Abstract
Purpose
Patients with unresectable locally advanced pancreatic cancer (LAPC) are still in dire need of effective therapies. We performed this cohort study in order to assess the efficacy and safety of high-intensity focused ultrasound (HIFU) ablation in treating patients with unresectable LAPC.
Patients and methods
Eighty-seven cases with unresectable LAPC from January 2014 to December 2016 were finally recruited according to the inclusion criteria. The primary end point of our study was OS of all the cases, and the secondary end points included 6-month and 12-month survival rate, tumor response rate, carbohydrate antigen (CA) 19-9 response rate, VAS, quality of life, and safety.
Results
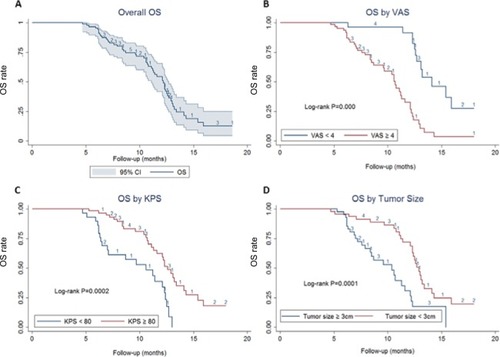
All the 87 patients received HIFU ablation successfully, and were included in the efficacy and safety analysis. With a median follow-up of 16 months, median OS was estimated to be 12.2 months, with 95 % CI of 11.1–12.7 months. The 6-month and 12-month survival rates were 94.25% (95% CI =86.74–97.57) and 50.85% (95% CI =38.17–62.21), respectively. Multivariate analysis revealed that patients with VAS <4, Karnofsky performance status ≥80, and tumor size <3 cm have a significant improvement in their OS (adjusted HR [aHR] =0.26 [95% CI =0.12–0.57], P=0.001; aHR =0.34 [95% CI =0.17–0.68], P=0.02; and aHR =0.39 [95% CI =0.20–0.78], P=0.007; respectively). Tumor responses were observed in 32 (36.8%) of 87 patients and CA 19-9 response rate was 56.2%. Global health status, physical function, emotional function, and cognitive function of patients were significantly improved after HIFU treatment, and symptoms of fatigue and pain were significantly reduced. A total of 28.7% (25/87) of patients reported adverse events (AEs), mainly including fatigue (14/87), abdominal pain (7/87), fever (7/87), nausea (5/87), and rash (4/87). No severe AEs and HIFU-related deaths were reported.
Conclusion
HIFU ablation might be a potentially effective and safe therapeutic option for the patients with unresectable LAPC.
Introduction
Pancreatic cancer is one of the most commonly diagnosed aggressive malignancies worldwide, with poor long-term prognosis.Citation1,Citation2 Patients with pancreatic carcinoma are often in the advanced stage and unresectable at the time of diagnosis, due to the difficulties in early diagnosis. Few of treatment options would be feasible for them. The latest report shows that pancreatic adenocarcinoma is the third cause of cancer-induced death worldwide, with a 5-year OS rate of 8%, while the survival is relatively better in the localized cases with the rate of 32%.Citation1 These patients with unresectable tumor lesions located in the celiac axis and the superior mesenteric artery without evidence of distant metastasis are defined as locally advanced pancreatic cancer (LAPC). Patients with LAPC account for ~30% of all the cases of pancreatic carcinoma,Citation3 with a median survival time of less than 10 months.Citation4
The front-line therapeutic options for LAPC patients are chemotherapy and radiotherapy, by which survival benefit is limited, and complications and adverse events (AEs) are frequent. Although some newer chemotherapy regimens like gemcitabine plus nab-paclitaxel,Citation5 dasatinib plus gemcitabine,Citation6 FOLFOX-6Citation7,Citation8 and FOLFIRINOXCitation9–Citation11 appear and have shown a substantial survival benefit in patients with LAPC, the long-term prognosis is still poor and the heterogeneity between these studies is significant.Citation12 Therefore, an optimal treatment for LAPC patients should provide survival benefit, alleviate pain, improve quality of life, but not cause severe compactions.
High-intensity focused ultrasound (HIFU) is an emerging noninvasive ablation procedure which can ablate various solid tumors including LAPC. It can focus ultrasound energy on the target lesions and induce tumor coagulation necrosis by thermal effect.Citation13 Several clinical trials of HIFU palliative therapy for pancreatic carcinoma cases have provided promising results.Citation14,Citation15 HIFU monotherapyCitation16–Citation21 or in combination with systemic chemotherapyCitation22,Citation23 has been proved to be able to relieve pain and might bring an additional survival benefit with rare severe AEs.
However, the efficacy of HIFU treatment remains to be confirmed by more research and clinical practice. This single-center, prospective, case series was performed to assess the clinical benefit and safety of HIFU treatment for LAPC cases.
Patients and methods
Patients
From January 2014 to December 2016, cytologically or pathologically confirmed unresectable LAPC patients were eligible for treatment in our center. The inclusion criteria were as follows: patients with age ≥18 years; patients with adequate hepatic, renal, and bone marrow function (white blood cell ≥3.9×109/L, absolute neutrophil count ≥1.5×109L, platelets ≥100×109/L, hemoglobin ≥10 g/dL, and serum creatinine ≤150 mmol/L); and patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1. In addition, some cases should be excluded including pregnancy, lactation, metabolic disease, prior cerebrovascular event, active second malignancy, and uncontrolled intermittent illness.
Each patient signed a document of informed consent before enrollment. The research obtained the approval of the ethics committee of Huadong Hospital affiliated to Fudan University and was done according to the Declaration of Helsinki.
Procedures
HIFU was performed using HIFUINT-9000 system (Shanghai A&S Sci-Tec Co., Ltd, Shanghai, China), which is a US-guided device.Citation22 Firstly, tumor location, size, and the morphological characteristics are identified by b-mode sonography, CT, or MRI; in the meantime, the influence of tumor on adjacent organs and blood vessels is also evaluated. Next, the detecting head of this system will complete the re-localization of the therapy area. Finally, the ablation energy focus is controlled to move sequentially along the three-dimensional axis until the target lesion is totally covered. The main HIFU parameters of treatment in this study were the following: input power, 5–10 kW/cm2; therapy depth, 2–15 cm; practice-focused sphere, 3 × 3 × 8 mmCitation3; unit transmit time (t1) : intermission time (t2) =1:2; and HIFU times at each lesion, 8–10 times. All of the parameters can be varied depending on the depth of the tumor.
Observation and measurement
The primary outcome of this single-center, prospective cohort study was OS of patients. The secondary end points were 6- and 12-month survival rates, tumor response rate, carbohydrate antigen (CA) 19-9 response rate, VAS, quality of life, and safety. The evaluation of tumors was conducted by a CT or MRI scan before HIFU ablation, and at 1, 3, 6, and 12 months post-HIFU treatment. The response was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1).Citation24 Serum levels of tumor biomarker CA 19-9 were measured before HIFU ablation and within 1 week after HIFU treatment, and CA 19-9 response was defined as >50% decline from baseline value. VAS pain scores and quality of life scores were also recorded and assessed before HIFU ablation, and at 1, 3, 6, and 12 months post-HIFU treatment. The European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-core 30 (EORTC-QLQ-C30) was adopted to evaluate the quality of life of patients. AEs were recorded, and the severity was graded in accordance with the Common Terminology Criteria for Adverse Events, version 4.Citation25
Statistical analyses
OS analysis of patients was conducted by the Kaplan–Meier method. To obtain more detailed descriptions of the survival, stratified analyses by the characteristics of cases were also performed. Potential clinicopathological factors influencing the OS rates were evaluated by log-rank test (univariate analysis) and then by Cox proportional hazards model (multivariate analysis). All the data analyses were performed by Stata 12.0 software (StataCorp LP, College Station, TX, USA). A P-value <0.05 indicated statistical significance.
Results
Patient baseline characteristics
In total, 87 patients were enrolled in this study. The cases included 41 males and 36 females, with a median age of 68 years (range: 38–80 years). Median Karnofsky performance status (KPS) was 80; 58 cases had a KPS score ≥80 and 29 cases <80. The median VAS prior to HIFU was 3 (range: 0–8); 70.1% of the cases had a VAS ≥4. The mean of maximum diameter was estimated to be 3.7 cm (range: 1.7–6.9 cm); 41 cases had a mean diameter ≥3 cm and 46 cases <3 cm. A majority (91%) of the tumors were located in the head and body of the pancreas. Many patients received other therapies before enrollment, including chemotherapy (26), radiotherapy (6), and surgery (12), however, some of them did not achieve remission, and others had relapsed in the short term. Details of the baseline characteristics of patients and tumors are described in .
Table 1 Patient baseline characteristics
OS and 6- and 12-month survival rate and the risk factors of OS
Median OS was estimated to be 12.2 months, with 95% CI of 11.1–12.7 months. The 6-month and 12-month survival was 94.25% (95% CI =86.74–97.57) and 50.85% (95% CI =38.17–62.21), respectively. The risk factors of survival were analyzed with a Cox proportional hazards model, which suggested that cases with VAS <4, KPS ≥80, and tumor size <3 cm have a significant improvement in the OS (HR =0.26 [95% CI =0.12–0.57], P=0.001; HR =0.34 [95% CI =0.17–0.68], P=0.02; and HR=0.39 [95% CI =0.20–0.78], P=0.007; respectively) after adjusting for age, VAS, KPS, and tumor size ( and ).
Table 2 Patients’ OS and the risk factor analysis of OS
Clinical response and CA 19-9 response
Among 87 patients administrated with HIFU ablation, complete response (CR), partial response (PR), stable disease (SD), and progression disease (PD) were observed in 7, 25, 36, and 19 patients, respectively. Objective response rate (ORR, CR+ PR) was found to be 36.8% (32/87). Disease control rate (CR+ PR+ SD) was observed to be 78.2% (68/87) ( and ).
Table 3 Maximum RECIST response, CA 19-9 response
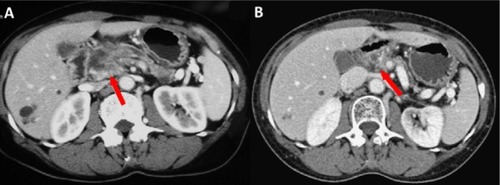
Figure 2 CT scans of a 63-year-old male patient with LAPC who received HIFU treatment.
Notes: (A) Before treatment, pancreatic cancer invaded local blood vessels. An irregular low-density mass with 2.6×3.0 cm could be seen in the head of the pancreas. The boundaries between the lesion and the hepatic artery, superior mesenteric vein, and the proximal end of the splenic vein were unclear, and the pancreatic duct was dilated. (B) One year after HIFU treatment, the pancreas atrophied, a small amount of pancreatic tissue was seen on the head of the pancreas, no obvious space-occupying lesions were seen, the gap of peripancreatic fat was clear, and there was no sign of pressure on the surrounding blood vessels.
Abbreviations: HIFU, high-intensity focused ultrasound; LAPC, locally advanced pancreatic cancer.
Results obtained for tumor biomarker CA 19-9 are also presented in . Patients achieved a CA 19-9 response at a median of 1 month (0.8–1.6). The mean nadir decrease from baseline was 4,077 U/mL. Nearly one-third of patients receiving HIFU treatment had a decrease exceeding 90% from the baseline level, and more than 50% of patients had a decrease exceeding 50% ().
VAS pain score
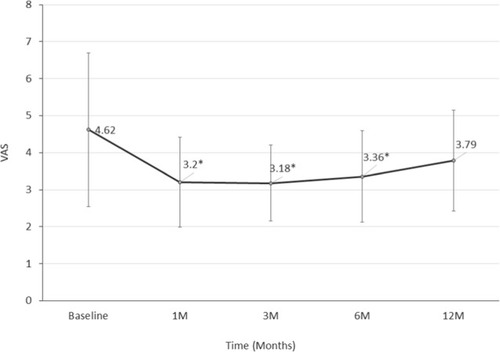
The mean VAS pain assessment score was 4.62 (±SD 2.07) at baseline, and was 3.20 (±SD 1.21), 3.18 (±SD 1.02), 3.36 (±SD 1.24), and 3.79 (±SD 1.36) at 1, 3, 6, and 12 months after HIFU ablation, respectively. As shown in , a significant decrease from baseline levels (P<0.05) was observed at 1, 3, and 6 months post-HIFU therapy.
Quality of life
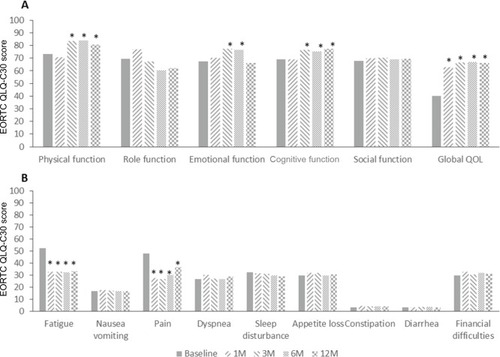
Function scores, global health scores, and symptom scores were converted into the centesimal system from 0 to 100. A higher score in both function and global health scales post-conversion indicated an increased ability of patients to do daily activities. Meanwhile, high scores of symptom scales indicated suffering of the patients. The results are shown in . Global health status, physical function, emotional function, and cognitive function of patients were significantly (P<0.05) improved after HIFU treatment. Symptom scales revealed that fatigue and pain were significantly (P<0.05) reduced after HIFU therapy.
Figure 4 QOL of patients was evaluated by EORTC QLQ-C30 questionnaire.
Notes: High levels of global health and function scales (A) indicated a higher ability, and high scores of symptom-scales (B) indicated suffering of the patients. *p<0.05 compared with Baseline.
Abbreviations: EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-core 30; ; M, months; QOL, quality of life.
Safety
All the reported AEs are listed in . Fatigue (14/87, 16.1%) is the most common AE, followed by abdominal pain (7/87, 8%), fever (7/87, 8%), nausea (5/87, 5.7%), and rash (4/87, 4.6%). Besides, elevated C-reactive protein levels, leucopenia and a subcutaneous nodule were reported by each of the two patients. It is worth mentioning that no patient complained of skin burns in our study. No cases of acute pancreatitis or peritonitis were observed during the follow-up period post-HIFU treatment. Moreover, no case withdrew from this research because of the side effects and no treatment-induced death was observed.
Table 4 Most frequent AEs (regardless of relationship to HIFU treatment)
Discussion
Patients with unresectable LAPC are still in dire need of effective therapies, because of the limited efficacy of current therapeutic options. These treatments should improve patients’ symptoms, relieve pain, and improve quality of life, rather than just achieving sufficient local tumor control. In this regard, HIFU ablation might be one of the appropriate therapies for patients with LAPC. During HIFU treatment, ultrasonic energy is focused on the target lesions, resulting in coagulative necrosis of lesions by thermal and cavitation effects.Citation26,Citation27 Moreover, HIFU treatment is reported to have the ability to enhance tumor immunity of patients.Citation28–Citation30
HIFU ablation is an emerging therapy with encouraging clinical results. As reported by numerous studies, it could be applied in patients with advanced pancreatic carcinoma safely and effectively.Citation19,Citation31,Citation32 In clinical practice, HIFU combined with chemotherapy is preferred, but not HIFU alone. However, in the present study, the patients did not receive chemotherapy. They refused chemotherapy either because of AEs or medical expenses associated with chemotherapy or fear of relapse following chemotherapy. ZhouCitation14 over-viewed 241 articles with a total of 653 cases on the HIFU monotherapy for LAPC and revealed that median OS was 10 months and pooled pain remission rate was 71.3% (). The AEs associated with HIFU treatment were rare and tolerable, especially for patients of older age and with poor ECOG PS. Therefore, HIFU ablation could be considered as an optimal treatment for elderly cases and for those with low ECOG PS, who are unavailable for chemotherapy or radiotherapy.
Table 5 Summary of recent studies of HIFU monotherapy for advanced pancreatic cancer
In our subjects, the median OS was found to be 12.2 months, with 95% CI of 11.1–12.7 months. As a golden standard treatment for LAPC, patients undergoing first-line treatment with gemcitabine were reported to have a median OS of 5.6–9.2 months in numerous high-quality randomized controlled trials.Citation33–Citation39 HIFU treatment might prolong the survival of patients to a greater extent, when compared with gemcitabine monotherapy. The 6-month and 12-month OS rates of 94.25% and 50.85%, respectively, in this study are comparable to the rates obtained with chemotherapy and radiotherapy in LAPC.Citation23,Citation40 In a study by Zhao et alCitation41 in 2017, the 6-month OS was found to be 44.4%–100% and 12-month OS was 11.1%–35.4% after HIFU treatment, which is numerically inferior to our results. Some factors such as sample size, statistical power, and baseline value might have contributed to this difference.
In terms of the AEs, during and after HIFU therapy, no serious adverse reactions were observed in this study. The treatment-related complication, as well as treatment cycle, was significantly reduced compared with chemotherapy or radiotherapy.Citation42,Citation43 However, recent reports have reported certain HIFU-related complications, especially skin burns, but our results did not show any evidence of complications due to skin burn. This can be attributed to the differences in the type of HIFU equipment used for treatment. HIFUNIT-9000 system used in this study adopts dual focus mode, and the energy upon the skin could be reduced effectively during operation compared with other equipment.
Several limitations of this study should be acknowledged. First, our results came from a single-center, non-blinded observation study, which would not provide the highest quality evidence of clinical practice. Second, our sample scale is relatively small, and the follow-up period is relatively short. Therefore, top-level designed trials with a larger sample size are needed. Nevertheless, our investigation has provided a reliable clinical evidence for the new direction of LAPC ablation treatment.
Conclusion
Our results further confirmed the efficacy and safety of HIFU treatment for patients with LAPC. HIFU might be one of the optimal therapies for LAPC. However, further well-designed double-blind, randomized controlled trials are warranted to evaluate the clinical efficacy of HIFU ablation, especially in combination with chemotherapy or radiotherapy.
Acknowledgments
The authors are grateful to all the staff at the study center who contributed to this study.
Author contributions
Design of the study: Hong Zhao and Kaimeng Hu. Data management and analyses: Yongshuo Ji, Junqiu Zhu, and Yanfei Zhu. Writing and revising the manuscript: Yu Zhang and Linglin Zhu. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- HeestandGMMurphyJDLowyAMApproach to patients with pancreatic cancer without detectable metastasesJ Clin Oncol201533161770177825918279
- HsuehCTPancreatic cancer: current standards, research updates and future directionsJ Gastrointest Oncol20112312312522811841
- YamadaSFujiiTYokoyamaYPhase I study of chemoradiotherapy using gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic cancerCancer Chemother Pharmacol201881581582129502139
- EvansTRJVan CutsemEMooreMJPhase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancerAnn Oncol201728235436127998964
- GhosnMFarhatFKattanJFOLFOX-6 combination as the first-line treatment of locally advanced and/or metastatic pancreatic cancerAm J Clin Oncol2007301152017278889
- GhosnMSaroufimAKattanJChahineGNasrFFarhatFSequential FOLFOX-6 and gemcitabine for locally advanced and/or metastatic pancreatic cancerMed Oncol20122942831283722392197
- LakatosGPetranyiASzűcsAEfficacy and Safety of FOL-FIRINOX in Locally Advanced Pancreatic Cancer. A Single Center ExperiencePathol Oncol Res201723475375928062950
- SukerMBeumerBRSadotEFOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysisLancet Oncol201617680181027160474
- NyweningTMWang-GillamASanfordDETargeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trialLancet Oncol201617565166227055731
- SultanaASmithCTCunninghamDStarlingNNeoptolemosJPGhanehPMeta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancerJ Clin Oncol200725182607261517577041
- OrsiFArnonePChenWZhangLHigh intensity focused ultrasound ablation: a new therapeutic option for solid tumorsJ Cancer Res Ther20106441442021358073
- ZhouYHigh-intensity focused ultrasound treatment for advanced pancreatic cancerGastroenterol Res Pract2014205325
- WuFHigh intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancerWorld J Gastroenterol20142044164801648825469016
- OrsiFZhangLArnonePHigh-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locationsAJR Am J Roentgenol20101953W245W25220729423
- WuFWangZBZhuHFeasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experienceRadiology200523631034104016055692
- WangKChenZMengZAnalgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancerInt J Hyperthermia201127210110721219135
- SungHYJungSEChoSHLong-term outcome of high-intensity focused ultrasound in advanced pancreatic cancerPancreas20114071080108621926543
- WangKZhuHMengZSafety evaluation of high-intensity focused ultrasound in patients with pancreatic cancerOnkologie2013363889223485995
- GaoHFWangKMengZQHigh intensity focused ultrasound treatment for patients with local advanced pancreatic cancerHepatogastroenterology2013601281906191024088318
- ZhaoHYangGWangDConcurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancerAnticancer Drugs201021444745220075714
- LiYJHuangGLSunXLZhaoXCLiZGThe combination therapy of high-intensity focused ultrasound with radiotherapy in locally advanced pancreatic carcinomaWorld J Surg Oncol2016146026927794
- TherassePArbuckSGEisenhauerEANew guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of CanadaJ Natl Cancer Inst200092320521610655437
- LiuYJZhuGPGuanXYComparison of the NCI-CTCAE version 4.0 and version 3.0 in assessing chemoradiation-induced oral mucositis for locally advanced nasopharyngeal carcinomaOral Oncol201248655455922289637
- SofuniAMoriyasuFSanoTThe current potential of high-intensity focused ultrasound for pancreatic carcinomaJ Hepatobiliary Pancreat Sci201118329530321360084
- HwangJHWangYNWarrenCPreclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumorsUltrasound Med Biol200935696797519201519
- WuFZhouLChenWRHost antitumour immune responses to HIFU ablationInt J Hyperthermia200723216517117578340
- LiuFHuZQiuLBoosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturationJ Transl Med20108720105334
- RanLFXieXPXiaJZXieFLFanYMWuFSpecific antitumour immunity of HIFU-activated cytotoxic T lymphocytes after adoptive transfusion in tumour-bearing miceInt J Hyperthermia201632220421026708472
- Vidal-JoveJPerichEDelCastillo MAUltrasound Guided High Intensity Focused Ultrasound for malignant tumors: The Spanish experience of survival advantage in stage III and IV pancreatic cancerUltrason Sonochem20152770370626044461
- GeHYMiaoLYXiongLLHigh-intensity focused ultrasound treatment of late-stage pancreatic body carcinoma: optimal tumor depth for safe ablationUltrasound Med Biol201440594795524462161
- SpanoJPChodkiewiczCMaurelJEfficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II studyLancet200837196302101210818514303
- BoradMJReddySGBaharyNRandomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients With Advanced Pancreatic CancerJ Clin Oncol201533131475148125512461
- CunninghamDChauIStockenDDPhase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancerJ Clin Oncol200927335513551819858379
- LoehrerPJSrFengYCardenesHGemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trialJ Clin Oncol201129314105411221969502
- ColucciGLabiancaRDi CostanzoFRandomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 studyJ Clin Oncol201028101645165120194854
- GonçalvesAGilabertMFrançoisEBAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancerAnn Oncol201223112799280522771827
- PhilipPABenedettiJCorlessCLPhase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205J Clin Oncol201028223605361020606093
- MarinovaMRauchMMückeMHigh-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensityEur Radiol201626114047405626886904
- ZhaoJZhaoFShiYDengYHuXShenHThe efficacy of a new high intensity focused ultrasound therapy for locally advanced pancreatic cancerJ Cancer Res Clin Oncol2017143102105211128620685
- Moureau-ZabottoLPhélipJMAfchainPConcomitant administration of weekly oxaliplatin, fluorouracil continuous infusion, and radiotherapy after 2 months of gemcitabine and oxaliplatin induction in patients with locally advanced pancreatic cancer: a Groupe Coordinateur Multidisciplinaire en Oncologie phase II studyJ Clin Oncol20082671080108518309942
- MahadevanAJainSGoldsteinMStereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancerInt J Radiat Oncol Biol Phys201078373574220171803
- MarinovaMHuxoldHCHenselerJClinical Effectiveness and Potential Survival Benefit of US-Guided High-Intensity Focused Ultrasound Therapy in Patients with Advanced-Stage Pancreatic CancerUltraschall Med Epub2018417
- XiongLLHwangJHHuangXBEarly clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancerJOP200910212312919287104
- LiPZZhuSHHeWHigh-intensity focused ultrasound treatment for patients with unresectable pancreatic cancerHepatobiliary Pancreat Dis Int201211665566023232639