Abstract
Background
The objective of this study was to evaluate the efficacy of a combination of gemcitabine and docetaxel (GD) as a second-line treatment for elderly patients with metastatic urothelial carcinoma (mUC).
Patients and methods
A total of 122 patients with mUC who were previously treated with platinum-based chemotherapy received second-line GD therapy from July 2010 to June 2016. This consisted of 800 mg/m2 gemcitabine and 40 mg/m2 docetaxel on days 1 and 8 in each 21-day cycle. Using pooled cumulative data, we divided patients into the following three groups based on age: <65 years (Group A), from 65 to 74 years (Group B), and ≥75 years (Group C), and then the data were retrospectively analyzed. All patients were evaluated for treatment-related toxicities and assessed at every cycle by imaging studies. Kaplan–Meier curves were used for survival and recurrence analyses. Furthermore, potential prognostic factors for progression-free survival (PFS) and overall survival (OS) were assessed via univariate and multivariate Cox regression analyses.
Results
The median follow-up period was 8.2 months (range: 2.1–100). The median number of treatment cycles was three (range: 1–16) in Group A, three (1–15) in Group B, and two (1–11) in Group C. The objective response rate was not significantly different between the three groups. In addition, PFS and OS from the start of second-line GD therapy were also not significantly different. According to univariate and multivariate analyses of the second-line GD-treated cohort, a good performance status was the only prognostic factor for PFS and OS. In Group C, myelosuppression including predominant neutropenia and anemia, fatigue, and nausea were the main common adverse events. However, the incidence of neutropenia and a reduction in platelets were not significantly different between the three groups. Treatment-related deaths did not occur in this study.
Conclusion
In this study, GD combination therapy as a second-line treatment for mUC resulted in favorable tumor responses and few treatment-related toxicities, even among elderly patients.
Introduction
Due to the aging of the population in industrialized nations, the percentage of the Japanese population aged ≥65 years is projected to increase from 22.1% in 2008 to 30.3% in 2025 as outlined in Japanese Government statistics. After smoking, age is an important risk factor in the development of bladder cancer.Citation1,Citation2 Therefore, as the population ages, the incidence of urothelial carcinoma (UC) of the urinary bladder is expected to increase in the coming years, and elderly patients will compose a larger number of metastatic UC (mUC) cases at diagnosis.
The gold standard for the treatment of patients with mUC is systemic cisplatin-based chemotherapy. A combination regimen of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) has been used for the past 2 decades.Citation3 A more recent alternative standard treatment for mUC is combination chemotherapy with gemcitabine and cisplatin (GC).Citation4,Citation5 However, long-term survival rates were deemed disappointing in long-term follow-up studies. Therefore, the rescue of patients who develop relapse after first-line cisplatin-based chemotherapy is an important issue.
There is no standard second-line treatment for mUC outside the United States, where atezolizumab, an anti-PDL1 antibody, was approved by the US Food and Drug Administration (FDA) in May 2016 for patients with prior first-line platinum-based chemotherapy.Citation6,Citation7 The use of gemcitabine and docetaxel (GD) combination therapy as a second-line treatment for patients with mUC after failure of first-line platinum-based chemotherapy has been reported in our previous study.Citation8 However, the efficacy of second-line therapy for elderly patients with mUC is unclear.
Several studies have described how elderly patients may be less tolerant to chemotherapy because of comorbidities and hematologic toxicities.Citation9,Citation10 Furthermore, elderly patients with mUC are underrepresented in clinical trials,Citation11–Citation13 and data regarding outcomes and adverse events (AEs) for second-line therapy are limited for this age group. Therefore, in this study, we retrospectively examined the effectiveness of second-line GD therapy for elderly patients with mUC by analyses of our pooled cumulative data.
Patients and methods
Patients
We enrolled eligible patients with histologically confirmed mUC of the urinary bladder or upper urinary tract and who were admitted to Nagoya City University Hospital and four affiliated institutions between July 2010 and June 2016. Patients had previously been surgically treated or had undergone biopsies of their primary lesions, and staging had been performed by enhanced computed tomography (CT). Patients diagnosed with mUC had also undergone more than one cycle of chemotherapy with GC (1,000 mg/m2 gemcitabine on days 1, 8, and 15 and 70 mg/m2 cisplatin on day 2) or gemcitabine and carboplatin (1,000 mg/m2 gemcitabine on days 1 and 8, and carboplatin area under the curve 4–5 mg/mL per minute on day 1), which was completed a minimum of 4 weeks prior to enrollment. Gemcitabine in these first-line regimens was not administered on days 8 and 15 if grade 3 toxicities occurred. In the study period, 124 patients underwent first-line chemotherapy, of whom, two patients, aged 71 and 78 years, discontinued the treatment because of a poor performance status caused by chemotherapy or cancer progression. Therefore, in total, we studied 122 patients who received second-line GD therapy. Using pooled cumulative data, we divided patients into the following three groups based on age: <64 years (Group A: n=45), from 65 to 74 years (Group B: n=56), and ≥75 years (Group C: n=21). Data were retrospectively analyzed. All patients were evaluated for the presence of any toxicity and were assessed at every cycle by imaging studies by enhanced CT. Patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 1 or lower as per World Health Organization (WHO) criteria: an adequate bone marrow reserve (white blood cell [WBC] count >3,500/µL, platelet count >100,000/ µL, and hemoglobin >10 g/dL). Other requirements included reasonable hepatic function (serum bilirubin ≤1.5 mg/dL) and an estimated life expectancy of ≥12 weeks. Prognostic comorbidity was estimated using the Charlson comorbidity index (CCI).Citation14 Ineligible patients included those with non-malignant systemic diseases such as an active infection that precluded them from receiving therapy or those with any clinically significant cardiac arrhythmia and/or congestive heart failure. All patients provided written informed consent prior to this clinical trial. The institutional chemotherapy review boards (ethical committees) of Nagoya City University Hospital and Nagoya City University (#1152) approved this study, which was conducted in accordance with the Declaration of Helsinki (according to the 2004 Tokyo revision).
Treatment schedule
The 122 selected patients were given an intravenous infusion of gemcitabine (800 mg/m2) for 30 minutes and docetaxel (40 mg/m2) for 60 minutes on days 1 and 8 according to the study by Dreicer et al.Citation15 This cycle was repeated every 21 days. Dexamethasone (6.6 mg administered intravenously for 30 minutes) was used as a premedication for docetaxel. The same GD doses were administered on days 1 and 8 of each cycle if patients displayed WBC and platelet counts of >3,000 and >75,000 µL/mL, respectively; treatment was discontinued in each cycle if the counts were lower. When grade 3 AEs occurred, a 10% dose reduction was performed in the next cycle, and GD treatment was continued until progression. The efficacy of the GD regimen as second-line chemotherapy was assessed in a follow-up analysis. Anti-emetics and analgesics for AEs were given as supportive care to patients.
Treatment evaluation
The estimated glomerular filtration rate (eGFR) was measured and calculated prior to each chemotherapy course, and hematological status and serum chemistries were measured twice a week during treatment. Radiology was used to assess tumor sizes, and physical examinations were also conducted. After each chemotherapy cycle, tumor sizes were remeasured. At least 4 weeks after the administration of chemotherapy, each patient’s response to treatment was evaluated. The cutoff for the relative dose intensity (RDI) in first-line chemotherapy was 90% in accordance with a previous study.Citation16 Death from the start of second-line therapy was the end point for the measurement of overall survival (OS), and progression-free survival (PFS) rates, and the time to failure from the start of second-line therapy was measured until the discontinuation of treatment, death, or progression. The Response Evaluation Criteria in Solid Tumors guidelines, version 1.1, were used to classify responses.Citation17 The National Cancer Institute Common Terminology Criteria for AEs, version 3.0,Citation18 were used to classify AEs. Tumor response, PFS, and OS were considered as end points of this study.
Statistics
Differences in categorical parameters were assessed using a Fisher’s exact test, ANOVA test, Tukey’s post hoc methods, Kruskal–Wallis test, and a chi-squared test, whichever was appropriate. Cumulative rates were estimated using the Kaplan–Meier method, and the significance of differences between curves was tested by the log-rank test. Univariate and multivariate analyses were conducted using the Cox proportional hazard regression model. A P value of <0.05 was considered statistically significant. All data were analyzed using EZR software (Saitama Medical Center, Jichi Medical University, Yakushiji, Japan).
Results
Treatment responses and outcomes of second-line GD chemotherapy
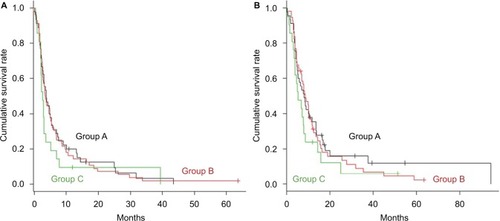
lists patients’ clinical characteristics. A statistical significance was not found between the three groups except for the median eGFR and RDI for first-line chemotherapy; a high ratio for the carboplatin-based regimen in Group C reflected the degradation of the eGFR. Patient’s responses were assessed after they underwent one or more chemotherapy courses and are described in and . The CCI score for Group C was significantly higher compared to those for Groups A and B. Second-line GD chemotherapy was performed for a median of three cycles (range: 1–16) for Group A, three cycles (range: 1–15) for Group B, and two cycles (range: 1–11) for Group C; the three groups did not show any significant differences. The objective response rate (ORR% of complete response [CR]+partial response [PR] cases per total cases) after second-line GD treatment was 22.2% for Group A, 14.3% for Group B, and 14.3% for Group C. The relative response rate (% of PR and stable disease [SD] cases per total cases) was 46.7% for Group A, 55.4% for Group B, and 38.1% for Group C. The median PFS from the start of second-line GD therapy was 3.7 months (95% CI: 2.6–5.3), 3.5 months (95% CI: 2.5–5.0), and 2.6 months (95% CI: 2.0–3.0) for Groups A, B, and C, respectively. The median response duration in PR cases for Group C was 5.4 months (range: 2.4–42.2). The median OS from the start of second- line GD therapy was 8.1 months (95% CI: 5.0–11.7), 8.5 months (95% CI: 6.8–10.3), and 5.3 months (95% CI: 3.4–7.9) for Groups A, B, and C, respectively. The survival rate after 1 year of follow-up from the start of second-line GD therapy was 30.2%, 29.0%, and 17.9% for Groups A, B, and C, respectively (). The median OS for the GD regimen from the start of first-line chemotherapy was 17.7 months (95% CI: 12.4–24.7), 17.5 months (95% CI: 14.4–19.5), and 12.7 months (95% CI: 9.3–18.2) for Groups A, B, and C, respectively. Significant differences in survival periods between the three groups were not found. The median follow-up period was 8.2 months (range: 2.1–100).
Table 1 Patients’ characteristics and oncological outcomes in first-line chemotherapy in the three age groups
Table 2 Patients’ characteristics and oncological outcomes in second-line chemotherapy in the three age groups
Figure 1 Kaplan–Meier curves of (A) progression-free survival and (B) overall survival in metastatic urothelial cancer patients after failure of first-line cisplatin-based chemotherapy.
Note: The 122 eligible patients who received second-line GD therapy were divided into the following three groups based on their age: <64 years (Group A: n=45), from 65 to 74 years (Group B: n=56), and ≥75 years (Group C: n=21).
Abbreviation: GD, gemcitabine and docetaxel.
Univariate and multivariate analyses for prognostic factors
Baseline parameters for the whole cohort were analyzed by univariate and multivariate analyses to elucidate predictive factors for PFS () and OS (). We found that ECOG-PS 0 at the start of second-line GD therapy was the only prognostic factor for both PFS (95% CI: 2.55–6.08, HR: 3.94 and 95% CI: 2.49–6.00, HR: 3.86, respectively) and OS (95% CI: 2.48–5.84, HR: 3.80 and 95% CI: 2.34–5.68, HR: 3.64, respectively) from the start of second-line treatment.
Table 3 Univariate and multivariate analyses of baseline parameters to find prognostic factors for progression-free survival in 122 patients treated with second-line GD chemotherapy
Table 4 Univariate and multivariate analyses of baseline parameters to find prognostic factors for overall survival in 122 patients treated with second-line GD chemotherapy
AEs in second-line GD chemotherapy
lists all AEs associated with second-line GD therapy in the three groups. Severe hematologic AEs that were the most frequently observed included decreased WBC (42.2% for Group A, 32.1% for Group B, and 42.9% for Group C) and neutropenia (42.2% for Group A, 35.7% for Group B, and 33.3% for Group C), followed by decreased platelets (24.4% for Group A, 30.4% for Group B, and 38.1% for Group C). Significant differences in the occurrence of these AEs between the three groups were not observed. However, the occurrence of anemia in Group C was found to be significantly increased when compared with Group A (19.0% and 6.7%, respectively, P<0.05). The frequencies of non-hematologic AEs, including grade 1–2 fatigue and nausea, were significantly higher in Group C compared with those of Groups A and B. However, grade 2 alopecia showed a significantly higher incidence in Group A compared with Groups B and C. Deaths related to treatment were not noted.
Table 5 Adverse events in 122 patients treated with second-line GD chemotherapy
Discussion
With industrial countries experiencing rapid increases in aging populations, a critical emerging issue is the proper treatment of increasing numbers of elderly patients with cancer. The WHO criterion for elderly patients is those ≥65 years of age. The median age of patients with mUC treated in clinical trials has consistently been between 61 and 64 years.Citation19–Citation21 However, the median age at the time of diagnosis of UC is >70 years, witĥ4% of the patients showing metastatic disease.Citation2 Thus, elderly patients with mUC are underrepresented in clinical trials. This underrepresentation has led to uncertainties in the expected level of tolerability of chemotherapy and outcomes following second-line chemotherapy. In this study, we therefore divided our patient cohort into three age groups to examine the efficacy of second-line GD chemotherapy between the groups. To date, only a few limited reports have described second-line chemotherapy in elderly patients with mUC. Noteworthy among these, Salah et alCitation22 pooled the individual data of elderly patients with mUC (over 70 years of age) from 10 studies for the assessment of second-line chemotherapy regimens and demonstrated that combination chemotherapy was associated with greater toxicity without any improvement in OS. However, a report that evaluated the effectiveness of a single regimen of second-line chemotherapy between elderly and younger patients with mUC is lacking. Our pooled data analyses of the response rate and prognostic outcomes of second-line GD chemotherapy for patients with mUC over 75 years of age showed that the ORR and median OS from the start of second-line therapy were 14.3% and 5.3 months (95% CI: 3.4–7.9), respectively, which were similar to the results found in younger patients.
In this study, gemcitabine was consistently used in two consecutive regimens as it was the main focus of our strategy of sequential chemotherapy. Recently, mechanisms of acquisition of chemoresistance to gemcitabine in urothelial cancer cells have been outlined.Citation23,Citation24 However, gemcitabine shows a synergistic effect when combined with different chemotherapeutic agents, and several studies have consistently used gemcitabine in sequential therapy,Citation25,Citation26 as in our chemotherapeutic strategies for mUC. As a result, GD therapy after the failure of gemcitabine and platinum-based combination therapy showed good anti-tumor effects: the total ORR waŝ20% and the median OS was 7.6 months for the entire cohort receiving second-line GD therapy. So far, the most extensively studied second-line combination chemotherapy regimen is gemcitabine and paclitaxel (GP). However, like other treatments, GP regimen has shown variable results. Our results are in line with recent reports demonstrating that in patients treated with a second-line combination regimen including GP regimen for mUC, the ORR was 8.6%–41.7% and median OS was 4.8–12.4 months.Citation8,Citation19,Citation21,Citation27–Citation31 Compared with these results, the oncological outcomes of our study in elderly patients were encouraging with regard to a durable response rate and survival, therefore, supporting the use of second-line GD therapy in elderly patients with a comorbidity.
It is well known that elderly patients may be less tolerant to chemotherapy because of comorbidities as well as decreased organ and hematologic functions; therefore, special attention must be paid to the occurrence of AEs. For example, in lung cancer, although the response rates of older and younger patients were comparable (80% vs 88%, respectively; P=0.11), severe hematologic toxicity, defined as grade 4–5, was significantly greater in elderly patients (84% vs 61%, respectively; P<0.01) following chemoradiation with a cisplatin and etoposide regimen.Citation32 In patients with UC who received gemcitabine plus cisplatin in a neoadjuvant setting, Jan et alCitation33 described that anemia, neutropenia, and thrombocytopenia were the main reasons for a dose reduction, with 7 out of 28 patients aged ≥65 years discontinuing chemotherapy because of decreased tolerability. In this study, the frequencies of grade 3–4 anemia as well as grade 1–2 fatigue and nausea were significantly higher in the ≥75-year-old group; however, they soon recovered after a transfusion and dose reduction. Furthermore, the GD regimen was well tolerated and could be safely used in patients who were previously treated for impaired renal function. In fact, patients with UC often have impaired renal function due to advanced age, prior platinum-containing chemotherapy, prior nephrectomy, and/or disease-related hydronephrosis. Despite the small patient sample of this study, in addition to an oncological outcome, our results show that second-line GD therapy may be a feasible and tolerable option for elderly patients even when renal function is insufficient and they have a comorbidity.
Nowadays, five immune checkpoint inhibitors have been approved by the FDA for the treatment of mUC in a second-line setting after platinum-based chemotherapy: atezolizumab,Citation6,Citation34 durvalumab,Citation35 avelumab,Citation36 nivolumab,Citation37 and pembrolizumab.Citation38 These showed an ORR of 15%–21.1% and median OS of ~10 months. Approximately 10% of severe treatment-related AEs were directly linked to an early immune response within a month after the initiation of treatment despite the similar characteristics of study populations, with a similar median age of 67 years. In general, these new immunotherapies are well tolerated and effective in elderly patients with mUC; however, data are, as yet, insufficient to reach any firm conclusion regarding treatment outcomes. Another important issue is how to select the best predictive markers associated with a clinical response in order to choose appropriate immunotherapies or chemotherapies because of the limitation of the response rate as outlined earlier. Therefore, based on this study, a prospective randomized trial as second-line therapy in patients with mUC comparing a GD regimen and pembrolizumab after first-line platinum-based chemotherapy was recently initiated (UMIN ID: R000037420). It is hoped that the results of this trial will be reported in due course.
In this study, several clinical parameters were measured in conjunction with PFS and OS, such as age, gender, eGFR, RDI, ECOG-PS, and the presence of visceral metastasis. Both univariate and multivariate analyses demonstrated that only ECOG-PS 0 at the start point of second-line GD therapy was a strong prognostic factor for PFS and OS. Thus, our findings suggest that rather than discontinuing chemotherapy entirely among those who are unable to tolerate full-dose therapy, elderly patients with mUC aged ≥75 years with a good ECOG-PS can still safely and adequately be treated by continuing with second-line GD chemotherapy and reducing doses. Bearing in mind the safety and other advantages of using second- line GD chemotherapy observed in this study, as well as its cost-effectiveness compared with the use of checkpoint inhibitors, further prospective trials, including this study, with the addition of biomarkers to help select patients are warranted to evaluate this strategic chemotherapeutic approach for elderly patients with mUC.
Several limitations were evident in this study. First, the patient cohort used was relatively small, and second, the study itself was undertaken in a retrospective manner. In spite of this, for patients with mUC previously treated with platinum- based therapy, combination therapy with GD appears to be well tolerated and shows activity against disease even in selected elderly patients ≥75 years of age.
Conclusion
This study concludes that for patients with mUC previously treated with platinum-based chemotherapy, combination therapy with GD appears to be well tolerated and shows activity against disease even in elderly patients.
Acknowledgments
The authors wish to state that they did not receive any financial support for this study.
Author contributions
Taku Naiki made critical revisions of the manuscript. Toshiki Etani, Takashi Nagai, Yutaro Tanaka, RyosUke Ando, Shuzo Hamamoto, Rika Banno, Daisuke Nagata, Noriyasu Kawai, and Yosuke Sugiyama carried out the acquisition of data and coordinated and helped to draft the manuscript. Keitaro Iida conducted statistical analyses concerning this study. Takahiro Yasui supervised this manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShariatSFSfakianosJPDrollerMJKarakiewiczPIMerynSBochnerBHThe effect of age and gender on bladder cancer: a critical review of the literatureBJU Int2010105330030819912200
- SpiessPEAgarwalNBangsRBladder Cancer, Version 5. 2017, NCCN Clinical Practice Guidelines in OncologyJ Natl Compr Canc Netw201715101240126728982750
- SternbergCNde MulderPSchornagelJHSeven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumoursEur J Cancer2006421505416330205
- von der MaaseHHansenSWRobertsJTGemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III studyJ Clin Oncol200018173068307711001674
- von der MaaseHSengelovLRobertsJTLong-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancerJ Clin Oncol200523214602460816034041
- Perez-GraciaJLLoriotYRosenbergJEAtezolizumab in Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: Outcomes by Prior Number of RegimensEur Urol Epub20171219
- PowlesTDuranIvan der HeijdenMSAtezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trialLancet20183911012274875729268948
- NaikiTKawaiNHashimotoYGemcitabine and docetaxel, an effective second-line chemotherapy for lung metastasis of urothelial carcinomaInt J Clin Oncol201419351652223749066
- SawhneyRSehlMNaeimAPhysiologic aspects of aging: impact on cancer management and decision making, part ICancer J200511644946016393479
- SehlMSawhneyRNaeimAPhysiologic aspects of aging: impact on cancer management and decision making, part IICancer J200511646147316393480
- CromePLallyFCherubiniAExclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT studyDrugs Aging201128866767721812501
- PallisAGRingAFortpiedCEORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumorsAnn Oncol20112281922192621266517
- HurriaALevitLADaleWImproving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology StatementJ Clin Oncol201533323826383326195697
- CharlsonMEPompeiPAlesKLMackenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- DreicerRManolaJSchneiderDJPhase II trial of gemcitabine and docetaxel in patients with advanced carcinoma of the urothelium: a trial of the Eastern Cooperative Oncology GroupCancer200397112743274712767086
- FléchonAFizaziKGourgou-BourgadeSGemcitabine and cisplatin after radical cystectomy for bladder cancer in an adjuvant setting: feasibility study from the Genito-Urinary Group of the French Federation of Cancer CentersAnticancer Drugs200617670570816917216
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- TrottiAColevasADSetserACTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatmentSemin Radiat Oncol200313317618112903007
- BellmuntJThéodoreCDemkovTPhase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tractJ Clin Oncol200927274454446119687335
- SrinivasSHarshmanLCA phase II study of docetaxel and oxaliplatin for second-line treatment of urothelial carcinomaChemotherapy200955532132619641314
- AlbersPParkSINiegischGRandomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]Ann Oncol201122228829420682548
- SalahSLeeJLRozziASecond-line Chemotherapy in Older Patients With Metastatic Urothelial Carcinoma: Pooled Analysis of 10 Second-line StudiesClin Genitourin Cancer2017154e563e57128065418
- SunLLuJNiuZA Potent Chemotherapeutic Strategy with Eg5 Inhibitor against Gemcitabine Resistant Bladder CancerPLoS One20151012e014448426658059
- NakanoTSaikiYKudoCAcquisition of chemoresistance to gemcitabine is induced by a loss-of-function missense mutation of DCKBiochem Biophys Res Commun201546441084108926196746
- DemolsAPeetersMPolusMGemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II studyBr J Cancer200694448148516434988
- NaikiTIidaKKawaiNA pilot study of gemcitabine and paclitaxel as third-line chemotherapy in metastatic urothelial carcinomaJ Rural Med201712210511129255527
- KanaiKKikuchiEOhigashiTGemcitabine and paclitaxel chemotherapy for advanced urothelial carcinoma in patients who have received prior cisplatin-based chemotherapyInt J Clin Oncol200813651051419093178
- KitamuraHTaguchiKKunishimaYPaclitaxel, ifosfamide, and nedaplatin as second-line treatment for patients with metastatic urothelial carcinoma: a phase II study of the SUOC groupCancer Sci201110261171117521323791
- KounoTAndoMYonemoriKWeekly paclitaxel and carboplatin against advanced transitional cell cancer after failure of a platinum- based regimenEur Urol20075241115112217433855
- MatsumotoKIrieASatohTOkazakiMIwamuraMBabaSGemcitabine and paclitaxel chemotherapy as a second-line treatment for advanced or metastatic urothelial carcinomaInt J Urol2007141110001004 discussion 100417956525
- IkedaMMatsumotoKTabataKCombination of gemcitabine and paclitaxel is a favorable option for patients with advanced or metastatic urothelial carcinoma previously treated with cisplatin-based chemotherapyJpn J Clin Oncol201141101214122021903707
- YuenARZouGTurrisiATSimilar outcome of elderly patients in intergroup trial 0096: Cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinomaCancer20008991953196011064352
- JanASDolanDELombardiKGuptaSTolerability of Gemcitabine Plus Cisplatin for Treatment of Urothelial Cancer in the Elderly PopulationClin Genitourin Cancer2016143e257e26326462439
- PowlesTDuránIvan der HeijdenMSAtezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trialLancet20183911012274875729268948
- PowlesTO’DonnellPHMassardCEfficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label StudyJAMA Oncol201739e17241128817753
- PatelMREllertonJInfanteJRAvelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trialLancet Oncol2018191516429217288
- SharmaPRetzMSiefker-RadtkeANivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trialLancet Oncol201718331232228131785
- BellmuntJde WitRVaughnDJPembrolizumab as Second- Line Therapy for Advanced Urothelial CarcinomaN Engl J Med2017376111015102628212060
