Abstract
Background
The prognosis of massive hepatocellular carcinomas (MHCCs; ≥10 cm) remains worse.
Purpose
The aim of this study was to evaluate the clinical benefits of transcatheter arterial chemoembolization (TACE) or TACE combined with percutaneous microwave coagulation therapy (PMCT) and the long-term survival rate of MHCC patients treated with these techniques.
Patients and methods
A retrospective study was performed using data involving 102 MHCC patients admitted to the Second Hospital of Nanjing from September 2010 to August 2015. The median interval between treatments and overall survival (OS) was hierarchically analyzed using log-rank tests. Multivariate analysis was done using Cox regression model analysis.
Results
The median survival time of MHCC patients was 3 months (range, 1–10 months) in the palliative group, 3 months (range, 1–39 months) in the TACE group, and 7.5 months (range, 3–30 months) in the TACE–PMCT group (P=0.038). The 6-, 12-, and 18-month OS rates for MHCC patients were 15%, 0%, and 0% in the palliative group, 30%, 25.63%, and 17.97% in the TACE group, and 50%, 41.67%, and 16.67% in the TACE–PMCT group, respectively (P=0.0467). In addition, TACE sessions had positive correlation with the survival time of MHCC patients (rho = 0.462, P<0.001). TACE treatment more than three times (HR =0.145, P<0.001) was an independent predictor of the survival of MHCC patients, which was identified by the Cox regression model analysis.
Conclusions
These results indicated that TACE–PMCT treatment in MHCC patients had advantages in prolonging OS and improving liver function. Multiple TACE treatments might be a suitable treatment for the MHCC patients.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Hepatocellular carcinoma (HCC) is one of the five most common causes of cancer-associated death worldwide. It is also an aggressive malignancy with poor prognosis.Citation1 First of all, surgical resection is an effective treatment for a solitary lesion without vascular invasion and with sufficient liver function reserve in HCC patients.Citation2 However, due to large tumor lesions, main blood vessels, including the portal vein, the hepatic artery and the vena cava are often infiltrated in patients with massive HCC (MHCC).Citation3,Citation4 In addition, most MHCC patients suffer from cirrhosis or liver dysfunction, which may also lead to difficulties in surgical intervention.Citation5 Even if surgical intervention is performed, MHCC patients might still have poor prognosis.Citation6–Citation8 Second, due to large tumor lesions and poor radiation tolerance of normal liver tissue, the curative effect of radiotherapy is also limited in MHCC patients.Citation9 Third, although capecitabine plus oxaliplatin regimenCitation10 and gemcitabine plus oxaliplatin regimenCitation11 could be safely administered with close monitoring and have moderate antitumor activity in patients with advanced HCC, they remain to be further investigated in MHCC patients.Citation12 In summary, although the abovementioned treatment is limited in MHCC patients, it is necessary to further explore the appropriate regimen therapy to prolong the survival time of MHCC patients and improve their quality of life.
Previous studies have demonstrated that interventional treatments such as transcatheter arterial chemoembolization (TACE) monotherapy or combined therapies could improve unresectable HCC patient prognosis.Citation13–Citation16 In addition, TACE is recommended as the standard of care for unresectable HCC at Barcelona Clinic Liver Cancer (BCLC) stage A–B.Citation17,Citation18 Percutaneous microwave coagulation therapy (PMCT) is a minimally invasive technique. PMCT produces high temperature by electrodes inserted into tumor tissue, which can lead to rapid coagulation and necrosis of tumor tissue, so as to achieve the goal of eliminating tumor.Citation5,Citation20 This method gradually became one of the most important treatments for HCC.Citation21,Citation22 Importantly, TACE could reduce the cooling effect of hepatic blood flow on microwave thermal coagulation by blocking the tumor vascular bed.Citation23 Therefore, TACE is expected to play a vital role in promoting tumor damage and improving the ability of PMCT to kill the tumor tissue in situ. To sum up, in theory, the therapeutic effect of TACE combined with PMCT on MHCC is better than that of TACE alone. However, in clinical practice, TACE combined with PMCT could prolong survival time and improve prognosis of MHCC patients. To the best of our knowledge, the benefits of TACE combined with PMCT for MHCC patients have not been well explored. In addition, the effect of TACE sessions on the prognosis of MHCC was unclear. Therefore, by comparing the efficacy and safety of TACE–PMCT treatment with TACE monotherapy in MHCC patients, the therapeutic regimen and TACE sessions suitable for MHCC patients will be elucidated in the present study. Importantly, treatment programs for 102 MHCC patients were determined through the BCLC proposal and patients’ informed consent.Citation24,Citation25 There fore, to reach the purpose of the abovementioned study, our study attempted to explore the predictive factors for MHCC patients through comprehensive retrospective analysis of medical history, imaging features, and laboratory results.
Patients and methods
Patient data
Patients
According to the inclusion and exclusion criteria of this study, 102 patients were enrolled including 84 males (82.4%) and 18 females (17.6%), aged 24–78 years, with a mean age of 52.45±11.15 years.
The inclusion criteria for the study population were as follows: 1) patients who were diagnosed with HCC according to the standards for the diagnosis and treatment of primary liver cancer established by the Ministry of Health of the People’s Republic of China by contrast imaging (computed tomography [CT] or magnetic resonance imaging [MRI] or contrast-enhanced ultrasonography [CEUS], and hepatic angiography) with positive tumor markers in combination with liver cirrhosis or tissue biopsy;Citation26 2) patients who had MHCC with the largest diameter tumor of at least 10 cm (mHCC); 3) patients who had no history of hepatic encephalopathy above third degree or ascites refractory to diuretics; 4) patients who had controlled diabetes mellitus and hypertension using medication; and 5) patients who had no multiple organ failure and no severe underlying diseases.
Patients were excluded from this study if they: 1) received other treatments in another hospital; 2) had missing data; 3) were not tracked adequately; 4) underwent liver transplantation and received previous hepatic interventional or surgical treatments; 5) had diffuse-type HCC; 6) previously received any other local invasive therapies, such as radio frequency ablation (RFA), 125I seed implantation, or percutaneous ethanol injection (PEI); and 7) had another type of malignant tumor.
Collection of data and primary end point assessment
Clinical data were collected from each patient at the time of MHCC diagnosis including gender, age, other chronic diseases (eg, hypertension and type 2 diabetes mellitus [T2DM]), Child–Pugh grade, and BCLC stage. Imaging features were also collected including tumor size, tumor number, cirrhosis, portal vein tumor thrombus (PVTT), intra-hepatic metastasis (IM), and extrahepatic metastasis (EM). All laboratory indicators were collected in the week before surgery. Laboratory results determined the alpha-fetoprotein (AFP), hepatitis B surface antigen (HbsAg), and hepatitis C virus antibody (HCV-Ab) levels. The main end point was survival time, which was defined as the duration from the time of primary treatment for MHCC to death or to August 2016, whichever was earlier. The secondary end point was outcomes during follow-up, including survival and death.
Treatments and follow-up
Eighty-one patients were initially treated with TACE. Hepatic artery angiography was performed using the Seldinger technique. TACE was performed using the following procedures. After using 5-French catheter selection to perform arteriography of the superior mesenteric, celiac, and common hepatic arteries, the hepatic artery was catheterized with a coaxial microcatheter. After the microcatheter was positioned into or as close as possible to the tumor-feeding branch, an emulsion of doxorubicin hydrochloride (Adriamycin) and iodized oil (lipiodol; Guerbet, Aulnay-sous-Bois, France) was slowly infused through the catheter. Oily TACE was performed as selectively as possible, and a microcatheter was routinely used. The doses of iodized oil and doxorubicin were determined according to the tumor size and tumor vascularity. The maximum doses of iodized oil and doxorubicin for a single session of TACE were 25 mL and 40 mg, respectively. Infusion of the lipiodol mixture was followed by particulate embolization with 300–500 µm-diameter Embosphere Microspheres (Merit Medical Systems, Inc., Rockland, MA, USA).
PMCT was initiated 1–3 weeks after TACE. PMCT was performed using the KY-2000 microwave therapy instrument (HengDa Electronic Co., Ltd., Xuzhou, China). The PMCT procedure was performed by an experienced hepatobiliary surgeon after local anesthesia using 2% lidocaine. The entire procedure was guided and constantly monitored using real-time ultrasound (MyLabTwice; Esaote Co., Ltd., Genoa, Italy). After anesthesia was achieved, a 15 cm 16 G electrode needle was inserted into the center of the nodule, and coagulation therapy was performed at 2,450 MHz with 60–80 W output for 8–20 minutes per ablation. The ablation was performed repeatedly until the tumors attained complete necrosis as monitored by real-time ultrasound, and the hyperechoic area overlapped the area of the tumor with a surrounding ≥1 cm safety margin. To relieve pain in the patients, dolantin (100 mg) was injected intramuscularly 5 minutes before the PMCT.
After treatment with TACE and PMCT, liver protection, anti-inflammatory, and sedation therapies were prescribed. A follow-up study by repeat CT (plain and enhanced) and serum AFP-level measurement was conducted once every 1–2 months.
Statistical analyses
The Kruskal–Wallis test was performed to analyze continuous variables, and the results were expressed as mean ± SD for normal distribution and M (Q1–Q3) for skewed distribution. For categorical variables, the chi-squared test and Fisher’s exact test were utilized. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Univariate analysis was performed by the Cox regression model. Multivariate analysis was carried out using the Cox proportional hazard model to generate adjusted HRs and 95% CIs. Any value of P<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 22.0 software (IBM Corporation, Armonk, NY, USA).
Ethical approval
All procedures in the current study were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki. The study was approved by the ethics committee of the Second Hospital of Nanjing, and the written informed consent for participation was obtained. This study had no influence on the subsequent management of patients.
Results
General condition
Eighty-one patients received 197 TACE treatments (mean, 2.4; range, 1–14) and 22 PMCT sessions (mean, 1.8; range, 1–5). In the TACE group, 69 patients received 166 TACE treatments (average, 2.4; range, 1–14). In the TACE–PMCT group, 12 patients received 31 TACE treatments (mean, 2.5; range, 1–7) and 22 PMCT sessions (mean, 1.8; range, 1–5). The median follow-up time was 41 months (range, 6–96 months).
The sample was predominantly males (82.4%), full grown adults with a long period of hepatitis B virus (HBV) infection (84.3%). Most patients (60.47%) received antiviral therapy. In the present study, the majority of the patients had cirrhosis (82.4%) and 48 patients (47.1%) had AFP levels more than 1,000 ng/mL. Most patients (63.7%) had Child–Pugh B functional status, whereas 30 patients (29.4%) and seven patients (6.9%) had Child–Pugh A and C functional status, respectively. Most patients (68.6%) had tumors classified as BCLC stage C, while 29 patients (28.4%) and three patients (2.9%) had tumors classified as BCLC stage B and D, respectively. The mean number of tumors was 2.31±0.94 (range 1–3), the mean tumor length was 12.94±2.53 cm (range 10–23.2 cm), and the mean tumor width was 10.33±2.29 cm (range 3.5–19.1 cm). In these 102 patients with MHCC, the detailed proportion of all patients with complications or other chronic diseases is summarized in .
Table 1 Characteristics of patients, tumor, complications, and treatment procedures
General characteristics of subjects in the three groups
According to the BCLC proposal and the patients’ informed consent, a total of 102 patients were included in this study. Twenty-one patients were treated with palliative treatment. Sixty-nine patients were treated with TACE alone, and 12 patients were treated with TACE–PMCT. The demographic and clinicopathological characteristics of the three groups are summarized in . The mean survival time of MHCC patients in the TACE–PMCT group was significantly longer than the other two groups, but the incidence of hydrothorax in TACE–PMCT was higher than the other two groups (P=0.038, P=0.022). Other laboratory and imaging parameters were not significantly different among the three groups including AFP levels, tumor length, tumor width, cirrhosis, PVTT, IM, EM, and complications except for hydrothorax (all of them, P>0.05). Gender, Child–Pugh grade, BCLC stage, antiviral therapy timing, hypertension, and T2DM were also not significantly different among the three groups (all of them, P>0.05).
Table 2 General characteristics of subjects in the three groups
Relationship between survival time and local invasive treatment strategies
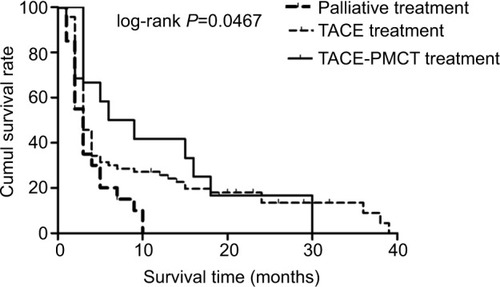
There were no fatal treatment-related complications for MHCC patients. This fact indicates that the local invasive treatments for MHCC patients receiving TACE or PMCT were safe in the short term. The median survival time was 3 months (range, 1–10 months) in the palliative group, 3 months (range, 1–39 months) in the TACE group, and 7.5 months (range, 3–30 months) in the TACE–PMCT group. The results showed that there were significant differences in the survival time of MHCC patients among the three groups (P=0.038). In addition, shows that the 6-, 12-, and 18-month survival rates for MHCC patients were 15%, 0%, and 0% in the palliative group, 30%, 25.63%, and 17.97% in the TACE group, and 50%, 41.67%, and 16.67% in the TACE–PMCT group, respectively. These results indicate significant differences among the three groups in OS rate.
Figure 1 The Kaplan–Meier survival curves for patients with MHCCs: significantly better survival of MHCC patients undergoing treatment with TACE plus PMCT (solid line) compared to MHCC patients undergoing TACE treatment (dashed line) and palliative treatment (bold dashed line).
Abbreviations: cumul, cumulative; MHCC, massive hepatocellular carcinoma; PMCT, percutaneous microwave coagulation therapy; TACE, transcatheter arterial chemoembolization.
In addition, there were 53 patients with PVTT. Of these patients, eleven patients received palliative treatment, 36 patients received TACE treatment, and six patients received TACE–PMCT treatment. The median survival time of these patients with PVTT was 2 months (range, 2–5 months) in the palliative group, 3 months (range, 2–13.5 months) in the TACE group, 6 months (range, 3–21 months) in the TACE– PMCT group. Although this result did not reach statistical significance (P=0.180), a trend was suggested that MHCC patients with PVTT receiving TACE–PMCT treatment had a longer survival time. Therefore, TACE–PMCT treatment was effective and safe for MHCC patients, even for those patients with PVTT.
Clinical features of MHCC patients based on the number of TACE treatments
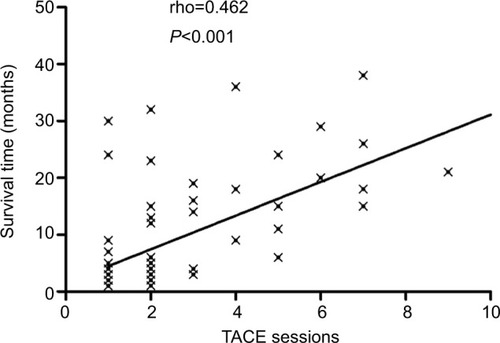
According to previous studies, TACE with lipiodol and gelatin sponge is highly effective for MHCC.Citation23,Citation27 However, it has not been confirmed whether the TACE treatments could impact on the survival time and OS rate in MHCC patients. In this study, the proportion of patients receiving TACE treatment was the highest, including the TACE group and TACE–PMCT group. shows that the number of TACE treatments was positively correlated with the survival time of MHCC patients in the TACE group and TACE–PMCT group. Next, TACE sessions were divided into two groups: TACE treatments more than three times group (n=66) and TACE treatments less than or equal to three times group (n=15). Then, we further studied the factors affecting the prognosis of MHCC.
Figure 2 Correlation analysis of MHCC patients: MHCC patients with multiple TACE sessions had longer survival time than single TACE treatment.
Note: Statistical analysis was performed with Spearman’s correlation analysis. Abbreviations: MHCC, massive hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization.
The data showed that the median survival time of MHCC patients was 3 months (range, 1–32 months) in the TACE treatments less than or equal to three times group and 20 months (range, 6–39) in the TACE treatments more than three times group. Therefore, patients in the TACE treatments more than three times group had significantly longer survival time than those in the TACE treatments less than or equal to three times group. Likewise, the data showed that HBV-DNA levels in the TACE treatments more than three times group were higher than those in the TACE treatments less than or equal to three times group (P=0.04). However, other parameters including HBsAg, AFP levels, Child–Pugh grade, BCLC stage, IM, EM, complications, and comorbidities had no significant differences between the two groups. Moreover, there were also no significant differences in patients’ age, gender, tumor numbers and size, and cirrhosis between the two groups. The detailed comparison of demographic and clinical characteristics of patients based on TACE sessions is summarized in .
Table 3 Demographic and clinical characteristics of patients according to TACE sessions
To sum up, the survival time of MHCC patients in the TACE treatments more than three times group was longer than those in the TACE treatments less than or equal to three times group. In addition, HBV-DNA levels of MHCC patients in the TACE treatments more than three times group were higher than those in the TACE treatments less than or equal to three times group. Therefore, we further studied the risk factors associated with the survival of MHCC.
Risk factors associated with the survival of MHCC by subgroup analyses
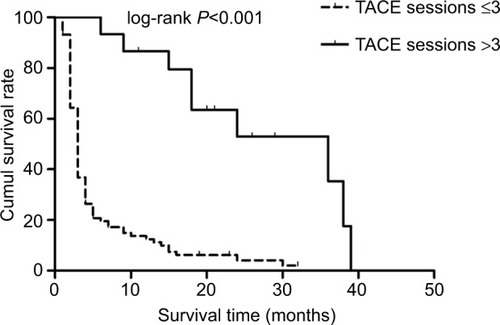
The Kaplan–Meier survival analyses were used to analyze the cumulative survival rate among the subgroups. shows that the 6-, 12-, and 18-month cumulative survival rates for MHCC patients treated with the TACE–PMCT were higher than those treated with TACE and palliative treatment. In addition, shows that the 6-, 12-, and 18-month cumulative survival rates for MHCC patients who received TACE treatments more than three times were higher than those who received TACE treatments less than or equal to three times (93.33%, 79.44%, 63.56% vs 19.54%, 12.45%, 6.23%, log-rank P<0.001).
Figure 3 The Kaplan–Meier survival curves for MHCC patients: significantly better survival of MHCC patients who received TACE treatments more than three times (solid line) compared to MHCC patients who received TACE treatments less than or equal to three times (dashed line).
Abbreviations: cumul, cumulative; MHCC, massive hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization.
Results of univariate survival analysis demonstrated that TACE treatments more than three times were a prognostic predictor. TACE treatments more than three times were associated with better OS rate. Other prognostic variables are presented in . Of these, non-spontaneous peritonitis, non-pulmonary metastasis, non-hepatic encephalopathy, TACE–PMCT vs palliative treatment, and TACE vs palliative treatment were related to better OS rate.
To eliminate the confounding factors, Cox proportional hazard model was used to evaluate the risk factors for the survival of MHCC patients. Variables included in the analysis were local invasive treatment, the number of TACE treatments, spontaneous peritonitis, pulmonary metastasis, and hepatic encephalopathy. summarizes that TACE treatments more than three times were independent protective factors for the survival of MHCC patients, namely palliative treatment and TACE treatments less than or equal to three times were risk factors for the survival of MHCC patients.
Table 4 Results of univariate and multivariate analyses in 102 HCC patients
Discussion
HCC is a frequent cancer that is among the leading causes of cancer deaths worldwide.Citation28 Most cases were detected until tumor progressed to large mass or to multiple lesions, at which point surgery was no longer a suitable treatment option.Citation29 MHCCs were defined as those with a maximum tumor diameter of ≥10 cm.Citation3,Citation30 For patients with large, unresectable tumor lesions, TACE was recommended as the suitable therapeutic option.Citation12 However, most of the prognosis was unsatisfactory.Citation9,Citation12,Citation13,Citation19 The mechanism of PMCT for HCC is based on the heating effect of microwaves and the sensitivity of the tumor to the heating action,Citation31 but the “heat sinking” effect limited the range of PMCT.Citation23 TACE could alleviate the adverse cooling effect induced by abundant blood flow on microwave thermal coagulation by blocking the tumor vascular bed.Citation20,Citation23 Seki et alCitation32 reported that the combination of TACE and PMCT might result in higher rates of necrosis and longer survival times compared to treatment with TACE or PMCT alone and improve the prognosis of HCC patients. Up to date, there are limited studies on the efficacy and safety of MHCC patients treated with TACE plus PMCT. In the present study, we mainly compared the effects of TACE–PMCT treatment with TACE treatment on the prognosis of MHCC patients.
It is well known that HCC possesses an abundant blood supply, especially in MHCC patients. TACE can obstruct the artery supply of the tumor through the hepatic artery. The iodine oil can fill up the portal vein surrounding the tumor and decrease the blood flow volume of the portal vein.Citation15–Citation18 PMCT produces high temperature by electrodes inserted into tumor tissue, which can lead to rapid coagulation and necrosis of tumor tissue, so as to achieve the goal of eliminating tumor.Citation5,Citation20 In addition, TACE can lead to ischemia and inflammatory edema of the tumor tissue, which could accelerate tumor necrosis and enhance the coagulation effect of microwaves.Citation16 This provides a theoretical basis for the combination of TACE and PMCT treatment for MHCC patients. In the present study, it was found that there were no fatal treatment-related complications for patients. It is indicated that MHCC patients who received local invasive treatments were safe in the short term. In addition, survival time of MHCC patients treated with TACE–PMCT was significantly longer than those treated with TACE and palliative. Importantly, the 6-, 12-, and 18-month OS rates for MHCC patients in the TACE–PMCT group were also higher than those in the TACE group and palliative group. Therefore, TACE–PMCT treatment was a safe and preferable treatment. MHCC patients receiving TACE–PMCT treatment could significantly prolong the survival time and improve the OS rates.
According to some studies, the complete necrosis rate of TACE only reaches 10–20% for HCC patients.Citation32 TACE can reduce tumor volume, induce tumor necrosis, and prevent tumor dissemination. However, a large area of chemoembolization may cause liver function impairment and even liver function failure.Citation12,Citation32 Therefore, multiple TACE procedures might be appropriate. By analyzing the survival time and the OS rate, it was found that MHCC patients who received TACE treatments more than three times had longer survival time and better OS rate than those who received TACE treatments less than or equal to three times. Herein, the larger tumor size could be controlled by repeated TACE treatment, and liver function could keep stable. Likewise, IMs or multicenter lesions could be controlled more easily by repeated PMCT in the absence of tumor-feeding vessels caused by the repeated TACE treatment. Therefore, liver dysfunction was decreased by the combination of TACE and PMCT treatment or repeated TACE treatment, making this therapy preferable to radical treatments.
Serum AFP levels were an important tumor biomarker of HCC. A few decades ago, Nomura et alCitation33 and Tangkijvanich et alCitation34 suggested that serum AFP levels could be used as an indicator to assess the clinical features and prognosis of HCC. Then, Choi et alCitation35 further demonstrated that serum AFP levels prior to RFA were important predictors of long-term outcomes in HCC. Afterward, Carr et alCitation36 reported that elevated AFP levels are associated with worse survival of patients with large tumors. More recent studies by Blank et alCitation37 and Terentiev and MoldogazievaCitation38 reported that preoperative serum AFP was an independent predictive factor among HBV–HCC patients following surgical resection. However, in our study, higher preoperative AFP level was not identified as an independent risk factor for the survival of MHCC patients. The reason for the different results may be due to different treatment modalities and the setting of AFP subgroup boundaries. In our study, serum AFP levels more than 1,000 ng/mL accounted for over a half of total MHCC patients. In addition, it is well known that the survival time of MHCC patients was significantly shorter than HCC patients with small or large tumor lesions. Therefore, the massive volume of tumor lesions accounts for crucial role in the prognosis of MHCC patients.
In general, MHCC patients treated with PMCT had mild adverse reactions. During and after treatment, local pain was common and tolerable. Subcapsular hematoma, internal bleeding, biliary duct damage, and so on were infrequent. However, hydrothorax and peritonitis were common complications caused by PMCT.Citation20 In this study, the higher incidence of hydrothorax in the TACE–PMCT group was found compared to other two groups, but hydrothorax can be resolved by applying albumin and diuretic. In addition, pulmonary metastasis, peritonitis, and hepatic encephalopathy were associated with the prognosis of MHCC patients by univariate analysis. However, it was confirmed that the tumor characteristics, complications, IM, EM, and comorbidities were not associated with the prognosis of MHCC patients by multivariate analysis in this study. These results were different to other previous studies on other types of HCC.Citation5,Citation39–Citation43 These results have not been reported in MHCC patients. The main reason might be associated with the features of MHCC including larger tumor volume, higher incidence of PVTT, and metastases.Citation3,Citation30
Limitations
Although some valuable and important conclusions have been obtained, there are still some limitations in this study. The first limitation of the present study was its retrospective nature. The data were collected retrospectively from the patient’s information system. However, there was one among-group difference in baseline characteristics, including the presence of hydrothorax. Moreover, due to this retrospective nature, a mild complication, for example, pain and low-grade fever, that did not need further treatment or was successfully treated with painkillers or physical hypothermia, may not have been documented in the patient’s information system and was therefore not traceable in a retrospective manner. The second limitation was that the selection of treatment modality inevitably depended not only on medical but also on nonmedical and/or economic considerations. However, no significant differences were found in the baseline characteristics among the three groups. Moreover, the patients in the three groups were treated strictly according to the study protocol. The third limitation was that the inclusion period of this study had a wide range from 2010 to 2015. In this period, diagnostic and treatment protocols might have been changed due to new insights and developments. However, subsequent treatments of the three groups were performed according to the same multidisciplinary treatment protocol by the same team.
Conclusion
TACE following PMCT is a safe and efficient treatment strategy in MHCC patients. In addition, multiple TACE treatments should be considered as an alternative treatment therapies for the unresectable MHCC. However, the results of this retrospective study need to amplify the sample to identify the benefits from TACE–PMCT treatments and be validated by prospective clinical trial.
Acknowledgments
This study was partially supported by grants from the Science and Technology Commission of Nanjing (no. 201605033 to Wei Ye), the Project of Six Talent Peaks of Jiangsu Province (no. WSN-177 to Wei Ye), the Project of Jiangsu Provincial Medical Youth Talent (Wei Ye), Nanjing Medical Science and Technology Development Foundation (no. YKK-17173 to Wei Ye), Medical Science and Technology Development Foundation, and Nanjing Department of Health (no. YKK-16192 to Lili Wang).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- WangXWangZWuLCombined measurements of tumor number and size helps estimate the outcome of resection of Barcelona clinic liver cancer stage B hepatocellular carcinomaBMC Surg2016162227094483
- AitkenKLHawkinsMAThe role of radiotherapy and chemora-diation in the management of primary liver tumoursClin Oncol2014269569580
- CarrBIGuerraVFeatures of massive hepatocellular carcinomasEur J Gastroenterol Hepatol201426110110823863262
- VautheyJNLauwersGYEsnaolaNFSimplified staging for hepatocellular carcinomaJ Clin Oncol20022061527153611896101
- KleinJDawsonLAHepatocellular carcinoma radiation therapy: review of evidence and future opportunitiesInt J Radiat Oncol Biol Phys2013871223223219567
- CarrBIGuerraVFeatures of massive hepatocellular carcinomasEur J Gastroenterol Hepatol201426110110823863262
- ChangYJChungKPChangYJChenLJLong-term survival of patients undergoing liver resection for very large hepatocellular carcinomasBr J Surg2016103111513152027550624
- GiulianteFde RoseAMGuerraVArditoFNuzzoGCarrBIClinical characteristics and survival of European patients with resectable large hepatocellular carcinomasJ Gastrointest Cancer201344332933523912605
- ZengZCTangZYFanJA comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinomaCancer J200410530731615530260
- BoigeVRaoulJLPignonJPMulticentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trialBr J Cancer200797786286717876335
- ZhuAXBlaszkowskyLSRyanDPPhase II study of gem-citabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinomaJ Clin Oncol200624121898190316622265
- LencioniRChemoembolization in patients with hepatocellular carcinomaLiver Cancer201211415024159570
- MurataSMineTSugiharaFInterventional treatment for unresectable hepatocellular carcinomaWorld J Gastroenterol20142037134531346525309076
- RayCEBrownACGreenTJSurvival outcomes in patients with advanced hepatocellular carcinoma treated with drug-eluting bead chemoembolizationAm J Roentgenol2015204244044725615768
- de StefanoGIodiceVSignorielloGScognamiglioUFarellaNP1325: Efficacy and safety of combined sequential treatment with RFA and sorafenib in patients with HCC in intermediate stage ineligible for TACE: A prospective randomized open studyJ Hepatol201562S852S852
- VeltriAMorettoPDoriguzziAPaganoECarraraGGandiniGRadiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC)Eur Radiol200616366166916228211
- KadalayilLBeniniRPallanLA simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancerAnn Oncol201324102565257023857958
- CilloUVitaleAGrigolettoFProspective validation of the Barcelona Clinic Liver Cancer staging systemJ Hepatol200644472373116488051
- LiapiEGeschwindJFChemoembolization for primary and metastatic liver cancerCancer J201016215616220404613
- SunHNiJJiangXThe effect of lipiodol deposition in HCC after TACE on the necrosis range of PMCTOnco Targets Ther2017103835384228814882
- SekiTWakabayashiMNakagawaTUltrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinomaCancer19947438178258039109
- YinXYXieXYLuMDPercutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factorsCancer200911591914192319241423
- XuLFSunHLChenYTLarge primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapyJ Gastroenterol Hepatol201328345646323216261
- Hernández-GuerraMHernández-CambaATurnesJApplication of the Barcelona Clinic Liver Cancer therapeutic strategy and impact on survivalUnited European Gastroenterol J201533284293
- PrajapatiHJKimHSTreatment algorithm based on the multivariate survival analyses in patients with advanced hepatocellular carcinoma treated with trans-arterial chemoembolizationPLoS One2017122e017075028170405
- Ministry of Health of the People’s Republic of CUpdated standards for the diagnosis and treatment of primary liver cancerZhonghua Gan Zang Bing Za Zhi201220419426 Chinese23230592
- XieLLSunCJLiXDWangYHWangCEArterial embolization of massive hepatocellular carcinoma with lipiodol and gelatin spongeIndian J Cancer201551Suppl 24951
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- FattovichGGiustinaGDegosFMorbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patientsGastroenterology199711224634729024300
- CarrBIGuerraVPancoskaPThrombocytopenia in relation to tumor size in patients with hepatocellular carcinomaOncology201283633934523006937
- SekiTWakabayashiMNakagawaTUltrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinomaCancer19947438178258039109
- SekiTTamaiTNakagawaTCombination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinomaCancer20008961245125111002219
- NomuraFOhnishiKTanabeYClinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patientsCancer1989648170017072477133
- TangkijvanichPAnukulkarnkusolNSuwangoolPClinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levelsJ Clin Gastroenterol200031430230811129271
- ChoiDLimHKRhimHPercutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factorsAnn Surg Oncol20071482319232917522947
- CarrBIGuerraVGianniniEGLow alpha-fetoprotein HCC and the role of GGTPInt J Biol Markers2014294395402
- BlankSWangQFielMIAssessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normalAnn Surg Oncol201421398699424232510
- TerentievAAMoldogazievaNTAlpha-fetoprotein: a renaissanceTumour Biol20133442075209123765762
- BholeeAKPengKZhouZRadiofrequency ablation combined with transarterial chemoembolization versus hepatectomy for patients with hepatocellular carcinoma within Milan criteria: a retrospective case-control studyClin Transl Oncol201719784485228070766
- ChoiYHChungJWSonKRNovel intraarterial therapy for liver cancer using ethylbromopyruvate dissolved in an iodized oilAcad Radiol201118447147821237678
- PaulSBGamanagattiSRGuptaAKTumor size determines the outcome of trans-arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (HCC): ongoing work at a tertiary care centre in IndiaJ Gastroen Hepatol201025A100A100
- NhuQKnowlesHPockrosPFrenetteCAn Unexpected Pulmonary Complication Following TACE With Low-Dose Doxorubicin-Eluting Beads and Small-Volume Lipiodol for a Small HCCAm J Gastroen-terol2015110S345S346
- GoelAFidelmanNYaoFRobertsJTerraultNPre-Transplant Transarterial Chemoembolization (TACE) for Hepatocellular Carcinoma (HCC) and Risk of Hepatic Artery Complications (HA-C) in Liver Transplant (LT) RecipientsLiver Transplant201218S256S256
