Abstract
Tumor vascular normalization alleviates hypoxia in the tumor microenvironment, reduces the degree of malignancy, and increases the efficacy of traditional therapy. However, the time window for vascular normalization is narrow; therefore, how to determine the initial and final points of the time window accurately is a key factor in combination therapy. At present, the gold standard for detecting the normalization of tumor blood vessels is histological staining, including tumor perfusion, microvessel density (MVD), vascular morphology, and permeability. However, this detection method is almost unrepeatable in the same individual and does not dynamically monitor the trend of the time window; therefore, finding a relatively simple and specific monitoring index has important clinical significance. Imaging has long been used to assess changes in tumor blood vessels and tumor changes caused by the oxygen environment in clinical practice; some preclinical and clinical research studies demonstrate the feasibility to assess vascular changes, and some new methods were in preclinical research. In this review, we update the most recent insights of evaluating tumor vascular normalization.
Background
The growth of solid tumors is closely related to active recruitment of blood vessel network – the so-called tumor angiogenesis. The tumor cells away from vessels experience hypoxia due to deficiency of blood and oxygen when the tumor is larger than 1–2 mm; as a result, tumor cells initiate the process of angiogenesis by generating excessive pro-angiogenic factors as an adaptive adjustment.Citation1,Citation2 Various molecules and genes have been identified to play a critical role in angiogenesis;Citation3,Citation4 under these circumstances, the equilibrium between angiogenic promoters and angiogenic inhibitors tilts in favor of the pro-angiogenic factors,Citation3–Citation6 which breaks the existing vascular silence and elicits the angiogenesis of tumor vessels.Citation7,Citation8 Professor Judah Folkman first proposed the theory of tumor angiogenesis in 1971,Citation1,Citation5 and a new therapy method that aimed to starve tumors by blocking their blood vessels was established. The first clinical trial of anti-angiogenesis was of interferon alpha for life-threatening hemangioma,Citation9 and the first anti-angiogenesis agent approved for clinical use is bevacizumab, which is used for treating colon cancer, lung cancer, kidney cancer, and brain cancer. However, some clinical trials showed that the efficacy of anti-angiogenesis monotherapy is limited,Citation10–Citation12 and antivascular endothelial growth factor (anti-VEGF) therapy cannot produce sustained shrinkage in certain tumors, such as colorectal and breast cancer tumors.Citation13 It is noteworthy that the combination of anti-angiogenesis therapy with systemic chemotherapy has often proven to be an effective strategy, with outcomes better than those of chemotherapy alone,Citation13–Citation19 which means that anti-angiogenesis in some way enhances the activity of cytotoxins. Why is this happening?
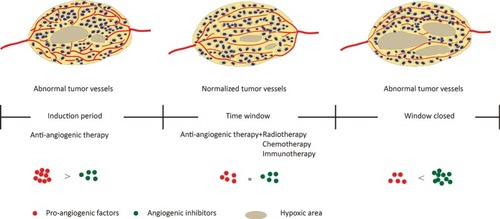
In response, Jain et alCitation7,Citation20,Citation21 proposed the “normalization of tumor blood vessels” hypothesis in 2001. In tumors, uncontrolled angiogenesis is associated with damage of the tumor vascular maturation process, leading to a heterogenous structure with tortuous, dilated, increased endothelial cell gap, and scarcity of pericyte coverage.Citation22,Citation23 These structural abnormalities contribute to dysfunction in tumor vessels, characterized by hypoperfusion, hyperpermeability, increased interstitial fluid pressure, and severe hypoxia.Citation7,Citation22,Citation24 As a result, blood supply deficiency and interstitial hypertension impede the delivery of cytotoxic agents to solid tumors,Citation4,Citation22,Citation25 and hypoxia favors tumor progression and acquired resistance both in radiotherapy and chemotherapy.Citation26–Citation28 The hypothesis of tumor vascular normalization posits that judicious use of anti-angiogenic therapy restores the abnormal structure and function of the tumor vasculature toward a more normal stateCitation22 and tumor blood flow (BF) and oxygenation transiently increase, thus providing an opportunity to improve radiotherapy, chemotherapy, and immunotherapyCitation13,Citation21,Citation25–Citation30 ().
Figure 1 Proangiogenic factors and angiogenic inhibitors tend to be in a state of equilibrium after moderate anti-angiogenesis therapy, forming a tumor vascular normalization time window; vascular structure and function are normalized, resulting in a remission of hypoxia, and increase radiotherapy, chemotherapy, and immunotherapy efficacy.
However, limitations exist in the clinical application of tumor vascular normalization – one of which is how to detect the time window simply and repeatedly. Tumor vascular normalization manifested as reduced blood vessel density, more regular vascular distribution, integrated pericyte coverage, and increased tumor perfusion. All these changes can be determined by histological detection. Vascular endothelial cells can be marked by CD 31 and CD 34,Citation14,Citation15,Citation25,Citation31 and markers for tumor pericytes include NG2, PDGFR-β, and α-SMA,Citation15,Citation25,Citation30 which are readily detected in pericytes in tumor angiogenesis. Tumor perfusion was determined by intravenous injection of fluorescein-labeled lectin or Evans blue,Citation26 and vascular permeability was measured by fluorescein-labeled dextran.Citation15 However, the above-mentioned methods are difficult to carry out in the clinic because it is almost unrepeatable in the same individual; thus, it is difficult to dynamically monitor the process of tumor vascular normalization, which limits the ability of guiding traditional therapy. Therefore, establishing a more convenient and repeatable detection method of vascular normalization is of great significance. In this review, we summarized the novel insights of detecting tumor vascular normalization from studies and discussed the questions that must be faced in the clinic ().
Table 1 Summary of techniques with the advantages and limitations
Computed tomography (CT)
Tumor vascular normalization is often manifested as changes in the vascular network architecture and perfusion. CT can visualize the vascular density, bifurcation, perfusion, and oxygen content of a tumor. CT for detecting tumor vessels has been proposed as early as the tumor vascular normalization proposed.Citation20 CT perfusion imaging is used to obtain the time–density curve of the region of interest (ROI) through a continuous scan after the contrast medium of bolus injection, and the hemodynamic parameters are calculated by software using mathematical models. Perfusion CT parameters of blood volume (BV), which may be related to vascular normalization, were positively correlated with microvessel density (MVD) in gastric adenocarcinoma.Citation32 BV and peak enhancement index (PEI) were positively correlated with MVD in lung tumor measured by pathology and CT.Citation31 Iodine concentration (IC) was widely used in clinical diagnosis of vascular imaging and various diseases; quantitative IC value detected by spectral CT correlated with the MVD and reflected angiogenesis in advanced gastric cancer.Citation33
With the development of functional imaging, tumor vessel assessment by contrast-enhanced CT makes the detection more comprehensive; the contrast agent provides indirect evidence for morphological and hemodynamic indices and extravasation in different tumor regions.Citation7,Citation34 Studies have revealed that the peak enhancement (PE) of the tumor and the enhancement ratio measured by dynamic multidetector CT correlate positively with the extent of angiogenesis, and dynamic-enhanced CT images may reflect the heterogeneity of tumor angiogenesis in view of the correlation between enhancement parameters and MVD.Citation35–Citation37 BV and permeability surface-area product (PSAP, measures of capillary leakage and functional vascular density) other than BF and transit time evaluated by multidetector CT correlated positively with MVD; however, there was no significant correlation between the imaging vascular parameters and the histological parameters pericyte coverage, vascular endothelial growth factor (VEGF) expression, and GLUT-1 expression.Citation38
An open-label Phase II study evaluated dynamic CT-based vascular parameters in patients with advanced non-small-cell lung cancer, and results showed that BF, BV, and permeability-surface area were significantly decreased after the induction dose of bevacizumab, but the change in mean transit time was more heterogeneous between patients.Citation39 Dynamic contrast-enhanced CT (DCE-CT) is a noninvasive technique to image vascular and interstitial tumor characteristics, which are used to observe tumor neo-angiogenesis and other vascular parameters. Early activity of cetuximab induced changes in tumor vascular, and interstitial characteristics were detected by DCE-CT.Citation40 More research is needed to verify the relationship between vascularization parameters and vascular normalization.
The ability of CT to detect tumor perfusion, vessel morphology, and response to anti-angiogenic therapy has been clinically validated; however, CT perfusion imaging is influenced by many factors, such as scanning solutions, image analysis software, and personal bias; thus, the sensitivity and reliability of perfusion CT need to be verified. At present, the normalization of tumor blood vessels by CT has not been clinically confirmed, and more data are needed to support this.
Magnetic resonance imaging (MRI)
MRI is a versatile tool to noninvasively evaluate changes in tumor angiogenesis over time. MRI can be performed repeatedly to measure morphology and function of the target dynamically in the short term;Citation41 therefore, it is an ideal platform for assessing changes in blood vessels.Citation42 Dynamic contrast-enhanced MRI (DCE-MRI) is a functional imaging, which has been used in clinical trials to assess the role of anti-angiogenic agents.Citation43
The MRI parameter Ktrans is a key factor displaying volume transfer coefficient of the contrast agent between plasma and extravascular space. Ktrans is used for characterizing vascular function and influenced by flow, permeability, or both.Citation44–Citation47 Changes in vascular heterogeneity quantified by Ktrans distribution have been shown in patients with primary rectal cancer and breast cancer.Citation48–Citation50 Tumor blood perfusion, which is another predictor of normalization, can also be detected using DCE-MRI by pharmacokinetic behavior of gadolinium (Gd)-based contrast agents.Citation51 Patients with an increase in tumor perfusion measured by MRI showed significantly higher vascular normalization index (three parameters: Ktrans, microvessel volume, and plasma collagen IV) compared with patients with stable or decreased perfusion.Citation44,Citation45 This result offers direct clinical evidence in support of the hypothesis that vascular normalization benefits outcomes, and the vascular normalization index is a potential indicator for progression-free survival and overall survival.Citation52 Advanced MRI showed that treatment with cediranib increased tumor blood perfusion in some patients with recurrent glioblastoma undergoing VEGF treatment, and these patients survived longer than patients whose tumor blood perfusion did not increase.Citation45 In patients with breast cancer, doxorubicin/cyclophosphamide plus sunitinib induced functional changes in tumor vasculature. The DCE-MRI functional parameters, including Ktrans, Vp (fractional plasma volume), Ve (fractional extravascular volume), and PS (vascular permeability), were significantly increased after one cycle of combined treatment except for perfusion and the increase in Ktrans with good histological response compared with decline subset.Citation53
In another prospective study, physiological MRI showed vascular integrity, and perfusion improved in patients with newly diagnosed glioblastoma who underwent cediranib plus chemoradiation therapy compared with those who underwent conventional chemoradiation alone.Citation54 The relative difference in the oxygen saturation levels – one of the indicators of tumor blood vessel normalization as measured by MRI – was linked with increased perfusion. T1- and T2-weighted MRI with the cerebra BV from the DSC-MRI modality to study tumor perfusion in glioblastoma showing parameter variations predict tumor perfusion normalization.Citation55 An intuitive description of the tumor vascular normalization by MRI in clinical practice demonstrates that relative tumor vascular size decreased in 1 day after cediranib treatment, and it remained decreased at day 28. At day 56, the relative vessel size reversed toward abnormal, suggesting the beginning of the closure of the vascular structural normalization window; at the same time, MRI detected a reduction in vascular permeability (as measured by the transfer constant Ktrans of Gd) at days 1 and 28, and vascular permeability remained decreased until day 112, indicating that vascular function normalized.Citation56 One study determined the appropriate time of chemotherapy administration after bevacizumab therapy in metastatic brain tumors in patients with breast cancer. The result showed reductions in the mean percentage change in MRI parameters peak, slope, iAUC60, and Ktrans at 1 hour and 24 hours, and the reductions in all parameters were significantly higher at 24 hours than at 1 hour, which suggested that the appropriate time frame of vascular normalization of bevacizumab was 24 hours.Citation57
Another hallmark of tumor vascular normalization is the alleviation of hypoxia in tumor tissue, and this change could be detected by MRI. Blood oxygenation level-dependent MRI (BOLD-MRI) is a functional MRI technique using the paramagnetic properties of deoxyhemoglobin, which can be seen as a natural contrast agent to map the local oxygen concentration. R2* values positively correlated with the level of CA IX, and HIF-1α demonstrates BOLD-MRI as a reliable predictor to reflect oxygen content.Citation58,Citation59 Using MRI to accurately monitor the changes in tumor tissue oxygen content can indirectly reflect the changes in vascular function.Citation60
Overall, MRI provides a very comprehensive and versatile platform to analyze the morphology and function of vascular normalization. However, the quality of a DCE-MRI vascular normalized study can easily be affected by various methodological and technological issues, such as treatment schedule, contrast medium, analysis performed, and types of tumors; therefore, further research and a unified standard need to be developed.
Emission computed tomography (ECT)
ECT is a computer imaging method that includes positron emission tomography (PET) and single-photon emission computed tomography (SPECT). PET is a molecular imaging technology that provides information about function and metabolism, including biomolecule metabolism, cell proliferation, receptors, and nerve mediators in vivo.Citation61 This technique is based on the high glucose metabolism in malignancies that have more accumulation of tracers labeled with radionuclides (such as Citation18F, Citation15O, and Citation11C). SPECT is a nuclear medicine tomographic imaging technique that uses gamma rays; it can be freely reformatted or manipulate images as required to provide true three-dimensional (3D) information.
PET with [Citation15O]H2O/[Citation18F] fluorodeoxyglucose (FDG) was used to analyze tumor BF and metabolism response to different doses of endostatin in clinical practice and display a biphasic concentration–BF curve.Citation62 Using [Citation15O]H2O/[Citation18F] FLT PET imaging demonstrated that transient antiangiogenic treatment improves tumor BF corresponding to a transient tumor vessel normalization time window, which improved delivery of erlotinib into the tumor.Citation63 Perfusion and metabolism changes after anti-angiogenesis treatment can be detected by dynamic [Citation18F]-FDG PET, and the time frame of potential tumor vasculature renormalization was monitored to allow optimal timing of follow-up treatment.Citation64 In preclinical studies, Citation18F-fluoromisonidazole (18F-FMISO) accumulation levels in renal cell carcinoma were significantly increased compared with the control group after sorafenib treatments, and this increase was dose-dependent, which is consistent with decreasing microvessels.Citation65
Like BOLD-MRI, 18F-FMISO PET provides information of changes in interstitial oxygen state, a surrogate parameter of vascular normalization, and might help track the normalization time window.Citation7,Citation60,Citation66–Citation70 In addition, PET has the potential to display angiogenesis on a molecular level based on the expression and functional activity of proteins.Citation71 One study used PET and Citation68Ga-NODAGA-c(RGDfK) imaging αvβ3 integrin – one of the most extensively examined targets of angiogenesis – to monitor tumor angiogenesis after bevacizumab treatment of squamous cell carcinoma xenografts. The result showed that Citation68Ga-NODAGA-c(RGDfK) uptake was significantly increased at day 7 of treatment and maintained until 3 weeks, accompanied with a transient normalization of blood vessel morphology and reduction of MVD and αvβ3 integrin expression.Citation71 Arg-Gly-Asp (RGD) peptides have a high affinity and selectivity for αvβ3; RGD-based PET tracers can measure the changes in neovascular density and integrin expression during antiangiogenic therapy.Citation72–Citation74 Similarly, PET also accurately traced vascular normalization and improved interstitial chemotherapy delivery; when [Citation18F]-FMISO-PET signal decreased, the tissue hypoxia reduction and the vascular structures are normalized; thus, it could be a potential biomarker in anti-angiogenic therapy.Citation66,Citation67
Using (99m)Tc-RGD on SPECT/CT to evaluate the tumor vessels determined the optimal dose regimen for bevacizumab and radiotherapy alone. Scintigraphic imaging showed a significantly increased RGD tumor uptake 2 hours after bevacizumab treatment compared with those 24 hours after bevacizumab treatment and controls, which identified the vascular normalization window and showed the effect of bevacizumab when administered 2 hours before radiotherapy.Citation75 99mTc-(CO)3 His-Annexin A5 SPECT probes a significant antitumor efficacy of irinotecan during normalization of tumor blood vessels caused by bevacizumab.Citation76
PET has been clinically used to detect the lesions of tumor metastasis and guide the staging and treatment program of the tumor. However, it failed to gain wider acceptance for routine clinical application for detecting tumor vasculature because of several limitations, for instance, the tracers’ accumulation in hypoxic tumors is slow, tracers have short half-life of the isotopes,Citation64 and a considerable amount of radioactive metabolites.Citation77 Data regarding SPECT in the clinical setting in response to antiangiogenic treatment are scarce. As a new advanced versatile detection platform, a large amount of clinical data and preclinical studies need to be collected and analyzed comprehensively to evaluate its value in detecting tumor vascular normalization.
Dynamic contrast-enhanced ultrasonography (DCE-US)
DCE-US is a functional technique using Doppler ultrasound with contrast medium and perfusion software, using a mathematical model to quantitatively evaluate BF and vascular morphological characteristics.Citation78–Citation80 For example, DCE-US has been introduced to improve the diagnosis of thyroid nodules, given the abundant vasculature of the thyroid gland.Citation81 In solid tumors, changes in tumor vascularization can be detected earlier than changes in tumor volume, and DCE-US has been proposed as an alternative method to evaluate vascular response to anti-angiogenic therapy and targeted therapy.Citation82–Citation87
Microvascular morphology can be delineated by contrast-enhanced ultrasound angiography (CEUA). Cannula centerlines extracted from ultrasound images can be more conveniently acquired vascular morphology parameters, such as vascular length, density, bifurcations, junction points, and other topologic features,Citation88 thus CEUA provides a quantitative basis for distinguishing malignant from normal tissue due to vascular heterogeneity.Citation89 3D contrast-enhanced ultrasonography could identify benign and malignant breast tumors and correlates with MVD and VEGF expression.Citation90 Combined analysis of quantitative vasculature, vascular morphology, and tortuosity by 3D power Doppler ultrasound can potentially serve as imaging biomarkers to predict the early response to anti-angiogenesis therapy.Citation91 DCE-US-based vascular morphology parameters exhibit a marked reduction in tumor vessels, bifurcations, and tortuosity, which are manifestations of tumor blood vessel normalization.Citation78
DCE-US imaging was used to estimate tissue perfusion, which is another index for tumor vascular normalization. Microbubble contrast agents (MCAs) will disintegrate and dissolve under ultrasound. The MCA reperfusion rate into the microvasculature will reflect BF. This technique has been used to map BF rate in many organs and tumors.Citation92 DCE-US could detect considerable changes in intratumoral BV through the functional parameters of area under the curve and peak intensity (PI), BF rate by time-to-peak intensity, wash-in rate (WIR), and wash-out rate (WOR), all of which may prove useful in determining tumor response to anti-angiogenesis or chemotherapy.Citation78 An earlier study used dynamic US to quantify dynamic changes in tumor blood vessels’ early response to bevacizumab treatment in patients with hepatocellular carcinoma, although there is no direct description of the alleviation in hypoxia and interstitial pressure due to the vascular normalization; however, the total area under the time–intensity curve that corresponds to BV and is probably the best indicator of tissue necrosis.Citation93 A clinical trial enrolled 539 patients in which DCE-US was carried out at baseline and 7 days after anti-angiogenic treatment. The trial identified the mean transit time of at least 12 seconds was the only criterion that correlated with freedom from progression of patients treated with bevacizumab at day 7, and this criterion might be linked to vascular normalization.Citation94 The hemodynamics of liver metastasis in patients with colorectal cancer who received anti-angiogenesis therapy was quantitatively evaluated by DCE-US. Dynamic monitoring showed that retention time (RT) increased continuously in the bevacizumab-sensitive tumors and transient reduction in bevacizumab-resistant tumor vessels. This heterogeneity might be related to the normalization of vasculature.Citation95 A recent study provided direct evidence by 3D power Doppler ultrasound that the possible timing of the normalization window was 20–24 hours after the administration of bevacizumab in human breast cancer.Citation96
In conclusion, tumor vascular parameters could be analyzed using various techniques. Among them, DCE-US has been proven to be safe, easily repeatable, low cost, and with reproducible results, which make it a feasible tool that can be relatively easy to implement to monitor tumor vascular normalization; however, the detection is influenced by intestine gas and is limited in its accuracy to monitor the BF of abdominal and pelvic tumors. Furthermore, in some tumors, the guidelines for evaluating the quality of results need to be established.
Plasma or serum marker
Since various proteins released by tumors are identified in the plasma or serum, blood is an ideal biological sample to predict disease progression, and blood proteomics have gained considerable interest in identification of novel reliable disease biomarkers.
Soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) is an endogenous antagonist of VEGF and placenta growth factor (PlGF) and is related to tumor vascular normalization. The plasma sVEGFR-1 correlates with antitumor and systemic efficacy of bevacizumab-based chemoradiation.Citation97 A transient decrease in plasma sVEGFR1 was measured at day 7 after the induction of bevacizumab,Citation39 which can be used as a candidate biomarker for predicting normalization of tumor blood vessels. Angiopoietin-1/2 (Ang1/2) plays a dynamic role in vessel formation, permeability, maturation, and maintenance.Citation98 Serum Ang2 is emerging as an endothelium activation marker in certain diseases, such as acute lung injury, hypertension-related myocardial infarction, sepsis, and many cancers,Citation99 and it has been reported to be overexpressed in tumors after anti-angiogenic therapies;Citation98 however, cediranib treatment induced transient decreases in Ang2 in plasma.Citation100 In tumor tissue, the Ang1/Ang2 ratio correlated with the degree of vascular normalization and with the survival of patients with glioblastoma.Citation101 Cediranib treatment led to transient normalization of the vasculature and was associated with a transient increase in plasma collagen IV at day 2, which reflects basement membrane thinning.Citation102 In clinical practice, circulating collagen IV level is one of the parameters to identify the tumor vascular normalization index with MRI.Citation44
Apelin/APJ signaling regulates pathological angiogenesis, and the secretion level of apelin was increased under hypoxic environment, which was mediated by HIF-1α;Citation103,Citation104 furthermore, apelin expression is upregulated in the process of new vascular formation but downregulated following vascular stabilization.Citation105,Citation106 A preclinical study found that apelin expression both in tumor tissue and in plasma would be transiently decreased during the vessel normalization window induced by bevacizumab and restored with the regression of vascular normalization.Citation107 Considering the relationship between hypoxia and apelin, this change is thought to be due to a temporary alleviation of hypoxia in the time window; however, the clinical usefulness of apelin to identify the tumor vascular normalization needs to be analyzed.
In addition, a series of factors, genes, or cells that exist in both blood and tumor tissue have been demonstrated as a remarkable marker in tumor tissue. Type 1 T helper (TH1) cells are associated with vessel normalization by secreting interferon-γ, and there is a positive feedback loop between TH1 and vascular normalization, accompanied with a decrease in the expression of Angpt2 and other VEGF-signature genes.Citation108 Moreover, Tian et alCitation108 identified that various angiogenesis-related genes positively or negatively correlate with survival, and they defined these genes as good- and poor-prognosis angiogenesis genes (GPAGs and PPAGs). The inversion of GPAGs and PPAGs correlates with “invasive vasculature”, and thus, these genes can be used as vessel normalization indicators.
Easy accessibility and sensitivity to changes in tumor vasculature make plasma and serum as promising markers for monitoring tumor vascular normalization during anti-angiogenic treatment. These markers suggest that potential combination regimens improve the effectiveness of chemotherapy drugs, and some of them may be applicable to other tumor types and warrant further testing.
Conclusion and outlook
Preclinical or clinical studies have shown that normalization of tumor blood vessels can improve the comprehensive therapy efficacy, including radiotherapy, chemotherapy, and immunotherapy. To ensure drug administration precisely within normalized time window, it is necessary to monitor the time window accurately; however, how to conduct effective clinical monitoring of vascular normalization time window is a key constraint. Although PET, MRI, and CT perfusion imaging have been used to evaluate the therapeutic efficacy of anti-angiogenesis therapy, no consensus has yet been reached. To date, no single method has been validated for detecting the complex process of normalization. At present, MRI and ultrasound are the most reported in the clinical literature, and their reliability and stability deserve more observation. PET and CT as the platform for the observation method have been widely used. Recently, we and other researchers have been gradually looking for serum or plasma markers of interest in monitoring, combining imaging methods to establish a more effective evaluation system that deserves further study.
Disclosure
The authors report no conflicts of interest in this work.
References
- FolkmanJTumor angiogenesis: therapeutic implicationsN Engl J Med197128521118211864938153
- CumsillePCoronelAConcaCQuiñinaoCEscuderoCProposal of a hybrid approach for tumor progression and tumor-induced angiogenesisTheor Biol Med Model20151211326133367
- ComunanzaVBussolinoFTherapy for Cancer: Strategy of Combining Anti-Angiogenic and Target TherapiesFront Cell Dev Biol2017510129270405
- JainRKAntiangiogenesis strategies revisited: from starving tumors to alleviating hypoxiaCancer Cell201426560562225517747
- CarmelietPJainRKAngiogenesis in cancer and other diseasesNature2000407680124925711001068
- BaeriswylVChristoforiGThe angiogenic switch in carcinogenesisSemin Cancer Biol200919532933719482086
- JainRKNormalization of tumor vasculature: an emerging concept in antiangiogenic therapyScience20053075706586215637262
- HuangDLanHLiuFAnti-angiogenesis or pro-angiogenesis for cancer treatment: focus on drug distributionInt J Clin Exp Med2015868369837626309490
- FolkmanJFighting cancer by attacking its blood supplySci Am199627531501548701285
- JainRKDudaDGClarkJWLoefflerJSLessons from phase III clinical trials on anti-VEGF therapy for cancerNat Clin Pract Oncol200631244016407877
- WuJMStatonCAAnti-angiogenic drug discovery: lessons from the past and thoughts for the futureExpert Opin Drug Discov20127872374322716277
- ShojaeiFAnti-angiogenesis therapy in cancer: Current challenges and future perspectivesCancer Lett2012320213013722425960
- GoelSWongAH-KJainRKVascular normalization as a therapeutic strategy for malignant and nonmalignant diseaseCold Spring Harb Perspect Med201223a00648622393532
- PetersonTEKirkpatrickNDHuangYDual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophagesProc Natl Acad Sci U S A2016113164470447527044097
- AdapalaRKThoppilRJGhoshKActivation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapyOncogene201635331432225867067
- TewariKSSillMWLongHJImproved survival with bevacizumab in advanced cervical cancerN Engl J Med Overseas Ed20143708734743
- SandlerAGrayRPerryMCPaclitaxel–Carboplatin Alone or with Bevacizumab for Non–Small-Cell Lung CancerN Engl J Med Overseas Ed20063552425422550
- GiantonioBJCatalanoPJMeropolNJBevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200J Clin Oncol200725121539154417442997
- SaltzLBClarkeSDíaz-RubioEBevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III studyJ Clin Oncol200826122013201918421054
- JainRKNormalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapyNat Med20017998798911533692
- HuangYGoelSDudaDGFukumuraDJainRKVascular normalization as an emerging strategy to enhance cancer immunotherapyCancer Res201373102943294823440426
- GoelSDudaDGXuLNormalization of the Vasculature for Treatment of Cancer and Other DiseasesPhysiol Rev20119131071112121742796
- ViallardCLarrivéeBTumor angiogenesis and vascular normalization: alternative therapeutic targetsAngiogenesis201720440942628660302
- MartinJDFukumuraDDudaDGBoucherYJainRKReengineering the Tumor Microenvironment to Alleviate Hypoxia and Overcome Cancer HeterogeneityCold Spring Harb Perspect Med2016612
- NingTJiangMPengQLow-dose endostatin normalizes the structure and function of tumor vasculature and improves the delivery and anti-tumor efficacy of cytotoxic drugs in a lung cancer xenograft murine modelThorac Cancer20123322923828920305
- CernigliaGJPoreNTsaiJHEpidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacyPLoS One200948e653919657384
- HuberPEBischofMJenneJTrimodal cancer treatment: beneficial effects of combined antiangiogenesis, radiation, and chemotherapyCancer Res20056593643365515867359
- JainRKDudaDGWillettCGBiomarkers of response and resistance to antiangiogenic therapyNat Rev Clin Oncol20096632733819483739
- LinMISessaWCAntiangiogenic therapy: creating a unique “window” of opportunityCancer Cell20046652953115607955
- HuangYYuanJRighiEVascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapyProc Natl Acad Sci U S A201210943175611756623045683
- YaoJZ-GangYangH-JiaoChenT-WuChenHuangJGastric adenocarcinoma: can perfusion CT help to noninvasively evaluate tumor angiogenesis?Abdom Imaging2011361152120336293
- KimEStamatelosSCebullaJBhujwallaZMPopelASPathakAPMultiscale imaging and computational modeling of blood flow in the tumor vasculatureAnn Biomed Eng201240112425244122565817
- MaERenAGaoBROI for outlining an entire tumor is a reliable approach for quantification of lung cancer tumor vascular parameters using CT perfusionOnco Targets Ther201692377238427175083
- ChenX-HRenKLiangPChaiYChenK-SGaoJ-BSpectral computed tomography in advanced gastric cancer: Can iodine concentration non-invasively assess angiogenesis?World J Gastroenterol20172391666167528321168
- CaYLeeKSKimEASolitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel densityRadiology2004233119119915304661
- WangJHMinPQWangPJDynamic CT Evaluation of Tumor Vascularity in Renal Cell CarcinomaAJR Am J Roentgenol200618651423143016632740
- HattoriYGabataTMatsuiOEnhancement patterns of pancreatic adenocarcinoma on conventional dynamic multi-detector row CT: Correlation with angiogenesis and fibrosisWorld J Gastroenterol200915253114312119575490
- GohVHalliganSDaleyFWellstedDMGuentherTBartramCIColorectal Tumor Vascularity: Quantitative Assessment with Multidetector CT-Do Tumor Perfusion Measurements Reflect Angiogenesis?Radiology2008249251051718812560
- HeistRSDudaDGSahaniDVImproved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancerProc Natl Acad Sci201511251547155225605928
- SchmitzSRommelDMichouxNDynamic contrast-enhanced computed tomography to assess early activity of cetuximab in squamous cell carcinoma of the head and neckRadiol Oncol2015491172525810697
- YangXKnoppMVQuantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a reviewJ Biomed Biotechnol201120111732848:11221541193
- HectorsSJJacobsILokJImproved Evaluation of Antivascular Cancer Therapy Using Constrained Tracer-Kinetic Modeling for Multiagent Dynamic Contrast-Enhanced MRICancer Res20187861561157029317433
- VriensDde Geus-OeiL-FHeerschapAvan LaarhovenHWMOyenWJGVascular and metabolic response to bevacizumab-containing regimens in two patients with colorectal liver metastases measured by dynamic contrast-enhanced MRI and dynamic 18F-FDG-PETClin Colorectal Cancer2011101E1E521609927
- SorensenAGBatchelorTTZhangW-TA “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patientsCancer Res200969135296530019549889
- SorensenAGEmblemKEPolaskovaPIncreased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusionCancer Res201272240240722127927
- JacksonAO’ConnorJPParkerGJJaysonGCImaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imagingClin Cancer Res200713123449345917575207
- ChLChenFHSchellingerhoutDLinYSHongJHLiuHLFlow versus permeability weighting in estimating the forward volumetric transfer constant (K(trans)) obtained by DCE-MRI with contrast agents of differing molecular sizesMagn Reson Imaging20173610511127989901
- de LussanetQGBackesWHGriffioenAWDynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancerInt J Radiat Oncol Biol Phys20056351309131516125874
- PadhaniARHayesCAssersohnLPrediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical resultsRadiology2006239236137416543585
- JohansenRJensenLRRydlandJPredicting survival and early clinical response to primary chemotherapy for patients with locally advanced breast cancer using DCE-MRIJ Magn Reson Imaging20092961300130719472387
- ParkIvon MorzeCLupoJMInvestigating tumor perfusion by hyperpolarized 13C MRI with comparison to conventional gadolinium contrast-enhanced MRI and pathology in orthotopic human GBM xenograftsMagn Reson Med201777284184726892398
- EmblemKEBjornerudAMouridsenKT(1)- and T(2)(*)-Dominant Extravasation Correction in DSC-MRI: Part II—Predicting Patient Outcome after a Single Dose of Cediranib in Recurrent Glioblastoma PatientsJ Cereb Blood Flow Metab201131102054206421505476
- WongALASundarRWangT-TPhase Ib/II randomized, open-label study of doxorubicin and cyclophosphamide with or without low-dose, short-course sunitinib in the pre-operative treatment of breast cancerOncotarget2016739640896409927577069
- BatchelorTTGerstnerEREmblemKEImproved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiationProc Natl Acad Sci U S A201311047190591906424190997
- KenSDeviersAFilleronTVoxel-based evidence of perfusion normalization in glioblastoma patients included in a phase I–II trial of radiotherapy/tipifarnib combinationJ Neurooncol2015124346547326189058
- BatchelorTTSorensenAGdi TomasoEAZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patientsCancer Cell2007111839517222792
- ChenB-BLuY-SLinC-HA pilot study to determine the timing and effect of bevacizumab on vascular normalization of metastatic brain tumors in breast cancerBMC Cancer201616146627412562
- WangYLiuMJinMLBlood Oxygenation Level-dependent Magnetic Resonance Imaging of Breast Cancer: Correlation with Carbonic Anhydrase IX and Vascular Endothelial Growth Factor. Chin Med J2017201713017176
- LiuMGuoXWangSBOLD-MRI of breast invasive ductal carcinoma: correlation of R2* value and the expression of HIF-1αEur Radiol201323123221322723835924
- GerstnerERZhangZFinkJRACRIN 6684: Assessment of Tumor Hypoxia in Newly Diagnosed Glioblastoma Using 18F-FMISO PET and MRIClin Cancer Res201622205079508627185374
- RanieriGMarechINiccoli AsabellaATyrosine-Kinase Inhibitors Therapies with Mainly Anti-Angiogenic Activity in Advanced Renal Cell Carcinoma: Value of PET/CT in Response EvaluationInt J Mol Sci20171891937
- HerbstRSMullaniNADavisDWDevelopment of biologic markers of response and assessment of antiangiogenic activity in a clinical trial of human recombinant endostatinJ Clin Oncol200220183804381412228200
- ChatterjeeSWieczorekCSchottleJTransient antiangiogenic treatment improves delivery of cytotoxic compounds and therapeutic outcome in lung cancerCancer Res201474102816282424675359
- KristianARevheimMEQuHDynamic 18F-FDG-PET for monitoring treatment effect following anti-angiogenic therapy in triple-negative breast cancer xenograftsActa Oncol20135271566157223984812
- YuWZhaoSZhaoYChanges in tumor oxygen state after sorafenib therapy evaluated by 18F-fluoromisonidazole hypoxia imaging of renal cell carcinoma xenograftsOncol Lett20171422341234628781672
- BekaertLValableSLechapt-ZalcmanE18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesisEur J Nucl Med Mol Imaging20174481383139228315948
- Hernández-AgudoEMondejarTSoto-MontenegroMLMonitoring vascular normalization induced by antiangiogenic treatment with 18F-fluoromisonidazole-PETMol Oncol201610570471826778791
- BarajasRFPampaloniMHClarkeJLAssessing Biological Response to Bevacizumab Using 18F-Fluoromisonidazole PET/MR Imaging in a Patient with Recurrent Anaplastic AstrocytomaCase Rep Radiol2015201511731361425793136
- MurakamiMZhaoSZhaoYANEvaluation of changes in the tumor microenvironment after sorafenib therapy by sequential histology and 18F-fluoromisonidazole hypoxia imaging in renal cell carcinomaInt J Oncol20124151593160022965141
- Jose BuenoMSanchezJColomerRQuintela-FandinoMBuenoMJAntiangiogenics and Hypoxic Response: Role of Fatty Acid Synthase InhibitorsCurr Drug Targets201617151735174627138758
- RylovaSNBarnuczEFaniMDoes imaging αvβ3 integrin expression with PET detect changes in angiogenesis during bevacizumab therapy?J Nucl Med201455111878188425278514
- ChenHNiuGWuHChenXClinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3Theranostics201661789226722375
- ShiJJinZLiuXPET Imaging of Neovascularization with 68Ga-3PRGD 2 for Assessing Tumor Early Response to Endostar Antiangiogenic TherapyMol Pharm201411113915392225158145
- TerrySYAbirajKLokJCan 111In-RGD2 monitor response to therapy in head and neck tumor xenografts?J Nucl Med201455111849185525349221
- BeckerSBohnPBouyeure-PetitACBevacizumab enhances efficiency of radiotherapy in a lung adenocarcinoma rodent model: Role of αvβ3 imaging in determining optimal windowNucl Med Biol2015421292393026410810
- VangestelCvan de WieleCvan DammeN(99)mTc-(CO) (3) His-annexin A5 micro-SPECT demonstrates increased cell death by irinotecan during the vascular normalization window caused by bevacizumabJ Nucl Med201152111786179422045708
- BourgeoisMRajerisonHGuerardFContribution of [64Cu]-ATSM PET in molecular imaging of tumour hypoxia compared to classical [18F]-MISO – a selected reviewNuclear Medicine Review2011142909522219149
- HoytKUmphreyHLockhartMRobbinMForero-TorresAUltrasound imaging of breast tumor perfusion and neovascular morphologyUltrasound Med Biol20154192292230226116159
- HudsonJMWilliamsRKarshafianRQuantifying vascular heterogeneity using microbubble disruption-replenishment kinetics in patients with renal cell cancerInvest Radiol201449211612324220251
- LassauNChamiLBenatsouBPeronneauPRocheADynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatmentEur Radiol200717Suppl 6(S6)8998
- MolinariFMantovaniADeandreaMLimonePGarberoglioRSuriJSCharacterization of single thyroid nodules by contrast-enhanced 3-D ultrasoundUltrasound Med Biol201036101616162520800947
- LassauNBonastreJKindMValidation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques studyInvest Radiol2014491279480024991866
- LassauNChapototLBenatsouBStandardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: the French multicenter Support for Innovative and Expensive Techniques StudyInvest Radiol2012471271171623095862
- LassauNChamiLChebilMDynamic contrast-enhanced ultrasonography (DCE-US) and anti-angiogenic treatmentsDiscov Med20111156182421276407
- FrampasELassauNZappaMVulliermeM-PKoscielnySVilgrainVAdvanced Hepatocellular Carcinoma: Early evaluation of response to targeted therapy and prognostic value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary resultsEur J Radiol2013825e205e21123273822
- LassauNKoscielnySAlbigesLMetastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonographyClin Cancer Res20101641216122520145174
- LassauNCoiffierBFaivreLStudy of Intrapatient Variability and Reproducibility of Quantitative Tumor Perfusion Parameters Evaluated With Dynamic Contrast-Enhanced UltrasonographyInvest Radiol2017523115427828787
- EisenbreyJRJoshiNDaveJKForsbergFAssessing algorithms for defining vascular architecture in subharmonic images of breast lesionsPhys Med Biol201156491993021248388
- GessnerRCAylwardSRDaytonPAMapping microvasculature with acoustic angiography yields quantifiable differences between healthy and tumor-bearing tissue volumes in a rodent modelRadiology2012264373374022771882
- ChenMWangWPJiaWRThree-dimensional contrast-enhanced sonography in the assessment of breast tumor angiogenesis: correlation with microvessel density and vascular endothelial growth factor expressionJ Ultrasound Med201433583584624764339
- ChangY-CHuangY-HHuangC-SChangR-FVascular morphology and tortuosity analysis of breast tumor inside and outside contour by 3-D power Doppler ultrasoundUltrasound Med Biol201238111859186922975041
- FeingoldSGessnerRGuracarIMDaytonPAQuantitative volumetric perfusion mapping of the microvasculature using contrast ultrasoundInvest Radiol2010451066967420808232
- LassauNKoscielnySChamiLAdvanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification--preliminary resultsRadiology2011258129130020980447
- LassauNCoiffierBKindMSelection of an early biomarker for vascular normalization using dynamic contrast-enhanced ultrasonography to predict outcomes of metastatic patients treated with bevacizumabAnn Oncol201627101922192827502701
- WuZYangXChenLAnti-angiogenic therapy with contrast-enhanced ultrasound in colorectal cancer patients with liver metastasisMedicine20179620e673128514289
- ChenDRLinCWangYFWindow of opportunity: A new insight into sequential bevacizumab and paclitaxel in two cases of metastatic triple-negative breast cancerExp Ther Med201510388588826622409
- DudaDGWillettCGAncukiewiczMPlasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancerOncologist201015657758320484123
- FueyoJHossainMBNguyenTGomez-ManzanoCNormalizing tumoral vessels to treat cancer: an out-of-the-box strategy involving TIE2 pathwayTransl Cancer Res20176S2S317S32028944168
- AugustinHGYoung KohGThurstonGAlitaloKControl of vascular morphogenesis and homeostasis through the angiopoietin–Tie systemNat Rev Mol Cell Biol200910316517719234476
- BatchelorTTDudaDGdi TomasoEPhase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastomaJ Clin Oncol201028172817282320458050
- SieMWagemakersMMolemaGMooijJJde BontESden DunnenWFThe angiopoietin 1/angiopoietin 2 balance as a prognostic marker in primary glioblastoma multiformeJ Neurosurg2009110114715518991494
- KamounWSLeyCDFarrarCTEdema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in miceJ Clin Oncol200927152542255219332720
- ZhangJLiuQFangZHypoxia induces the proliferation of endothelial progenitor cells via upregulation of Apelin/APLNR/MAPK signalingMol Med Rep20161321801180626676468
- WuLChenLLiLApelin/APJ system: A novel promising therapy target for pathological angiogenesisClinica Chimica Acta20174667884
- AliYFEl-MorshedySImamAAThe role of serum apelin in retinopathy of prematurityClin Ophthalmol20171138739228260850
- KidoyaHTakakuraNBiology of the apelin-APJ axis in vascular formationJ Biochem2012152212513122745157
- ZhangLTakaraKYamakawaDKidoyaHTakakuraNApelin as a marker for monitoring the tumor vessel normalization window during antiangiogenic therapyCancer Sci20161071364426475217
- TianLGoldsteinAWangHMutual regulation of tumour vessel normalization and immunostimulatory reprogrammingNature2017544764925025428371798
