Abstract
Background
Non-small-cell lung cancer (NSCLC) comprises about 85% of all lung cancers and is usually diagnosed at an advanced stage with poor prognosis. The IL-6/STAT3 signaling pathway plays a pivotal role in NSCLC biology. Rhein is a lipophilic anthraquinone extensively found in medicinal herbs. Emerging evidence suggests that Rhein has significant antitumor effects, supporting the potential uses of Rhein as an antitumor agent.
Methods
Cell viability and colony formation were performed to examine Rhein’s potent anti-proliferative effect in human NSCLC cell lines PC-9, H460 and A549. Flow cytometry-based assay was employed to study whether Rhein could affect cell apoptosis and cycle. The expression level of P-STAT3, apoptosis and cycle-related proteins Bcl-2, Bax, MDM2, CDC2, P53 and CyclinB1 were detected by Western blotting. The xenograft models were used to evaluate the in vivo effect of Rhein.
Results
We found that Rhein could significantly reduce the viability and stimulate apoptosis in human NSCLC cells in a dose-dependent manner. Western blot analysis results suggested that the antitumor effect of Rhein might be mediated via STAT3 inhibition. Rhein upregulated the expression of the proapoptotic protein Bax and downregulated the expression of the antiapoptotic protein Bcl-2. In addition, Rhein induced the arrest of NSCLC cells in the G2/M phase of the cell cycle and dose dependently inhibited the expression of cycle-related proteins. The Rhein also inhibited tumor growth in H460 xenograft models.
Conclusion
Rhein shows potent efficacy against NSCLC through inhibiting the STAT3 pathway. Our results also suggest that Rhein has a promising potential to be used as a novel antitumor agent for the treatment of NSCLC.
Introduction
Lung cancer is the leading cause of death from cancer worldwide and is responsible for nearly one in five cancer deaths.Citation1 Only 17.7% of all patients with lung cancer can live ≥5 years after diagnosis.Citation2 Non-small-cell lung cancer (NSCLC) represents about 85% of all lung cancers.Citation3 Up to 69% of the advanced NSCLC patients could have a potentially actionable molecular target.Citation4 However, for patients with advanced NSCLC who do not fit an approved molecular targeted therapy, the standard first-line treatment remains platinum-based doublet therapy. Although targeted drugs against epidermal growth factor receptor (EGFR) have been increasingly developed for the treatment of NSCLC, unfortunately, nearly all patients eventually have disease progression due to acquired resistance.
As an important member of the signal transducer and activator of transcription family (STAT), STAT3 is associated with malignant transformation and tumor progression.Citation5,Citation6 Constitutive activation of STAT3-meditated signal pathway plays pivotal roles in tumor cell growth, survival, apoptosis, angio-genesis and metastasis.Citation7,Citation8 Growing evidence demonstrates that constitutively activated STAT3 contributes to tumor development and progression in the majority of cancers, including breast, prostate, ovary, lung, gastric, pancreatic, melanoma and blood cancers.Citation9–Citation12 STAT3 is persistently activated in 22%–65% of NSCLC.Citation13–Citation15 Several studies suggest that the high expression of P-STAT3 is a strong predictor of poor prognosis in patients with NSCLC. Previous findings reported that the STAT3 pathway was associated with intrinsic resistance to chemotherapeutic agents in several malignancies.Citation16,Citation17 You et al showed that ionizing radiation induces phosphorylation of JAK2 and STAT3, and higher expression of STAT3 was found in the nucleus of radioresistant NSCLC cells.Citation18
STAT3 is also involved in one of the EGFR downstream pathways.Citation19 EGFR can directly phosphorylate STAT3, and activation of STAT3 has also been reported in NSCLC cell lines harboring activated EGFR mutations.Citation14,Citation20,Citation21 Studies also showed that EGFR inhibitors acting on cancer cells can activate the IL-6/JAK/STAT3 signaling pathway, thereby leading to drug resistance.Citation22,Citation23,Citation24 Although the response rate to EGFR tyrosine kinase inhibitor (TKI) iŝ80% in EGFR-mutant patients, progression-free survival is only about 1 year, as most patients eventually develop acquired resistance to the TKIs.Citation24 Several reports found that inhibition of STAT3 suppressed the growth of cancer cells and enhanced the sensitivity to antitumor agents in multiple types of cancer.Citation26,Citation27 Therefore, STAT3 has been considered a potential target for NSCLC therapy.
Currently, studies have focused on the antitumor properties of natural products because of their confirmed pharmacological properties and few side effects. Rhein is a lipophilic anthraquinone extensively found in medicinal herbs Rheum palmatum L., Cassia tora L. and so on, which have been used medicinally for >1,000 years.Citation25 Rhein has many pharmacological effects, including hepatoprotective, nephroprotective, anti-inflammatory, anticancer, antioxidant and antimicrobial activities. Although several studies have reported the mechanisms and pathways of the antitumor effect of Rhein, the direct molecular targets and specific mechanism remain unclear.Citation25 Diacerein, which is known to be completely metabolized into Rhein by humans and animals, is clinically prescribed for the treatment of osteoarthritis.Citation26 In this study, we focus on the specific molecular mechanism of action of Rhein and Diacerein that exert their antitumor effects by inhibiting STAT3.
Materials and methods
Cell culture
Human NSCLC cell lines PC-9, H460 and A549 were obtained from Shanghai Institute of Biosciences and Cell Resources Center (Chinese Academy of Sciences, Shanghai, People’s Republic of China). All the cells were cultured in Roswell Park Memorial Institute-1640 media (Thermo Fisher Scientific, Waltham, MA, USA) with 10% FBS (Thermo Fisher Scientific). Cells were cultured in a humidified cell incubator with an atmosphere of 5% CO2 at 37°C.
Antibodies and reagents
The antibodies against P-STAT3, STAT3, Bax, GAPDH, P53 and Cyclin B1 were purchased from Cell Signal Technology (Danvers, MA, USA). The antibodies against Bcl-2, MDM2, CDC2, horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). MTT and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The caspase-3 colorimetric assay kit was purchased from Abcam Co. (Cambridge, MA, USA). The Annexin V-fluo-rescein isothiocyanate (FITC) apoptosis Detection Kit I and propidium iodide (PI) were purchased from BD Pharmingen (Franklin Lakes, NJ, USA). The Dual-Luciferase Report Assay Kit was obtained from Promega Biotech Co., Ltd (Madison, WI, USA). Rhein and Diacerein were purchased from Aladdin Biochemical Technology Company (Shanghai, People’s Republic of China). The compounds used in vitro were dissolved in DMSO. A Bradford protein assay kit, polyvinylidene fluoride (PVDF) membrane and enhanced chemiluminescence kit were obtained from Bio-Rad Laboratories Inc. (Hercules, CA, USA). A protease phosphatase inhibitor mixture was obtained from Applygen Technologies (Beijing, People’s Republic of China). Acrylamide (30%), Coomassie Brilliant Blue, tetramethylethylenediamine, Tris-glycine, sodium dodecyl sulfate, prestained protein marker and nonfat dry milk were from Bio-Rad Laboratories.
MTT cytotoxicity assay
MTT assay was utilized to measure human NSCLC cells’ cytotoxicity and viability. Cells (5×103 cells/well) were plated in 96-well plates and allowed to attach overnight. After appropriate treatment, an MTT solution was added at 25 µL/well and incubated for 4 hours at 37°C. The formazan crystals were dissolved in 150 µL DMSO and the OD was measured using a microplate reader at 490 nm. The cell viability was calculated according to the following formula: viability = (average OD values of treatment wells/average OD values of vehicle control wells) ×100%. Also, the IC50 values were determined by GraphPad Pro Prism 7.0.
Colony formation assay
Human NSCLC cells (1,000 cells/well) were seeded in six-well plates at 37°C in 5% CO2 atmosphere overnight. DMSO (control), Rhein (30, 60, 100 µM) or Diacerein (30, 60, 100 µM) was added to the cells for 7 days. The culture medium was replaced with fresh medium every 2 days to keep the cells growing for 7 days. Colonies were washed with PBS, fixed with 4% paraformaldehyde at room temperature for 15 minutes, washed with purified water for 3 times and stained with crystal violet for 10 minutes. Each experiment was done in triplicate for three independent experiments.
Western blot analysis
Cancer cells were seeded in six-well plates at a density of 500,000 cells/well and then incubated overnight. DMSO (control), Rhein (30, 60, 100 µM) or Diacerein (30, 60, 100 µM) was added into the six-well plates. Treated cells were washed with PBS and harvested using ice-cold RIPA lysis buffer with 1% phenylmethanesulfonyl fluoride. Proteins were separated by 10% SDS-PAGE and transferred onto a PVDF membrane, and then the blots were blocked for 2 h at room temperature with fresh 5% nonfat milk. The blots were incubated with specific primary antibodies. After washing, the membranes were incubated with the relevant secondary antibodies; visualization of bands was by enhanced chemiluminescence.
Cell apoptosis assay
PC-9 and H460 cells were seeded into six-well plates and allowed to grow to 80% confluency in complete medium. Then the cells were treated with DMSO (control), Rhein (30, 60, 100 µM) or Diacerein (30, 60, 100 µM) for 48 hours to evaluate the effects of these compounds on apoptosis. Cells were collected, washed twice in ice-cold PBS and then resuspended in binding buffer according to the instructions of the apoptosis kit. The treated cells (as described above) were simultaneously incubated with fluorescein-labeled Annexin V and PI. Annexin V-binding buffer was then added to the mixture before fluorescence was measured on a FACSCalibur (BD Biosciences, Baltimore, MD, USA). Data were analyzed using Flowjo software.
Cell cycle assay
Cells were seeded into six-well plates for 24 hours and then treated with DMSO or Rhein (30, 60, 100 µM) for 24 hours. Cells were then labeled with PI, and the cell cycle was analyzed on a FACSCalibur.
STAT3 luciferase report assay
The STAT3 luciferase reporter plasmid (pGLSTAT3-Luc) was used to detect STAT3 activation. The H460 cells were seeded in 24-well plates 24 hours before transfection. Then, the cells were co-transfected with pGLSTAT3-Luc and pRL-TK, a plasmid encoding Renilla luciferase, using Lipofectamine 3000 (Thermo Fisher Scientific) for 6 hours. Finally, the cells were treated with the indicated concentrations of Rhein for 24 hours. Luciferase activity was assessed by SpectraMax ID3 (Molecular Devices, San Jose, CA, USA). The inhibition of STAT3 activation by Rhein was calculated as the ratio between the value of firefly and Renilla luciferase activity. Experiments were performed in triplicate.
In vivo antitumor study
The Wenzhou Medical University Animal Policy and Welfare Committee approved all mouse experiments. The H460 cells (5×106 cells were mixed with an equal volume of PBS and Matrigel in 100 µL) were implanted on the hind flank of mice (athymic nude female mice, 5–6 weeks old). Once the tumor volumes reached ~50 mm3, mice were divided into three experimental groups (six mice per group, no differences in mean body weights or tumor volumes between the groups). Tumor volume was measured as V=(L×W×W)/2, in which L and W represent length and width of tumor, respectively. Animals were sacrificed on day 10, 4 hours after the last Rhein treatment. The tumors, heart, liver, kidney and lung were removed for use in the histology and Western blot analysis.
Statistical analysis
Data were expressed as mean ± SD of three independent experiments. The statistical differences between different groups were analyzed by the Student’s t-test or one-way ANOVA in Graph-Pad Pro7.0 (GraphPad Software, Inc., La Jolla, CA, USA). P-values <0.05 were considered indicative of significance.
Results
The effect of Rhein on NSCLC cell proliferation by the MTT and colony formation assays
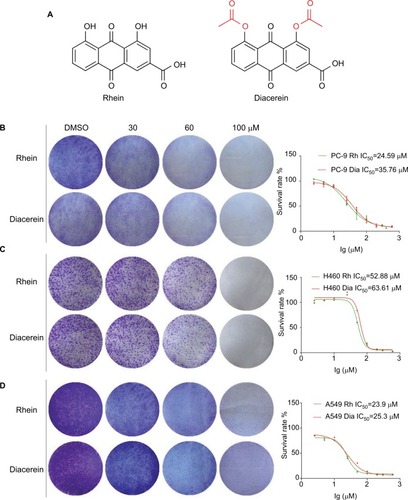
To investigate the effect of Rhein and Diacerein () on human NSCLC cell lines, cell viability was evaluated by the MTT assay. The results show that cell viability was suppressed in a dose-dependent manner in the NSCLC cell lines. For comparison, the IC50 values of Rhein and Diacerein were 24.59 and 35.76 µM in PC-9 cells (), 52.88 and 63.61 µM in H460 cells () and 23.9 µM and 25.3 µM in A549 cells (), respectively. Furthermore, compared with control cells, a dose-dependent decrease in colony formation by PC-9, H460 and A549 cells was detected in colony formation assay. These results suggest that Rhein and Diacerein significantly inhibited the growth and proliferation of human NSCLC cells, inducing cell death in a dose-dependent manner.
Figure 1 Rhein inhibits viability and colony formation of NSCLC cells.
Notes: (A) Chemical structure of Rhein and Diacerein. (B) Effects of Rhein and Diacerein inhibit cell viability and colony formation of PC-9. Cells viability: PC-9 cell lines were treated with various concentrations of Rhein and Diacerein for 48 hours, and were analyzed by MTT assay and the IC50 values were calculated. Colony formation: PC-9 cells were treated with a concentration gradient of Rhein and Diacerein for 24 hours and incubated for 1 week; then, the clones were fixed with ice-cold methanol and stained with crystal violet. (C) Effects of Rhein and Diacerein inhibit cell viability and colony formation of H460. (D) Effects of Rhein and Diacerein inhibit cell viability and colony formation of A549.
Abbreviation: NSCLC, non-small-cell lung cancer.
Rhein inhibits the STAT3 signaling pathway
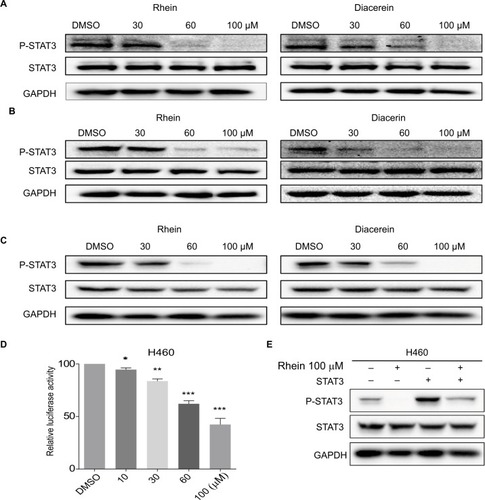
Based on the important role of STAT3 in the process of NSCLC and the structural similarity of Rhein with the four known inhibitors of STAT3 (Napabucasin, STA-21, LLL12 and LY5), we thought that Rhein may affect the phosphorylation of STAT3.Citation11,Citation21 We tested the inhibiting ability of Rhein on STAT3 phosphorylation (P-STAT3) in NSCLC cells. Not unexpectedly, Rhein and Diacerein markedly reduced the P-STAT3 protein level in H460, A549 and PC-9 cell lines. Rhein and Diacerein decreased P-STAT3 protein expression in NSCLC cells in a concentration-dependent manner after 24 hours of treatment; under the same condition, STAT3 protein expression remained unchanged (). To further confirm the STAT3 inhibitory effect, we also detected the constitutive activation of STAT3 in H460 cells using STAT3 luciferase reporter assay. In agreement with the data obtained using Western blot analysis, we found Rhein significantly blocked P-STAT3 activation in a dose-dependent manner ().
Figure 2 Western blot analysis of P-STAT3 in PC-9, A549 and H460 cells.
Notes: (A) H460 cell lines were treated with a concentration gradient of Rhein and Diacerein. The expression of P-STAT3 was found by Western blot analysis. (B) PC-9 cell lines were treated with a concentration gradient of Rhein and Diacerein. The expression of P-STAT3 was found by Western blot analysis. (C) A549 cell lines were treated with a concentration gradient of Rhein and Diacerein. The expression of P-STAT3 was found by Western blot analysis. (D) H460 cells were transfected with luciferase reporter gene plasmid and treated with Rhein for 24 hours. The results were normalized to the Renilla luciferase activity. The bars indicate the mean ± SD. Statistically significant differences (Student’s t-test), *P<0.05; **P<0.01; ***P<0.001. (E) The STAT3 plasmid was transfected into H460 cells and then treated with Rhein for 24 hours. Cells were then lysed and subjected to immunoblotting with indicated antibodies. GAPDH was used as a loading control.
Rhein induces apoptosis in human NSCLC cells
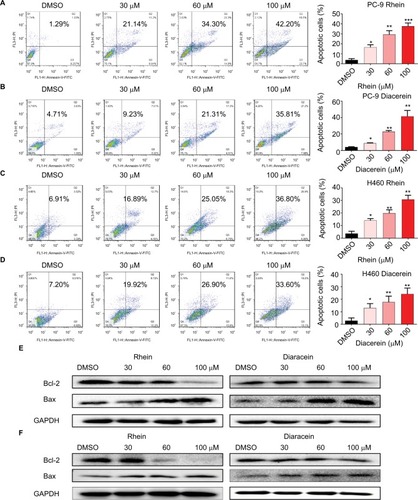
To evaluate the apoptosis-inducing effects of Rhein and Diacerein in H460 and PC-9 cell lines, the cancer cells were treated with Rhein and Diacerein for 48 hours, stained with Annexin V-FITC and PI and the apoptotic cells were evaluated by flow cytometry analysis. The data showed that Rhein and Diacerein dose dependently induced cell apoptosis in PC-9 and H460 cell lines, respectively (). In addition, Western blot analysis data further revealed that Rhein and Diacerein increased the expression of the proapoptotic protein Bax and decreased the level of the antiapoptotic protein Bcl-2 in human NSCLC cells in a dose-dependent manner (). Our results also revealed that Rhein could regulate the expression of cell death-related proteins, which blocked the process of human NSCLC cell proliferation.
Figure 3 Rhein induced apoptosis of NSCLC cells.
Notes: (A) PC-9 cell lines were treated with a concentration gradient of Rhein for 48 hours and stained with Annexin V and propidium iodide. (B) PC-9 cell lines were treated with a concentration gradient of Diacerein for 48 hours and stained with Annexin V and propidium iodide. (C) H460 cell lines were treated with a concentration gradient of Rhein for 48 hours and stained with Annexin V and propidium iodide. (D) H460 cell lines were treated with a concentration gradient of Diacerein for 48 hours and stained with Annexin V and propidium iodide. Representative histograms from flow cytometry analysis in the two human NSCLC cells treated with various concentrations of Rhein and Diacerein. Assays were performed in triplicate. *P<0.05, **P<0.01, and ***P<0.001. (E) Western blot analysis of apoptosis-related proteins. The expression of Bcl-2 and Bax in PC-9 cells. (F) The expression of Bcl-2 and Bax in H460 cells.
Abbreviations: NSCLC, non-small-cell lung cancer.
Rhein induces G2/M cell cycle arrest in human NSCLC cells
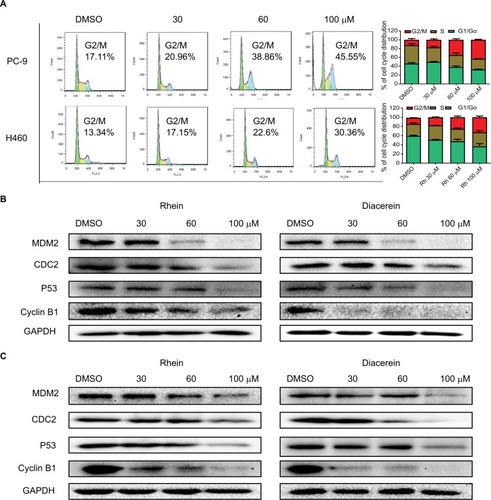
The cell cycle is a process involving a series of cellular events that lead to cell division and ultimately to proliferation. The entire cell cycle can be divided into four stages including the G1 phase, S phase, G2 phase and M phase (the division stage).Citation27 To evaluate the cell cycle arrest associated with Rhein, PC-9 and H460 cell lines were treated with Rhein for 24 hours. The percentage of Rhein-treated and untreated NSCLC cells in the G2/M phase was measured by flow cytometry analysis. Rhein significantly induced the arrest of NSCLC cells in the G2/M phase of the cell cycle compared with the untreated control group (), following reduced cell growth and increased apoptosis. The Western blot analysis indicated that treatment with Rhein and Diacerein dose dependently inhibited the expression of MDM2, Cyclin B1, CDC2 and P53 in H460 and PC-9 cells (). These results indicate that the inhibition of cell proliferation by Rhein is partly associated with the induction of G2/M phase arrest in H460 and PC-9 cells.
Figure 4 Rhein induces G2/M cell cycle arrest in human NSCLC cell lines.
Notes: (A) Induction of cell cycle arrest in PC-9 and H460 cells analyzed by flow cytometry after treatment with a concentration gradient of Rhein for 24 hours. Representative histograms from flow cytometry analysis in the two human NSCLC cells treated with various concentrations of Rhein. Assays were performed in triplicate. (B) Western blot analysis of cycle-related proteins MDM2, CDC2, Cyclin B1 and P53 in PC-9. (C) Western blot analysis of cycle-related proteins MDM2, CDC2, Cyclin B1 and P53 in H460 cells.
Abbreviation: NSCLC, non-small-cell lung cancer.
Rhein inhibits the growth of NSCLC xenograft models
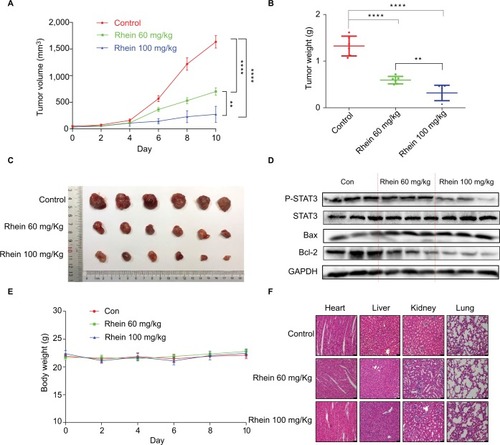
Given the potent cytotoxic activity of Rhein against human NSCLC cells, we next assessed the therapeutic efficacy of Rhein in H460 xenograft models. Mice were treated with two different doses of Rhein (60 and 100 mg/kg; intraperitoneally) every other day. Rhein led to a supra-additive reduction in growth in xenograft models (). Mechanistically, we found that STAT3 phosphorylation was inhibited in Rhein treatment groups (). In addition, Rhein treatment markedly increased apoptosis as indicated by the expressions of Bax and the Bcl-2 (). Importantly, the body weight of the mice was stable (). Moreover, Rhein treatment caused no gross toxicities on the heart, liver, kidney and lung compared with the control group (), demonstrating excellent safety profiles.
Figure 5 Antitumor activity of Rhein in H460 xenograft animal model.
Notes: (A) The result of tumor volume. (B) The tumor weight (n=6). (C) Representative images of the tumor tissue in control and treatment groups (n=6). (D) The tumor tissues were extracted and a Western blot assay was performed. (E) Mice body weight. (F) Kidneys, livers, lungs and hearts tissues from three groups were sectioned at 5 μm and the slides were stained with H&E. All images were obtained by microscope with 20× magnification. **P<0.01****P<0.0001.
Discussion
Recent studies highlight the importance of STAT3 in NSCLC through regulating tumor cell proliferation, survival, tumor invasion and angiogenesis.Citation28,Citation29 Several studies have implicated STAT3 activation in EGFR resistance.Citation22,Citation23 These suggest that persistent STAT3 may lead to primary resistance to targeted therapies. Thus, the IL-6/JAK/STAT3 signaling pathway seemed to be linked to primary resistance to treatments, including cytotoxic chemotherapy, radiotherapy and targeted therapy. However, up to now, no direct STAT3 inhibitor has been approved for clinical use.
The discovery of small molecular compounds for treating NSCLC is important to improve patient outcome. In this study, we have demonstrated that Rhein inhibited the STAT3 signaling pathway () and thereby induced apoptosis () and reduced the growth of human NSCLC cells in vitro ( and ). More importantly, mice treated with Rhein showed robust inhibition of tumor growth in vivo (). Diacerein as a prodrug of Rhein, a known drug for osteoarthritis, also showed the potential for inhibiting IL-6/STAT3 cancer signaling pathway. Consistent with our results, molecular investigations indicated that Diacerein-instigated apoptosis was associated with inhibition of IL-6/STAT3.Citation30,Citation31 Natural products with satisfactory clinical efficacy and low toxicity can be used as replacement therapies for many clinical diseases, and such correlative research is one of the hot topics in modern medicine. Notably, we found Rhein had few side effects on the mouse body at our therapeutic concentration (). Thus, the discovery of Rhein and Diacerein could speed up the development of clinical therapies for the IL-6/STAT3-dependent cancers.
Collectively, our studies demonstrate that Rhein exhibits the inhibitory potential for NSCLC growth by inhibiting STAT3 activation. Also, of course, besides potential direct usage for clinic trials, the two compounds can also serve as lead compounds for optimization to speed the development of drugs selectively targeting the IL-6/STAT3 cancer signaling pathway. Additionally, the pharmacokinetics, pharmacodynamics and toxicity of Rhein will be further comprehensively evaluated. We also plan to investigate the combination of Rhein and other clinical cancer drugs to improve the antitumor potency of Rhein and verify its effect on NSCLC.
Acknowledgments
This work was financially supported by the Medical Scientific Research Fund of Zhejiang Province (2019322308), National Key R&D Program of China (2017YFA0506000), National Natural Science Foundation of China (81622043), Zhejiang Provincial Natural Science Foundation of China (LR16H310001, LY18H160047 and LY17H160055) and Wenzhou Science and Technology Project (Y20170280). We thank Dr Huameng Li for helpful discussions and assistance in writing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- YatabeYKerrKMUtomoAEGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic surveyJ Thorac Oncol201510343844525376513
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin2012621102922237781
- HirschFRScagliottiGVMulshineJLLung cancer: current therapies and new targeted treatmentsLancet20173891006629931127574741
- YuHLeeHHerrmannABuettnerRJoveRRevisiting STAT3 signalling in cancer: new and unexpected biological functionsNat Rev Cancer2014141173674625342631
- YuHPardollDJoveRSTATs in cancer inflammation and immunity: a leading role for STAT3Nat Rev Cancer200991179880919851315
- ChunJLiRJChengMSKimYSAlantolactone selectively suppresses STAT3 activation and exhibits potent anticancer activity in MDA-MB-231 cellsCancer Lett2015357139340325434800
- DevarajanEHuangSSTAT3 as a central regulator of tumor metastasesCurr Mol Med20099562663319601811
- YuHJoveRThe STATs of cancer – new molecular targets come of ageNat Rev Cancer2004429710514964307
- ZhangTLiSLiJNatural product pectolinarigenin inhibits osteo-sarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibitionCell Death Dis2016710e242127735939
- BaiEYangLXiangYL61H46 shows potent efficacy against human pancreatic cancer through inhibiting STAT3 pathwayCancer Manag Res20181056558129606890
- ZhengHHongHZhangLNifuratel, a novel STAT3 inhibitor with potent activity against human gastric cancer cellsCancer Manag Res2017956557229138596
- CortasTEisenbergRFuPKernJPatrickLDowlatiAActivation state EGFR and STAT-3 as prognostic markers in resected non-small cell lung cancerLung Cancer200755334935517161498
- GaoSPMarkKGLeslieKMutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomasJ Clin Invest2007117123846385618060032
- HauraEBZhengZSongLCantorABeplerGActivated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancerClin Cancer Res200511238288829416322287
- BarréBVigneronAPerkinsNRoninsonIBGamelinECoqueretOThe STAT3 oncogene as a predictive marker of drug resistanceTrends Mol Med200713141117118707
- IkutaKTakemuraKKiharaMOverexpression of constitutive signal transducer and activator of transcription 3 mRNA in cisplatin-resistant human non-small cell lung cancer cellsOncol Rep200513221722215643501
- YouSLiRParkDDisruption of STAT3 by niclosamide reverses radioresistance of human lung cancerMol Cancer Ther201413360661624362463
- ZhongZWenZDarnellJEJrSTAT3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6Science1994264515595988140422
- AlvarezJVGreulichHSellersWRMeyersonMFrankDASignal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptorCancer Res20066663162316816540667
- ZhaoCLiHLinHJYangSLinJLiangGFeedback activation of STAT3 as a cancer drug-resistance mechanismTrends Pharmacol Sci2016371476126576830
- LeeHJZhuangGCaoYDuPKimHJSettlemanJDrug resistance via feedback activation of STAT3 in oncogene-addicted cancer cellsCancer Cell201426220722125065853
- Van SchaeybroeckSKalimuthoMDunnePDADAM17-dependent c-MET-STAT3 signaling mediates resistance to MEK inhibitors in KRAS mutant colorectal cancerCell Rep2014761940195524931611
- CostaDBNguyenKSChoBCEffects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinibClin Cancer Res200814217060706718981003
- ZhouYXXiaWYueWPengCRahmanKZhangHRhein: a review of pharmacological activitiesEvid Based Complement Alternat Med201520151110
- DebordPLouchahiKTodMCournotAPerretGPetitjeanOInfluence of renal function on the pharmacokinetics of diacerein after a single oral doseEur J Drug Metab Pharmacokinet199419113197957446
- JangWKimTKooJSKimSKLimDSMechanical cue-induced YAP instructs Skp2-dependent cell cycle exit and oncogenic signalingEMBO J201736172510252828673931
- JiangRJinZLiuZSunLWangLLiKCorrelation of activated STAT3 expression with clinicopathologic features in lung adenocarcinoma and squamous cell carcinomaMol Diagn Ther201115634735222208386
- XuYHLuSXA meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancerEur J Surg Oncol201440331131724332948
- BhartiRDeyGBanerjeeISomatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapyCancer Lett201738829230228025102
- BhartiRDeyGOjhaPKDiacerein-mediated inhibition of IL-6/IL-6R signaling induces apoptotic effects on breast cancerOncogene201635303965397526616855
