recurrence (P=0.001) in SEOC. There was a significant correlation between β-catenin with recurrence in SEOC (P=0.035). Platinum resistance (P=0.003) and Gal-3 expression (P<0.001) were independent risk factors for poorer overall survival (OS). OS of the strongly positive Gal-3 group was significantly lower than that of the negative and weakly positive groups (log-rank test, P=0.001). OS of the positive β-catenin group was lower than that of the negative β-catenin group (log-rank test, P=0.034). Downregulating Gal-3 expression attenuated the protein expressions of Wnt/β-catenin pathway in ovarian cancer cell lines.
Conclusion
Gal-3 might activate Wnt/β-catenin signaling pathway in SEOC. Hence, Gal-3 may serve as a prognostic factor for SEOC. Targeting Gal-3 may be a promising new treatment approach for SEOC.
Introduction
Although the incidence of ovarian cancer is low, it is the most lethal tumor of the gynecologic genital tract, while serous epithelial ovarian cancer (SEOC) is the most common type of ovarian cancer. For several decades, pathologists have acknowledged that many lesions diagnosed as serous ovarian tumors are in fact of fallopian tube origin.Citation1 Every year in the USA, there are ~22,240 new ovarian cancer patients, and approximately 14,070 patients die from this disease.Citation2 Due to the lack of specific symptoms in the early stages and the absence of highly sensitive screening methods, most patients with SEOC are diagnosed at advanced stages. Cytoreductive surgery and platinum-based chemotherapy are currently the standard treatment regimens for SEOC. Although the majority of SEOC patients exhibit excellent reactivity to the existing platinum-based chemotherapy regimens, >80% of patients will experience recurrence due to platinum resistance.Citation3 Upon relapse, patients are classified according to the time since their last treatment with a platinum agent. Patients who relapse within 6 months of completion of initial platinum therapy are considered to have primary platinum resistance. These women have a poor prognosis, and response rates to subsequent lines of therapy range from 7% to 20%.Citation4 Therefore, the elucidation of the molecular mechanisms that are involved in the pathogenesis of these neoplasms may improve survival rates through better therapy.
Galectin-3 (Gal-3) belongs to the galectin family. This protein has a conserved amino acid sequence and can specifically recognize β-galactose. Gal-3 is a multifunctional protein that functions in many pathways and is involved in the development and progression of a variety of tumors. It has been shown that Gal-3 may also be associated with chemoresistance in vitro.Citation5–Citation7 In our previous studies, we reported that Gal-3 might influence the chemosensitivity of SEOC cells through the NF-κB signaling pathway.Citation8 Recently, other studies showed that Gal-3 contains binding sequences for β-catenin,Citation9 which is a key protein in the cancer stem cell–Wnt/β-catenin signaling pathway.Citation10 Therefore, the cancer-promoting mechanism of Gal-3 might be associated with the Wnt/β-catenin signaling pathway. This hypothesis was previously supported by studies on tongue squamous cell carcinoma and colon cancer.Citation11,Citation12 However, in SEOC, whether the cancer-promoting and chemoresistance mechanisms of Gal-3 are mediated by the Wnt/β-catenin signaling pathway is still unknown.
Thus, we aimed to evaluate the correlations between the clinicopathological features and Gal-3 or β-catenin expression in patients with SEOC. Moreover, we assessed the association between Gal-3 and β-catenin to preliminarily investigate the role of Gal-3 in the Wnt/β-catenin signaling pathway in SEOC.
Materials and methods
Ethical approval for the study protocol
This study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital (No. 2016-94). Written informed consent was obtained from all patients.
Human ovarian cancer cell lines and culture
SKOV3 and OVCAR3 cells were purchased from the College of Stomatology, Shanghai Jiao Tong University, and cultured respectively in DMEM and 1640 mediums (Gibco, Carlsband, CA, USA), which contained 10% fetal bovine serum (Gibco), at 37°C with 5% CO2.
Patients and tissue samples
Ninety-four paraffin-embedded, formalin-fixed specimens, which included 74 SEOC tissues and 20 tubal epithelial tissues, were obtained from the Department of Pathology, SunYat-sen Memorial Hospital between January 2008 and January 2011. Fallopian tube epithelial tissues, which served as a control, were obtained at the time of hysterosalpingo-oophorectomy for benign disease including endometrioma, myoma, abnormal uterine bleeding, or pelvic organ prolapse. All patients underwent primary maximum cytoreductive surgery followed by intravenous paclitaxel (135–175 mg/m2) plus carboplatin (AUC 5–6) or cisplatin (70 mg/m2) combination chemotherapy every 3 weeks for 6–8 cycles. Patients were divided into two groups according to their sensitivity to first-line platinum-based chemotherapy. Platinum sensitivity was defined as a platinum-free interval ≥6 months, and platinum resistance was defined as a platinum-free interval <6 months.
Immunohistochemistry
Isolated tumors were fixed in 10% neutral-buffered formalin for 48 hours and embedded in paraffin according to standard protocols. Sections (thickness, 5 µm) were deparaffinized and rehydrated in a graded series of alcohol solutions. For antigen retrieval, the slides were immersed in ethylenediamine tetra-acetic acid (1 mmol/L, pH 8.0) and boiled for 15 minutes in a microwave oven. Endogenous peroxidase activity was quenched by immersing the slides in 3% H2O2 at room temperature for 10 minutes, and nonspecific binding was blocked by incubation with 5% bovine serum albumin (Gibco) for 30 minutes. Tubal epithelial tissues were used as a negative control because most SEOC actually originate in the fallopian tube. Sections were then incubated with anti-Gal-3 (mouse anti-Gal-3 monoclonal antibody; 1:150 dilution; Abcam, Cambridge, UK) and anti-β-catenin (rabbit β-catenin monoclonal antibody; 1:100 dilution; Cell Signaling Technology, Danvers, MA, USA) antibodies overnight at 4°C. After washing in PBS, the sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Streptavidin-HRP kit, SP-9000, ZSGB Biotech, Beijing, China) at room temperature for 30 minutes. After three washes in PBS, the antibody complexes were stained with 3, 3′-diamino benzidine and then counterstained with hematoxylin. Finally, the slides were dehydrated and evaluated.
Semiquantitative method
The immunohistochemistry (IHC) evaluation of the tissues was conducted by two pathologists who assessed the number of positive cells and the intensity of staining. Positive results were determined according to a semiquantitative point system. Staining intensity was scored as follows: 0 (negative), 1 (weak), and 2 (strong). The percentage of Gal-3-positive cells was scored as follows: 0 (0%), 1 (1%–25%), 2 (26%–50%), and 3 (>50%). The staining intensity score and the percent stained score were then multiplied to obtain the total score. A total score ≥4 was considered a strong expression, a total score <4 was considered a weak expression, and no detectable staining was considered negative.Citation13 β-catenin staining was evaluated according to Maruyama’s method.Citation14,Citation15 If >10% of cancer cells were positively stained in the cytoplasm and/or nuclei, the cells were considered β-catenin positive. In contrast, cancer cells with only membranous staining were considered β-catenin negative.
Small interfering RNA
Ovarian cancer cells were transferred with scramble small interfering RNA duplexes (sense: 5′-UUCUUCGAACGUGUCACGUTT-3′; antisense: 3′-ACGUGACACGUUCGGAGAATT-3′) using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) following its protocol. Negative control cells were treated with Stealth RNAi Negative control Duplex (Invitrogen).
Western blot analyses
Cells were plated into a six-well plate and extracted into proteins using radio-immunoprecipitation assay and cocktail (Beyotime, Shanghai, China). Twenty-five microliters of protein was loaded and separated by using 8%–10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Beyotime) and transferred to polyvinylidene fluoride membranes (Beyotime). The membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 for 1 hour at room temperature. The blots were probed with the relevant primary antibodies overnight at 4°C, washed in TBST, and probed with a secondary antibody for 1 hour. All antibodies were bought from Cell Signaling Technology.
Statistical analyses
Statistical analyses were calculated using SPSS 19.0. The cross-table analysis was employed to analyze the differences in the clinicopathologic characteristics between patients with high and low Gal-3 and β-catenin expressions. Overall survival (OS) was calculated according to the Kaplan–Meier method, while the log-rank test was used for comparisons. The Cox proportional hazards regression model was used for univariate and multivariate analyses. The Spearman correlation analysis was used to analyze the relationship between Gal-3 and β-catenin expressions. A two-sided P-value ≤0.05 was considered statistically significant.
Results
Gal-3 expression in SEOC
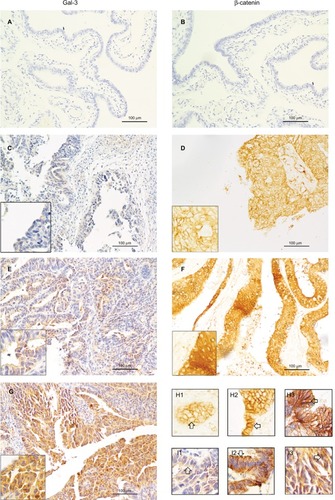
To confirm the expression of the Gal-3 protein in SEOC, we performed IHC and detected Gal-3 protein (indicated by yellow-brown granules) in the cytoplasm and nucleus (). As shown in , only 19 cases among the 74 SEOC cases were negative for Gal-3 expression (25.68%), while 55 cases exhibited positive expression (74.32%). The tubal epithelium did not express the Gal-3 protein. These results showed that Gal-3 is not expressed in normal epithelium and that its aberrant positive expression may indicate epithelial neoplasia.
Table 1 Expressions of Gal-3 and β-catenin in normal ovarian tissue and in SEOC
Figure 1 Galectin-3 and β-catenin in surgical specimens of tubal epithelium and SEOC patients. (×200).
Notes: (A) Negative expression of Gal-3 in tubal epithelium. (B) Negative expression of β-catenin in tubal epithelium. (C) Negative expression of Gal-3 in SEOC. (D) Negative expression of β-catenin in SEOC. (E) Weak positive expression of Gal-3 in SEOC. (F) Positive expression of β-catenin in SEOC. (G) Strong positive expression of Gal-3 in SEOC. (H1) β-catenin is expressed in the cell membrane in SEOC, which is considered negative expression. (H2 and 3) β-catenin is expressed in the cytoplasm and nucleus in SEOC, which is considered positive expression. Gal-3 expressed in the membrane (I1), cytoplasm (I2), and nucleus (I3) of SEOC. The arrows show the negative/positive expression cells.
Abbreviations: Gal-3, galectin-3; SEOC, serous epithelial ovarian cancer.
β-catenin expression in SEOC
To identify the expression of the β-catenin protein, IHC was performed in 74 SEOC tissue samples. As shown in , 37 cases that were negative for β-catenin expression were found among the 74 SEOC patients, which accounted for 50%. β-catenin staining in only the cell membranes represented negative β-catenin expression, while β-catenin staining in the cytoplasm and nucleus represented positive expression. The normal tubal epithelium did not express the β-catenin protein (). These results showed that β-catenin was abnormally expressed in the cytoplasm and nucleus in a portion of SEOC tissues, while β-catenin was expressed entirely in the membrane of normal epithelial tissues.
Association of Gal-3 and β-catenin with the clinicopathological features of SEOC
Interestingly, as shown in , high Gal-3 expression was found to be significantly associated with high clinical stage (P=0.034), platinum resistance (P=0.03), recurrence (P=0.001), and high histological grade (P=0.037). We also found that positive β-catenin expression was associated with recurrence (P=0.035).
Table 2 Relationship between Gal-3 and β-catenin expressions and the clinicopathological features of 74 patients with SEOC (cross-table analysis)
High expression of Gal-3 and β-catenin was associated with a worse prognosis in SEOC patients
The clinicopathological data and the prognosis of the 74 patients were analyzed using univariate variation (Cox regression) analysis. As shown in , age (P=0.057), differentiation (grade) (P=0.004), platinum resistance (P=0.054), and Gal-3 expression (P=0.003) were factors that affected the OS of SEOC patients. Further multivariate analysis showed that platinum resistance and Gal-3 expression were statistically significantly correlated with OS.
Table 3 Univariate and multivariate analyses for the OS of patients with SEOC (Cox proportional hazards regression model)
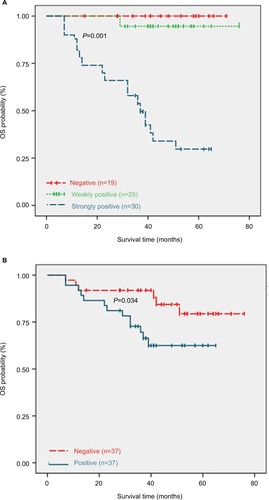
As shown in , high Gal-3 expression was significantly associated with a poor prognosis. The OS of the patients whose tumors were strongly positive for Gal-3 was significantly lower than that of the patients whose tumors were weakly positive and negative for Gal-3 (log-rank test, P=0.001). Expression of β-catenin was significantly associated with a poor prognosis (log-rank test, P=0.034). SEOC patients with positive expression of Gal-3 and β-catenin have a worse prognosis, and Gal-3 expression and platinum resistance are independent risk factors for OS.
Figure 2 Kaplan–Meier OS curves of the patients.
Notes: (A) Patients with strong positive expression of Gal-3 had a significantly poorer OS than those with weak positive and negative expressions (log-rank test, P=0.001). (B) Patients with β-catenin-positive SEOC tended to have a poorer OS (log-rank test, P=0.034).
Abbreviations: Gal-3, galectin-3; OS, overall survival; SEOC, serous epithelial ovarian cancer.
The correlation of Gal-3 and β-catenin expression in SEOC
To determine whether the expression of Gal-3 and that of β-catenin are correlated in SEOC, we performed statistical analysis using Spearman correlation analysis and found () that the expression of Gal-3 and β-catenin showed a significant positive correlation in SEOC (r=0.304 and P=0.001). The results showed that β-catenin expression was positive when Gal-3 expression was stronger. Similarly, when Gal-3 expression was weakly positive or negative, β-catenin expression decreased accordingly or was undetectable.
Table 4 The relationship between Gal-3 and β-catenin in SEOC (Spearman correlation analysis)
Downregulating Gal-3 expression attenuates the protein expressions of Wnt/β-catenin pathway in ovarian cancer cell lines
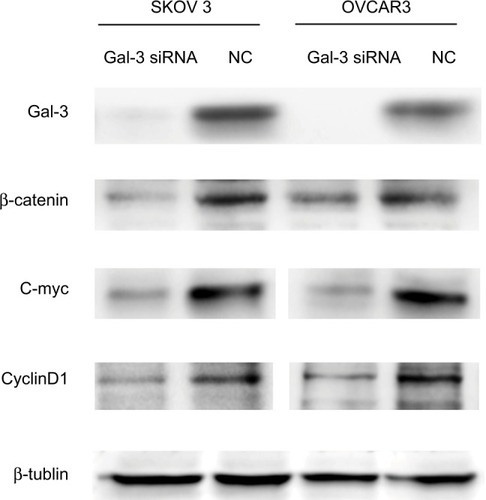
Western blot analyses were conducted after downregulation of Gal-3 in SKOV3 and OVCAR3 cells. The results showed the expression of β-catenin was decreased, and the downstream molecules of Wnt/β-catenin pathway – c-myc and cyclin D1 – were attenuated ().
Figure 3 Protein expressions of Wnt pathway molecules after downregulation of Gal-3 in ovarian cancer cell lines.
Notes: Downregulating Gal-3 expression attenuates β-catenin expression in ovarian epithelial cancer cells SKOV3 and OVCAR3; c-myc and cyclin D1 which are both downstream proteins in Wnt pathways are downregulated.
Abbreviations: Gal-3, galectin-3; NC, negative control; siRNA, small interfering RNA.
Discussion
Our study showed that Gal-3 was highly expressed in SEOC, was correlated with platinum resistance and recurrence, and was an independent risk factor for prognosis. These findings suggest that Gal-3 may be a novel predictor of platinum resistance and poor prognosis in patients with SEOC. β-catenin was also extensively expressed in SEOC and was positively correlated with Gal-3 expression. Western blot analyses on SEOC cell lines showed that Gal-3 could regulate Wnt/β-catenin pathway, which together suggests that Gal-3 might activate Wnt/β-catenin pathway in SEOC.
Gal-3 is expressed in a variety of malignant tumors, including gastric cancer, colon cancer, and liver cancer.Citation16–Citation18 Gal-3 is also highly expressed in SEOC; however, the correlation between its expression and the clinicopathological features of the disease is currently controversial. Our study showed that Gal-3 was correlated with FIGO (International Federation of Gynecology and Obstetrics) stage, grade, platinum resistance, and recurrence. The study conducted by Lee et al indicated that Gal-3 expression was correlated with tumor histology.Citation19 However, there were no differences in Gal-3 expression in comparisons among benign, borderline, and malignant mucinous and serous tumors. Brustmann showed that Gal-3 expression was not significantly correlated with the FIGO stage and grade.Citation20 Also, the expression of Gal-3 gradually decreased in ovarian serous cystadenocarcinoma, borderline serous cystadenoma, and serous cystadenoma. These two results were different from the results of our study, which might be due to the sample size. Kim et al suggested that Gal-3 was a significant independent risk factor for the prognosis of ovarian cancer.Citation21 They had performed IHC in 71 ovarian cancer patients’ tissue samples and found that there was no significant correlation between Gal-3 and chemosensitivity. But, in vitro experiments showed that downregulation of Gal-3 could enhance sensitivity to paclitaxel treatment. We believe that the contrary results are due to the nonhomogeneity of the tissue samples. The 71 samples include serous, endometrioid, and mucinous ovarian carcinoma, but it is well known that Gal-3 expresses differently in these three types of carcinomas. SEOC is the most common type of ovarian cancer, so we focused on SEOC patients’ samples and selected SEOC cell lines in our research to avoid nonhomogeneity and bias in different histological types of ovarian carcinoma.
The accumulation of β-catenin in the cytoplasm and nucleus is a common event in malignant tumors, as β-catenin is a key protein in the Wnt/β-catenin signaling pathway. Increased intracellular expression and nuclear translocation of β-catenin are indicators of activation of the Wnt/β-catenin signaling pathway.Citation22,Citation23 Bodnar et al showed that strong positive expression of β-catenin on the cell membrane was an independent risk factor for shorter progression-free survival (PFS) and poorer OS in patients with advanced epithelial ovarian cancer while the research included only 24 cases of SEOC, which in our study was 74.Citation22 Their further multivariate analysis showed that only resistance to first-line chemotherapy was an adverse independent prognostic factor for OS. In our study, the definition of “positive expression” of β-catenin was quite distinct from Bodnar’s. We took intracellular and nuclear translocation of β-catenin as “positive expression” instead of membrane expression. Lee et al studied β-catenin expressions in high-grade and low-grade SEOC, respectively, and found a statistically significant correlation between β-catenin nuclear localization and high-grade SEOC.Citation24 Due to the limited sample size, there were no significant differences of OS between patients with or without β-catenin nuclear staining. Also, they did not study the linkage between β-catenin expression and the clinicopathological features such as recurrence, chemoresistance, and stage. In our study, positive β-catenin expression (cytoplasmic and nuclear staining) was associated with recurrence. β-catenin was primarily expressed in the cytoplasm, nucleus, and cell membrane, with slight expression in the nuclear envelope, while in normal tubal epithelial cells, there was only membrane staining. All these findings suggest that the Wnt/β-catenin signaling pathway is abnormally activated in SEOC, especially in recurrent cases.
Studies showed that Gal-3 might regulate β-catenin in tongue squamous cell carcinoma, colon cancer, and thyroid cancer.Citation11,Citation12,Citation25 Nevertheless, Ahmed et al proposed a negative correlation between Gal-3 and β-catenin expressions in brain meningioma.Citation26 However, their definition of “positive staining” of β-catenin was very different from that in our study. Membranous staining was deemed negative in our study, and this may be the reason for the inconsistent results. To date, no relevant reports have been published on SEOC. For the first time, we performed a preliminary investigation into the relationship between Gal-3 and β-catenin in SEOC. Bivariate Spearman correlation analyses revealed a positive correlation between Gal-3 and β-catenin expressions (r=0.304 and P=0.001). The correlation coefficient between the two molecules was not sufficiently high, but the trend was statistically obvious and may be due to the small sample size. Downregulating Gal-3 expression in SKOV3 and OVCAR3 cells attenuated the protein expressions of Wnt/β-catenin pathway and indicated that Gal-3 may mediate Wnt/β-catenin pathway in SEOC.
We speculated that Gal-3 could cause an accumulation of β-catenin in the cytoplasm and nucleus, then Wnt/β-catenin signaling pathway was activated and its downstream molecules were upregulated. Given the widespread transcriptional activity of c-myc and its multiple oncogenic roles, Gal-3 may contribute to tumor proliferation, transformation, invasion, and metastasis.Citation27 Alteration of cyclin D1 induces tumor cell G1/S phase transition, promotes proliferation, and attenuates cell apoptosis.Citation28 Losing control of G1/S phase transition is the key step of the cancer. Kornmann et al first reported that inhibition of cyclin D1 not only suppressed cancer cell growth but also potentiated the antiproliferative effect of cisplatinum.Citation29 So, we speculated that Gal-3 could mediate resistance to first-line chemotherapy via Wnt/β-catenin pathway, which in turn resulted in recurrence and patient death.
There are no clinical tests or results on urine or serum Gal-3 levels in SEOC patients. Due to the lack of a test kit, the application of Gal-3 in clinical diagnoses is also limited. Our future research will be focused on two aspects: 1) the mechanism and interactions of Gal-3 and Wnt/β-catenin pathway molecules in vitro and in vivo and 2) serum tests of Gal-3 levels in SEOC patients and comparison with CA125, HE4 in sensitivity and specificity in diagnosis of SEOC.
Conclusion
We demonstrated that Gal-3 and β-catenin are overexpressed in SEOC, and Gal-3 is associated with platinum resistance and a poor prognosis. In addition, Gal-3 might mediate the abnormal activation of the Wnt/β-catenin signaling pathway in SEOC. Hence, Gal-3 might become an effective indicator for the prediction of recurrence and platinum resistance in SEOC, and targeting Gal-3 may be a promising new treatment approach for SEOC.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81602290) and the Science and Technology Planning Project of Guangzhou (No. 201601020102).
Disclosure
The authors report no conflicts of interest in this work.
References
- DubeauLThe cell of origin of ovarian epithelial tumoursLancet Oncol20089121191119719038766
- SiegelRLMillerKDJemalAStatistics C. Cancer statistics, 2016CA Cancer J Clin201666173026742998
- DavisATinkerAVFriedlanderM“Platinum resistant” ovarian cancer: what is it. Who to treat and how to measure benefit?Gynecol Oncol2014133362463124607285
- SlaughterKHolmanLLThomasELPrimary and acquired platinum-resistance among women with high grade serous ovarian cancerGynecol Oncol2016142222523027208536
- DumicJDabelicSFlögelMGalectin-3: an open-ended storyBiochim Biophys Acta20061760461663516478649
- OishiTItamochiHKigawaJGalectin-3 may contribute to Cisplatin resistance in clear cell carcinoma of the ovaryInt J Gynecol Cancer20071751040104617433067
- LinCIWhangEEAbramsonMAGalectin-3 regulates apoptosis and doxorubicin chemoresistance in papillary thyroid cancer cellsBiochem Biophys Res Commun2009379262663119124005
- LuHLiuYWangDGalectin-3 regulates metastatic capabilities and chemotherapy sensitivity in epithelial ovarian carcinoma via NF-κB pathwayTumour Biol2016378114691147727012551
- ShimuraTTakenakaYFukumoriTImplication of galectin-3 in Wnt signalingCancer Res20056593535353715867344
- de SousaEMeloFVermeulenLWnt signaling in cancer stem cell biologyCancers20168760
- WangLPChenSWZhuangSMLiHSongMGalectin-3 accelerates the progression of oral tongue squamous cell carcinoma via a Wnt/β-catenin-dependent pathwayPathol Oncol Res201319346147423519607
- SongSMazurekNLiuCGalectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3 beta activityCancer Res20096941343134919190323
- LiHFLiuYQShenZJDownregulation of MACC1 inhibits invasion, migration and proliferation, attenuates cisplatin resistance and induces apoptosis in tongue squamous cell carcinomaOncol Rep201533265166025421538
- MaruyamaKOchiaiAAkimotoSCytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancerOncology200059430230911096342
- LiangJZhouHPengYβ-Catenin expression negatively correlates with WIF1 and predicts poor clinical outcomes in patients with cervical cancerBiomed Res Int20162016492390327843945
- JiangSSWengDSWangQJGalectin-3 is associated with a poor prognosis in primary hepatocellular carcinomaJ Transl Med20141227325260879
- MiyazakiJHokariRKatoSIncreased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodesOncol Rep2002961307131212375039
- EndoKKohnoeSTsujitaEGalectin-3 expression is a potent prognostic marker in colorectal cancerAnticancer Res20052543117312116080575
- LeeJHZhangXShinBKLeeESKimIMac-2 binding protein and galectin-3 expression in mucinous tumours of the ovary: an annealing control primer system and immunohistochemical studyPathology200941322923319291534
- BrustmannHEpidermal growth factor receptor expression in serous ovarian carcinoma: an immunohistochemical study with galectin-3 and cyclin D1 and outcomeInt J Gynecol Pathol200827338038918580315
- KimMKSungCODoIGOverexpression of Galectin-3 and its clinical significance in ovarian carcinomaInt J Clin Oncol201116435235821327452
- BodnarLStanczakACierniakSWnt/β-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancerJ Ovarian Res201471624499657
- López-KnowlesEZardawiSJMcneilCMCytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patientsCancer Epidemiol Biomarkers Prev201019130130920056651
- LeeCMShvartsmanHDeaversMTbeta-Catenin nuclear localization is associated with grade in ovarian serous carcinomaGynecol Oncol200388336336812648588
- WeinbergerPMAdamBLGourinCGAssociation of nuclear, cytoplasmic expression of galectin-3 with beta-catenin/Wnt-pathway activation in thyroid carcinomaArch Otolaryngol Head Neck Surg2007133550351017515507
- AhmedRASheblAMHabashyHOExpression levels of β-catenin and galectin-3 in meningioma and their effect on brain invasion and recurrence: a tissue microarray studyCancer Biol Med201714331932628884048
- ZhangLZhouHLiXEya3 partners with PP2A to induce c-Myc stabilization and tumor progressionNat Commun201891104729535359
- AlvesMRE MeloNCBarros-FilhoMCDownregulation of AGR2, p21, and cyclin D and alterations in p53 function were associated with tumor progression and chemotherapy resistance in epithelial ovarian carcinomaCancer Med Epub2018529
- KornmannMDanenbergKDArberNBegerHGDanenbergPVKorcMInhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genesCancer Res199959143505351110416617
