Abstract
Purpose
Triple-negative breast cancer (TNBC) is more than a single disease. Identifying biomarkers to further subdivide TNBC patients with distinct outcome is of great importance. It has been reported that single-nucleotide polymorphisms (SNPs) in Aurora kinase A (AURKA) or Aurora kinase B (AURKB) are associated with the risk and survival of several cancers. But till now, there is no research about these polymorphisms in TNBC patients.
Materials and methods
In this study, we investigated the association between polymorphisms in AURKA or AURKB gene and prognosis of TNBC patients treated with taxane-based adjuvant chemotherapy. A total of 273 TNBC patients were enrolled. Haploview 4.2 software was used to identify Tag SNPs. Genotyping was conducted using the MassARRAY MALDI-TOF system.
Results
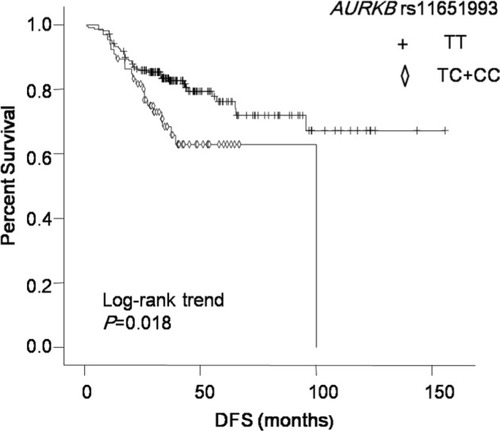
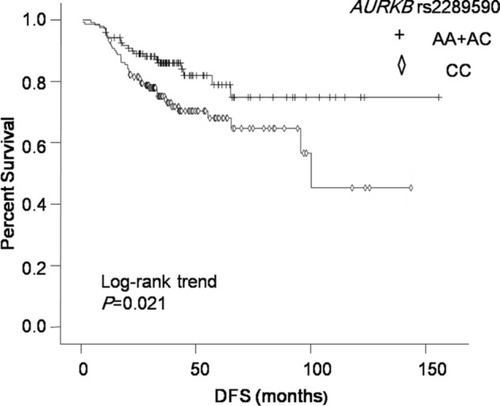
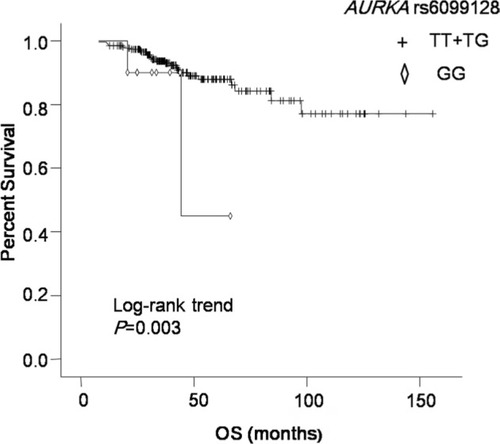
We found that AURKA rs6099128 GG genotype carriers had significantly worse overall survival (OS) than TT+ TG genotype carriers (P = 0.003, HR = 12.499, 95% CI = 2.357–66.298). AURKB rs11651993 TT genotype carriers had better disease-free survival (DFS) than TC + CC genotype carriers (P = 0.018, HR = 1.876, 95% CI = 1.116–3.154). AURKB rs2289590 CC genotype carriers had worse DFS than CA + AA genotype carriers (P = 0.021, HR = 0.536, 95% CI = 0.315–0.912). After subgroup analysis, rs11651993 TC + CC genotype predicted worse DFS in subgroups of age ≤ 50, post-menopausal, grade unknown (UK), tumor size >2 cm, and lymph node negative. Rs2289590 CA + AA genotype could predict favorable DFS in pre-menopausal, grade 3 and lymph node-positive patients.
Conclusion
We first demonstrated that polymorphisms in AURKA or AURKB gene might predict the OS or DFS of TNBC patients treated with taxane-based adjuvant chemotherapy.
Keywords:
Introduction
Breast cancer is the most common cancer and is the leading cause of cancer death in women around the world.Citation1 It is a very heterogeneous disease and is divided into several subgroups that have different clinicopathological characteristics and prognosis.Citation2 Triple- negative breast cancer (TNBC) is defined as lacking expression of estrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor 2 (HER2). It is characterized by aggressive behavior, onset at young age, and early relapse.Citation3,Citation4 TNBC is insensitive to endocrine and HER2-targeted therapy, and therefore, chemotherapy remains the mainstay of treatment. Although some clinical trials found that TNBC is more sensitive to platinum-based chemotherapy,Citation5,Citation6 taxane/anthracycline-based regimens are still standard and preferred regimens for TNBC patients in the adjuvant setting.Citation7 However, not all TNBC patients respond to chemotherapy, which suggests that TNBC is more than a single disease.Citation8 Identifying biomarkers to further subdivide TNBC patients with distinct outcome is of great importance.
Aurora kinase A (AURKA) and Aurora kinase B (AURKB) are members of the Aurora kinase subfamily of conserved serine/threonine kinases.Citation9 AURKA localizes to the duplicate centrosomes from the beginning of S phase, shifts to the bipolar spindle microtubules during mitosis, and, finally, moves to perinuclear materials of the daughter cells at the end of mitosis.Citation10 By contrast, AURKB starts at early G2 and localizes to the chromosomes in prophase, the centromere in prometaphase and metaphase, the central spindle in anaphase, and the mid-body in cytokinesis.Citation11 AURKA plays a critical role in centrosome duplication and maturation.Citation12,Citation13 AURKB plays a key role during mitosis by regulating chromosomal alignment, segregation, and cytokinesis, as the catalytic protein of the chromosomal passenger complex (CPC).Citation11 Deregulation of Aurora kinases leads to impairment of mitotic spindle checkpoints causing abnormal spindle assembly.Citation9
It has been reported that single-nucleotide polymorphisms (SNPs) in AURKA or AURKB are associated with the risk and survival of several cancers including breast cancer,Citation14 esophageal cancer,Citation15 and so on. But till now, there is no research about polymorphisms in AURKA or AURKB and prognosis of TNBC patients. In our study, we first demonstrated that polymorphisms in AURKA or AURKB gene were associated with the survival of TNBC patients.
Materials and methods
Study subjects
Between January 2004 and December 2012, 273 primary TNBC patients treated with taxane-based adjuvant chemotherapy were enrolled in this study. Blood samples were collected from each patient. Patients were followed up until July 30, 2017, to collect data on recurrence and death. The disease-free survival (DFS) time was defined as the time from the date of diagnosis until the date of first locoregional recurrence, first distant metastasis, or death from any cause (whichever came first). Overall survival (OS) was defined as the time from the date of diagnosis to the date of death from any reason or last follow-up.
Formalin-fixed, paraffin-embedded breast cancer tissue samples were obtained from the patients. Immunohistochemistry (IHC) performed with anti-ER and anti-PR antibodies was used to evaluate the ER and PR status. A positive ER and PR status was defined by nuclear staining of more than 1% according to the guidelines issued by the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAPs) in 2010.Citation16 To determine the HER2 status, IHC or fluorescence in situ hybridization (FISH) was performed.Citation17 Tumors negative for ER, PR, and HER2 were defined as TNBCs.
Ethics statement
This investigation was approved by the institutional review board of the Chinese Academy of Medical Sciences Cancer Hospital. It was conducted in accordance with the ethical standards of the Declaration of Helsinki and following the national and international guidelines. Written informed consent was obtained from all patients.
SNP selection and genotyping
Genotype data from AURKA and AURKB gene regions encompassing 10 kb of upstream and 3 kb of downstream flanking sequences were extracted from the HapMap Chinese Han population (Hapmap Data Rel 27, Phase II + III, http://www.HapMap.org). Haploview 4.2 software (http://www.broadinstitute.org/mpg/haploview) was used to identify Tag SNPs. The inclusion criteria were SNPs known in ethnic Han Chinese people and with a minor allele frequency (MAF) of 0.05. Finally, a total of 11 candidate SNPs were selected for genotyping, and information for these SNPs is listed in . Primers and probes were designed using MassARRAY Typer 4.0 software.
Table 1 Information for the SNPs genotyped in this study
Peripheral blood samples (5 mL) were collected from each subject on recruitment. Genomic DNA was isolated by the routine phenol–chloroform method. Each DNA sample was diluted to a working concentration of 10 ng/mL for genotyping. Genotyping was conducted using the MassARRAY MALDI-TOF System (Sequenom Inc., San Diego, CA, USA)Citation18,Citation19 at once by the method described in the Sequenom Genotyping Protocol. Twenty percent duplicate samples and negative controls (without DNA) were included for quality assurance of genotyping. Concordance for duplicate samples was 100%. The analysts who carried out the genotyping were blinded to the group information on each sample.
Statistical analyses
SPSS version 18.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. The 5-year DFS rates and 5-year OS rates were estimated by the Kaplan–Meier product limit method for each of the different genotypes and expressed in percentages. Comparisons were made with the log-rank test. HRs of recurrence/metastasis and death with 95% CIs were estimated by using the Cox model. The multivariate analysis was adjusted for age (≤50 vs >50), histological grade (1–2 vs 3 vs unknown [UK]), tumor size (≤2 cm vs >2 cm), lymph node status (with vs without regional lymph node metastasis), and vascular invasion (with vs without vascular invasion). We performed subgroup analysis for polymorphisms, which were associated with OS or DFS of patients in multivariate analysis. Since there were only 10 patients with rs6099128 GG genotype, we did not perform subgroup analysis for rs6099128. All statistical tests were two sided, and P < 0.05 was considered significant.
Results
Clinical characteristics and survival of TNBC patients
A total of 273 patients were enrolled in this study. The median age at diagnosis is 48 years (range, 22–75 years). The 5-year OS rate was 87.1%, and the 5-year DFS rate was 72.8%. Among total patients, 142 (52.0%) and 131 (48.0%) patients were at pre- and post-menopausal stages, respectively. Seventy (25.6%) patients presented with grade 1–2 and 152 (55.7%) with grade 3 tumors. Eighty-four (30.8%), 137 (50.2%), and 52 (19.0%) subjects were diagnosed at stage I, II, and III, respectively. The relationship between clinicopathological characteristics and survival of these patients is summarized in . Patients with grade 1–2 tumors had a significantly higher 5-year OS rate than those with grade 3 tumors (96.8% vs 79.5%, P = 0.039, HR = 3.640, 95% CI = 1.071–12.373). Tumor size and lymph node status were significantly related to both DFS and OS. No significant association was observed between age or menopausal status and TNBC survival. After multivariate analysis, tumor size (P = 0.024, HR = 3.149, 95% CI = 1.166–8.509) and lymph node status (P < 0.001, HR = 11.058, 95% CI = 3.287–37.206) were demonstrated to be independent prognostic factors.
Table 2 Clinicopathological characteristics and survival of TNBC
Table 3 AURKA or AURKB genotypes and DFS
Table 4 AURKA or AURKB genotypes and overall survival
Polymorphisms in AURKA or AURKB gene and survival of TNBC patients
The results of relationship between polymorphisms in AURKA or AURKB and TNBC survival in different genetic models are summarized in and . In univariate analysis, AURKA rs10485805 GA genotype carriers had worse prognosis than GG genotype carriers (P = 0.043, HR = 2.177, 95% CI = 1.024–4.628). But after multivariate analysis, there was no association between rs10485805 genotype and OS. In multivariate analysis, AURKA rs6099128 GG genotype carriers had significantly worse OS than TT+ TG genotype carriers (P = 0.003, HR = 12.499, 95% CI = 2.357–66.298; ). Two polymorphisms in AURKB were significantly associated with DFS in both univariate and multivariate analyses, including AURKB rs11651993 and AURKB rs2289590 ( and ). AURKB rs11651993 TT genotype carriers had better DFS than TC + CC genotype carriers (P = 0.018, HR = 1.876, 95% CI = 1.116–3.154). AURKB rs2289590 CC genotype carriers had worse DFS than CA + AA genotype carriers (P = 0.021, HR = 0.536, 95% CI = 0.315–0.912).
Figure 1 Kaplan–Meier curve of OS for patients with different AURKA rs6099128 genotypes.
Abbreviation: OS, overall survival.
Polymorphisms in AURKA or AURKB gene and survival of TNBC in different subgroups
In multivariate analysis, AURKA rs6099128 was associated with OS, but since there were only 10 patients with GG genotype, we did not explore the relationship in subgroups. As shown in , we explored the relationship between AURKB rs11651993 or rs2289590 and survival of TNBC in different subgroups. AURKB rs11651993 TC + CC genotype predicted worse DFS in subgroups of age ≤ 50 years, post-menopausal, grade UK, tumor size >2 cm, and lymph node negative. It also predicted shorter OS in subgroups of age > 50 years and grade 3. AURKB rs2289590 CA + AA genotype could predict favorable DFS in pre-menopausal, grade 3, and lymph node-positive patients. No significant relationship was observed between rs2289590 and OS in any subgroups.
Table 5 Subgroup analysis of polymorphisms and survival
Discussion
Polymorphisms in AURKA or AURKB have been reported to be associated with prognosis of some kinds of cancers. Up to now, there is no such comprehensive research that investigated the association between polymorphisms in both AURKA and AURKB and the outcome of TNBC patients. In our study, we first demonstrated that AURKA rs6099128 GG genotype carriers had significantly worse OS than TT + TG genotype carriers, AURKB rs11651993 C allele was associated with worse DFS, and AURKB rs2289590 A allele was significantly associated with better DFS.
AURKA gene is an oncogene located on chromosome 20q13. Polymorphisms in AURKA gene are associated with risk and intrinsic subtype of breast cancer.Citation20,Citation21 In the study by Ruan et al,Citation22 AURKA rs10485805 was associated with risk of breast cancer under the recessive genetic model (OR = 0.38, 95% CI = 0.18–0.82, P = 0.014); but there is no relationship between rs2298016 and risk in Chinese population. Taylor et alCitation23 found that rs6099128 had reduced ORs for luminal A (OR = 0.76, 95% CI = 0.60–0.95) and basal-like breast cancer (OR = 0.54, 95% CI = 0.37–0.80). Only a few studies in the literature reported the association between polymorphisms in AURKA and OS of breast cancer. Shi et alCitation24 found that in the Swedish population, for rs8173, the BC-specific survival was worse in women with at least one G allele, when they had tumors smaller than 2 cm (HR = 2.74, 95% CI = 1.08–6.98) or stage 0–I tumors (HR = 6.94, 95% CI = 1.45–33.22). However, in our research, there is no relationship between genotypes of rs8173 and survival of TNBC. Maybe it is because of the different ethnic groups and different subtypes of BC. Among the six SNPs of AURKA investigated in our study, rs1468056 and rs2236207 had never been reported. We found that they were not associated with OS of TNBC. We first demonstrated that AURKA rs6099128 GG genotype carriers had significantly worse OS than TT + TG genotype carriers (P = 0.003, HR = 12.499, 95% CI = 2.357–66.298).
High AURKA expression was strongly associated with decreased survival of breast cancer (P = 0.0005), and it was an independent prognostic marker in the study by Nadler et al.Citation25 In TNBC patients with AURKA high expression, the risk of distant recurrence peaked at the first 3 years and declined rapidly thereafter, whereas patients with AURKA low expression showed a relatively constant risk of recurrence during the entire follow-up period. Univariate and multivariate analysis showed that overexpression of AURKA predicted poor OS (P = 0.002) and progression-free survival (P = 0.012) in TNBC.Citation26 AURKA had been reported to interact and phosphorylate several important proteins involved in stress response and cell cycle checkpoint after DNA damage.Citation27 Overexpression of AURKA led to diminished transcriptional activity and increased degradation of p53, causing checkpoint defects and genetic instability and ultimately facilitating cancer development and progression.Citation28
AURKB gene is located on chromosome 17p13. In German population,Citation29 synonymous AURKB rs2241909 (885A>G) polymorphism resulted in an increased familial breast cancer risk for carriers of the homozygous 885G genotype (OR = 1.45, 95% CI = 1.05–2.0, P = 0.02). There have been debating data regarding the role of the AURKB expression in cancer prognosis. Zhang et alCitation30 found that AURKB expression was correlated with the proliferation index (P < 0.001) and p53 expression (P = 0.014) in breast cancer tissues. Higher expression of AURKB is significantly correlated with the poor survival in these cases (P = 0.038). A multivariate Cox regression analysis demonstrated that AURKB expression is an independent prognostic indicator of breast cancer DFS (HR = 1.39, 95% CI = 1.04–1.86). While in the study by Nadler et al,Citation25 AURKB expression was not associated with the survival of breast cancer patients. None of the polymorphisms in AURKB of our study has been reported in TNBC. We first found that rs11651993 TC + CC genotype predicted worse DFS in subgroup of age ≤ 50 years, post-menopausal, grade UK, tumor size >2 cm, and lymph node negative. It also predicted shorter OS in subgroup of age > 50 years and grade 3. Rs2289590 CA + AA genotype could predict favorable DFS in pre-menopausal, grade 3, and lymph node-positive patients.
All patients in our study received taxane-based adjuvant chemotherapy. Taxanes are microtubule targeting agents (MTAs), which are most widely used drugs in adjuvant setting for TNBC patients. The cytotoxic action of these compounds is mediated primarily through their binding of β-tubulin monomers, leading to microtubule stabilization, thus blocking their depolymerization and subsequently triggering cell cycle arrest at the G2/M phase.Citation31 Considering the role of Aurora kinases in spindle formation and the reported extent of their deregulation in cancer, we hypothesize that polymorphisms in AURKA or AURKB might contribute to taxane resistance and then influence the survival of cancer patients. Zhang et alCitation30 demonstrated that in breast cancer patients who received neoadjuvant chemotherapy (containing sequential taxane and anthracycline-based regimens), elevated expression of AURKB contributed to chemoresistance (P = 0.011). In taxane-resistance breast cancer cell lines, expression of AURKA was significantly higher. Knockdown of AURKA not only markedly decreased the expression of P-gp but also downregulated the P-gp function in resistant breast cancer cells. The results indicated that AURKA plays a crucial role in paclitaxel-resistant breast cancer.Citation32
However, the underlying mechanisms of these SNPs on survival of TNBC are not yet clear and need to be further investigated. By silico analysis of prediction of binding motifs, Mesic et alCitation33 indicated that polymorphic sites in AURKA and AURKB could bind different transcription factors. As for AURKA rs8173, when the G allele was present, C/EBPalpha, C/EBPbeta, and NF-1 transcription factor binding motifs were recognized, whereas when the same region contained the C allele, additional E2F and RAR-β transcription factor motifs were identified. In the case of AURKB rs2289590, when the A allele was present, PEA3 and TFII-I binding motifs were recognized. In contrast, when the C allele is present, PEA3, TFII-I, and YY1 binding motifs were identified. Since YY1 expression level and/or activity is associated with unchecked cell proliferation, resistance to apoptosis, metastasis, and tumor cell resistance to chemotherapeutics,Citation34 the binding of an additional YY1 protein in the presence of C allele may alter the level of AURKB expression and then influence the survival, which might be a possible explanation for our result that AURKB rs2289590 A allele was significantly associated with better DFS.
Previous studies enrolled different subtypes of breast cancer patients, and the regimens are not consistent. In our study, only TNBC patients were enrolled, which can better explain the correlation between polymorphisms in AURKA or AURKB and survival. There are some limitations of our study. First, this was a retrospective study, so selection bias might exist. Second, the sample size was relatively small, and the prospective large-scale studies are needed to confirm the conclusions. Finally, since we did not explore the mechanisms, further studies on the biological mechanisms are warranted.
Conclusion
For the first time in TNBC patients receiving taxane-based adjuvant chemotherapy, we demonstrated that AURKA rs6099128, AURKB rs11651993, and AURKB rs2289590 were associated with the survival. Since most polymorphisms have never been reported, more research are needed to verify our results.
Acknowledgments
This study was funded by Natural Science Foundation of Jiangxi Province of China (No. 20151BAB205043).
Disclosure
The authors declare no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer Statistics, 2017CA cancer J Clin201767173028055103
- BlowsFMDriverKESchmidtMKSubtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studiesPLoS Med201075e100027920520800
- HafftyBGYangQReissMLocoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancerJ Clin Oncol200624365652565717116942
- PerezEAMoreno-AspitiaAAubrey ThompsonEAndorferCAAdjuvant therapy of triple negative breast cancerBreast Cancer Res Treat2010120228529120094772
- VernieriCMilanoMMennittoAAntitumor activity and safety profile of weekly carboplatin plus paclitaxel in metastatic breast cancer: a ten-year, monocentric, retrospective studyBreast Cancer Res Treat2017165236537328616768
- FerreiraARMetzger-FilhoOSarmentoRMBBinesJNeoadjuvant Treatment of Stage IIB/III Triple Negative Breast Cancer with Cyclophosphamide, Doxorubicin, and Cisplatin (CAP Regimen): A Single Arm, Single Center Phase II Study (GBECAM 2008/02)Front Oncol2017732929416986
- La BelleAKhatibJSchiemannWPVinayakSRole of Platinum in Early-Stage Triple-Negative Breast CancerCurr Treat Options Oncol201718116829110096
- SaraivaDPGuadalupe CabralMJacintoABragaSHow many diseases is triple negative breast cancer: the protagonism of the immune microenvironmentESMO Open201724e00020829018573
- FuJBianMJiangQZhangCRoles of Aurora kinases in mitosis and tumorigenesisMol Cancer Res20075111017259342
- SugimotoKUranoTZushiHMolecular dynamics of Aurora-A kinase in living mitotic cells simultaneously visualized with histone H3 and nuclear membrane protein importinalphaCell Struct Funct200227645746712576638
- XuZOgawaHVagnarelliPINCENP-aurora B interactions modulate kinase activity and chromosome passenger complex localizationJ Cell Biol2009187563765319951914
- MarumotoTZhangDSayaHAurora-A - a guardian of polesNat Rev Cancer200551425015630414
- KovarikovaVBurkusJRehakPBrzakovaASolcPBaranVAurora kinase A is essential for correct chromosome segregation in mouse zygoteZygote201624332633726174602
- QinKWuCWuXTwo nonsynonymous polymorphisms (F31I and V57I) of the STK15 gene and breast cancer risk: a meta-analysis based on 5966 cases and 7609 controlsJ Int Med Res201341495696323803310
- PanJYAjaniJAGuJAssociation of Aurora-A (STK15) kinase polymorphisms with clinical outcome of esophageal cancer treated with preoperative chemoradiationCancer2012118174346435322213102
- HammondMEHayesDFDowsettMAmerican Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancerJ Clin Oncol201028162784279520404251
- WolffACHammondMEHicksDGRecommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline updateJ Clin Oncol201331313997401324101045
- XiuLZhangCWuZPengJEstablishment and Application of a Universal Coronavirus Screening Method Using MALDI-TOF Mass SpectrometryFront Microbiol20178151028848521
- CheungKWPengQHeLRapid and Simultaneous Detection of Major Drug Resistance Mutations in Reverse Transcriptase Gene for HIV-1 CRF01_AE, CRF07_BC and Subtype B in China Using Sequenom MassARRAY® SystemPLoS One2016114e015364127092551
- FletcherOJohnsonNPallesCInconsistent association between the STK15 F31I genetic polymorphism and breast cancer riskJ Natl Cancer Inst200698141014101816849685
- CoxDGHankinsonSEHunterDJPolymorphisms of the AURKA (STK15/Aurora Kinase) Gene and Breast Cancer Risk (United States)Cancer Causes Control2006171818316411056
- RuanYSongAPWangHGenetic polymorphisms in AURKA and BRCA1 are associated with breast cancer susceptibility in a Chinese Han populationJ Pathol2011225453554321598251
- TaylorNJBensenJTPooleCGenetic variation in cell cycle regulatory gene AURKA and association with intrinsic breast cancer subtypeMol Carcinog201554121668167725328151
- ShiHBevierMJohanssonRSingle nucleotide polymorphisms in the 20q13 amplicon genes in relation to breast cancer risk and clinical outcomeBreast Cancer Res Treat2011130390591621630024
- NadlerYCampRLSchwartzCRimmDLKlugerHMKlugerYExpression of Aurora A (but not Aurora B) is predictive of survival in breast cancerClin Cancer Res200814144455446218628459
- XuJWuXZhouWHAurora-A identifies early recurrence and poor prognosis and promises a potential therapeutic target in triple negative breast cancerPLoS One201382e5691923437271
- MarumotoTHondaSHaraTAurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cellsJ Biol Chem200327851517865179514523000
- TentlerJJIonkinaAATanACp53 Family Members Regulate Phenotypic Response to Aurora Kinase A Inhibition in Triple-Negative Breast CancerMol Cancer Ther20151451117112925758253
- TchatchouSWirtenbergerMHemminkiKAurora kinases A and B and familial breast cancer riskCancer Lett2007247226627216762494
- ZhangYJiangCLiHElevated Aurora B expression contributes to chemoresistance and poor prognosis in breast cancerInt J Clin Exp Pathol20158175175725755770
- LiYTangKZhangHZhangYZhouWChenXFunction of Aurora kinase A in Taxol-resistant breast cancer and its correlation with P-gpMol Med Rep20114473974621584498
- MonzóMRosellRSánchezJJPaclitaxel resistance in non- small-cell lung cancer associated with beta-tubulin gene mutationsJ Clin Oncol19991761786179310561216
- MesicAMarkocicERogarMJuvanRHudlerPKomelRSingle nucleotide polymorphisms rs911160 in AURKA and rs2289590 in AURKB mitotic checkpoint genes contribute to gastric cancer susceptibilityEnviron Mol Mutagen201758970171128843004
- GordonSAkopyanGGarbanHBonavidaBTranscription factor YY1: structure, function, and therapeutic implications in cancer biologyOncogene20062581125114216314846