Abstract
Purpose
Contrast-enhanced ultrasound (CEUS) is a new technique used to distinguish benign from metastatic superficial lymph nodes (LNs) with variable accuracy. The objective of this meta-analysis was to evaluate the accuracy of CEUS for the diagnosis of superficial metastatic LNs.
Methods
A comprehensive literature search of PubMed, Web of Science, Medline, Embase, and the Cochrane Library was performed until April 2018. Nine articles reporting the diagnosis of 436 LNs by CEUS were investigated. The Mantel–Haenszel and DerSimonian–Laird methods were used to analyze pooled sensitivity, specificity, diagnostic OR, positive likelihood ratio (LR), and negative LR, and a summary receiver operating characteristic (SROC) curve was also evaluated.
Results
The pooled sensitivity and specificity of CEUS for the differential diagnosis of benign and metastatic superficial LNs were 0.88 (95% CI, 0.83–0.92) and 0.80 (95% CI, 0.74–0.85), respectively. The pooled positive LR, negative LR, and diagnostic OR were 4.36 (95% CI, 2.38–7.99), 0.17 (95% CI, 0.10–0.31), and 32.75 (95% CI, 11.08–96.84), respectively. SROC area under the curve (AUC) was 0.9288. The subgroup analysis excluding outliers implied that the heterogeneity was almost eliminated, and the pooled sensitivity and specificity were 0.87 (95% CI, 0.81–0.92) and 0.79 (95% CI, 0.71–0.85), respectively. The SROC AUC was 0.8950.
Conclusion
CEUS, a novel imaging modality for the characterization of superficial LNs, shows a high sensitivity and specificity in the diagnosis of superficial metastatic LNs. The different diagnostic standards, contrast agents, and contrast modes contribute to a considerable level of heterogeneity among studies. A uniform standard for distinguishing between benign and metastatic LNs is needed for further clinical application.
Introduction
In a wide number of clinical situations, including prognosis prediction, carcinoma staging, selecting, and monitoring treatment for patients with carcinoma and lymphoma, it is crucial to distinguish between benign and malignant lymph nodes (LNs).Citation1,Citation2 Currently, several diagnostic imaging modalities are applied to characterize LNs, including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). As reported by Liao et al,Citation3 the pooled sensitivity of CT, MRI, PET, and US was 52%, 65%, 66%, and 66%, respectively, while the pooled specificity was 93%, 81%, 87%, and 78%, respectively. However, currently, there is no imaging modality capable of accurately staging nodal disease. In this context, US-guided fine-needle aspiration cytology (FNAC) can improve the diagnostic accuracy; FNAC has proven to be the most accurate imaging modality for the detection of cervical LN metastases.Citation4 Compared with other modalities, US is easy to use, widely available, and less expensive and does not involve exposure to ionizing radiation. Currently, US is regarded as a valuable tool for the diagnosis of metastatic LNs,Citation5,Citation6 which are characterized by hypoechoic mass, the absence of echogenic hilum, round shape, and blood vessels that are predominant in periphery.Citation6–Citation8
Contrast-enhanced ultrasound (CEUS), a novel technique for evaluating tissue perfusion in real time, has been widely used for the evaluation of lesions in the liver, kidney, pancreas, spleen, ovary, thyroid, breast, and prostate, as well as for sentinel nodes identification in patients with breast cancer.Citation9–Citation12 CEUS helps radiologists to detect and characterize focal lesions; its contrast pools, as well as its sensitivity and specificity in diagnosing liver lesions, are similar to contrast CT, which is more costly and involves higher levels of exposure to ionizing radiation. Recently, CEUS has been used to distinguish benign from malignant superficial LNs,Citation13–Citation15 revealing that CEUS has a higher sensitivity in identifying minor regions of hypovascularity or hypervascularity not detectable by Doppler techniques, thus leading to a better evaluation in LNs categorization.Citation16 Compared with other imaging modalities, such as CT and MRI, gray-scale US combined with CEUS is capable of evaluating the shape, margins, internal structure, and vascularization of LNs.Citation17 It has improved the accuracy of ultrasonography for the diagnosis of superficial lymphadenopathy compared with conventional US.Citation18,Citation19 Nevertheless, Schulte-Altedorneburg et alCitation20 have found that the use of US agent does not improve the diagnostic accuracy in identifying malignant LNs. The main limitation of CEUS is the practice pattern because the interpretation of sonographic images depends on individual skill and the experience of the radiologist. In addition, CEUS is not suitable for patients who have allergic reaction to contrast agents or pregnant women.Citation21
A series of studies assessing the accuracy of CEUS in distinguishing benign from malignant superficial LNs have been published, with the sensitivity ranging from 76% to 99% and the specificity ranging from 55% to 98%.Citation13,Citation15,Citation22–Citation32 The aim of this meta-analysis was to evaluate the overall accuracy of CEUS for the differential diagnosis between benign and metastatic superficial LNs.
Methods
Search strategy
A comprehensive search of PubMed, Web of Science, Medline, Embase, and the Cochrane Library was performed for relevant literature published from January 1980 to April 2018. The search terms were (lymph node OR lymph nodes) and (ultrasound OR sonography OR ultrasonography OR ultrasonic OR US) and (contrast enhanced OR CE OR contrast enhancement OR echo enhanced OR contrast imaging) and (surgery OR biopsy OR pathology OR histology). All references from the selected studies were reviewed to identify additional relevant articles to expand our study. Two authors (Mei Mei and Ligang Ye) independently searched and extracted the data following the same process. All disagreements were resolved by mutual agreement. Articles written by the same authors were scrutinized to avoid duplicate data. In case there was any unclear information, the authors were contacted for further clarification.
The local ethics committee and institutional review board approved this study.
Study selection
The following studies were included: 1) studies using CEUS for differential diagnosis between benign and metastatic superficial LNs among adults with carcinoma; 2) studies using a reference standard of US-guided biopsy or surgical histology; 3) articles written in English; and 4) studies providing adequate data to construct a 2×2 contingency table for true-positive, true-negative, false-positive, and false-negative results.
The following studies were excluded: 1) studies using CEUS for identifying sentinel LNs by subcutaneous injection of contrast agent; 2) studies with incomplete data available to construct a 2×2 contingency table; 3) retrospective studies; 4) studies that recruited patients with lymphoma; 5) studies using CEUS for evaluating LNs in pediatric patients; 6) studies with duplicate data; 7) reviews, editorials, and letters that did not report their own data; and 8) case reports.
Quality of studies
The revised tool for the quality assessment of diagnostic accuracy studies (QUADAS-2) was used to assess the quality of included studies.Citation33 This tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Two investigators assessed the quality of the selected studies independently. All disagreements were resolved by mutual consensus. For each domain, the risk of bias and concerns about applicability were labeled as low risk, high risk, and unclear risk.
Statistical methods
This meta-analysis of the evaluation of accuracy of CEUS was performed by calculating the pooled sensitivity, specificity, posi tive likelihood ratio (LR), negative LR, and diagnostic ORs. We constructed a 2×2 contingency table for each selected study, and a value of 0.5 was added to all cells with zero. Pooled results were calculated by using the Mantel–Haenszel method (fixed-effect model) when there was no significant heterogeneity; otherwise, the DerSimonian–Laird method (random-effect model) was used. The heterogeneity among included studies was analyzed by Cochran’s Q test. Inconsistency (I2) was calculated to express the variability attributable to heterogeneity across the studies. I2>25% was considered significant for heterogeneity when the random-effect model (DerSimonian–Laird method) should be used.Citation34 A summary receiver operating characteristic (SROC) curve was produced by the Moses–Shapiro–Littenberg method to explore the nonlinear relationship between sensitivity and 1 – specificity.Citation35 The value of area under the curve (AUC) was calculated; an AUC close to 0.5 indicated a poor test, whereas an AUC of 1.0 indicated a perfect diagnostic test.Citation36
Additionally, we performed the meta-regression analysis to explore the possible sources of heterogeneity among the included studies with the following covariates: number of LNs (<50 vs ≥50), interpretation of CEUS (qualitative vs quantitative), contrast agent (SonoVue vs Levovist), and contrast mode (harmonic vs color/power Doppler). P<0.05 was considered statistically significant. Furthermore, outliers were identified by inspection of the plots of sensitivity, specificity, positive LR, and negative LR, and SROC curve. A subgroup analysis was performed by excluding outliers. Spearman correlation analysis was conducted to assess threshold effect. A strong positive correlation between the log of sensitivity and the log of 1 – specificity manifested the presence of threshold effect.
For studies using both the qualitative and quantitative diagnostic standards, sensitivity analysis was conducted by using either one of the two or both. Deeks’ asymmetry test was used, and a funnel plot of diagnostic log OR vs 1/sqrt (effective sample size) was constructed for the publication bias assessment.Citation37 A slope coefficient with P<0.05 indicated the presence of publication bias.
The pooled sensitivity, specificity, positive LR, negative LR, diagnostic OR, and SROC AUC, and meta-regression results were analyzed using software Meta-DiSc, version 1.4 (Ramón y Cajal Hospital, Madrid, Spain). The publication bias assessment was performed using Stata version 11.0 (Stata Corporation, College Station, TX, USA).
Results
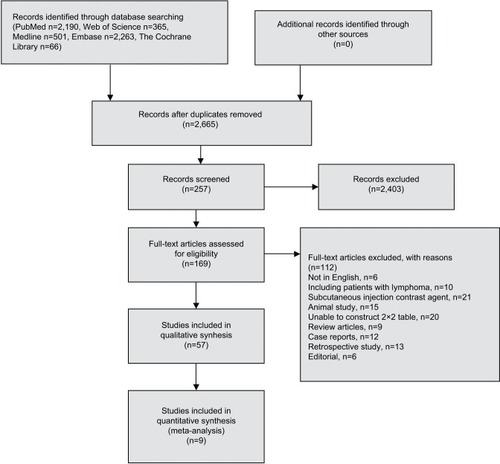
The predefined search strategy yielded 2,665 studies, among which nine were eligible for analysis. No articles were found by searching the reference lists. The detailed flowchart of literature research is presented in , while the characteristics of the selected studies are shown in . A total of 436 LNs analyzed using CEUS were investigated in this meta-analysis. Among them, 319 LNs were in the neck, 100 were in the axilla, and 17 were in the groin region. In addition, 23 LN metastases were from melanoma, 63 were from squamous cell carcinoma, 85 were from breast cancer, 61 were from ENT cancer, 37 were from oral cavity carcinoma, 8 were from adenocarcinoma, 8 were from small-cell malignancy, and 18 were from poorly differentiated carcinoma. SonoVue and Levovist are the two main contrast agents, and various imaging strategies were applied to US including color/power Doppler and contrast harmonic-enhanced US. The selected studies all used intravenous contrast agent. Among the nine studies, three studies used qualitative interpretation of CEUS, including inhomogeneous nature, early impregnation, perfusion defects, peripheral perfusion, or mixed patterns with heterogenic hyperperfusion;Citation15,Citation26,Citation32 three studies used quantitative interpretation of CEUS;Citation27,Citation28,Citation31 and the remaining three studies used both the qualitative and quantitative interpretation of CEUSCitation13,Citation22,Citation25.
Table 1 Characteristics of the included studies
Quality assessment of studies
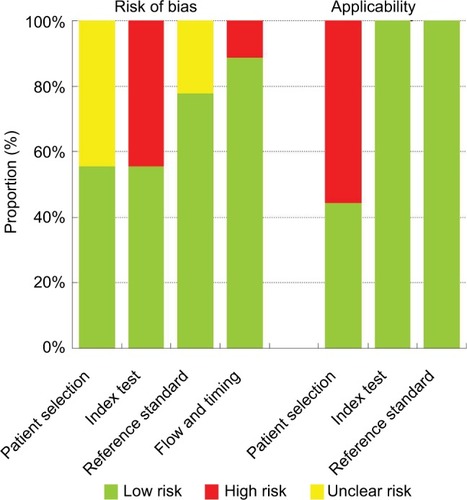
The quality assessment of the selected studies using QUA-DAS-2 tool is shown in and . Concerning the patients’ selection bias, four studies did not explicitly report consecutive patients.Citation13,Citation22,Citation26,Citation27 Regarding applicability concerns for the patient selection, four studies were labeled as having high bias because only patients with a definite carcinoma were included.Citation22,Citation25,Citation26,Citation31 Regarding risk of bias for the index test, four studies did not prespecify the diagnostic threshold.Citation25,Citation27,Citation28,Citation31 All studies met the predefined criteria such as qualitative diagnostic standards, quantitative diagnostic standards, or both. There were no concerns about reference standard applicability because all studies applied pathological evaluation. Nevertheless, two studies were labeled as “unknown” because the blinded status was not specifically mentioned.Citation15,Citation27 For the flow and timing domain, one study was labeled as having high risk because not all subjects were included in the analysis.Citation22.
Table 2 Quality assessment of the included studies using QUADAS-2
Meta-analysis
The pooled sensitivity (random-effect model) and specificity (random-effect model) of CEUS for the differential diagnosis of benign and metastatic superficial LNs were 0.88 (95% CI, 0.83–0.92) and 0.80 (95% CI, 0.74–0.85), respectively ( and ). Significant heterogeneity was found for the sensitivity (I2=46.6%) and specificity (I2=77.6%). The pooled positive LR (random-effect model), negative LR (random-effect model), and diagnostic OR (random-effect model) for diagnosing LNs by CEUS were 4.36 (95% CI, 2.38–7.99), 0.17 (95% CI, 0.10–0.31), and 32.75 (95% CI, 11.08–96.84), respectively (–). Significant heterogeneity was found for positive LR (I2=75.4%), negative LR (I2=50.0%), and diagnostic OR (I2=64.0%). SROC AUC was 0.9288 (standard error [SE] =0.0379) (). The pooled sensitivity, specificity, positive LR, negative LR, and diagnostic OR were similar to the studies reporting application of either one of the two diagnostic standards or both such as the studies by Poanta et al,Citation19 Ouyang et al,Citation25 and Cui et al.Citation22.
Figure 3 Forest plot showing the pooled sensitivity of CEUS for diagnosis of superficial metastatic LNs.
Abbreviations: CEUS, contrast-enhanced ultrasound; df, degrees of freedom; LNs, lymph nodes; LR, likelihood ratio.
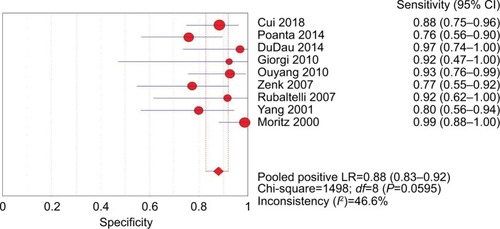
Figure 4 Forest plot showing the pooled specificity of CEUS for diagnosis of superficial metastatic LNs.
Abbreviations: CEUS, contrast-enhanced ultrasound; df, degrees of freedom; LNs, lymph nodes; LR, likelihood ratio.
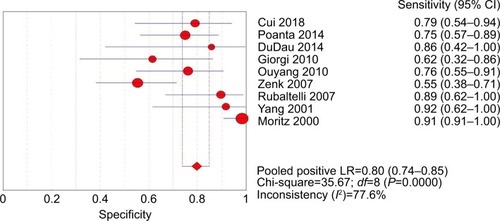
Figure 5 Forest plot showing positive LR of CEUS for diagnosis of superficial metastatic LNs.
Abbreviations: CEUS, contrast-enhanced ultrasound; df, degrees of freedom; LNs, lymph nodes; LR, likelihood ratio.
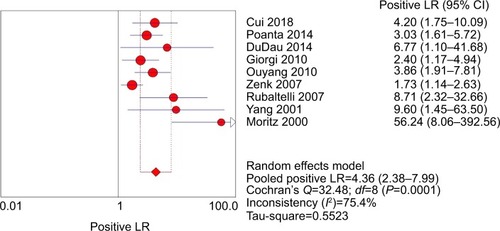
Figure 6 Forest plot showing negative LR of CEUS for diagnosis of superficial metastatic LNs.
Abbreviations: CEUS, contrast-enhanced ultrasound; df, degrees of freedom; LNs, lymph nodes; LR, likelihood ratio.
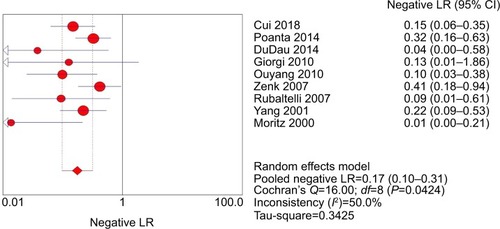
Figure 7 Forest plot showing the diagnostic OR of CEUS for diagnosis of superficial metastatic LNs.
Abbreviations: CEUS, contrast-enhanced ultrasound; df, degrees of freedom; LNs, lymph nodes.
Figure 8 SROC curve of CEUS for diagnosis of superficial metastatic LNs.
Abbreviations: AUC, area under the curve; CEUS, contrast-enhanced ultrasound; LNs, lymph nodes; SE, standard error; SROC, summary receiver operating characteristic.
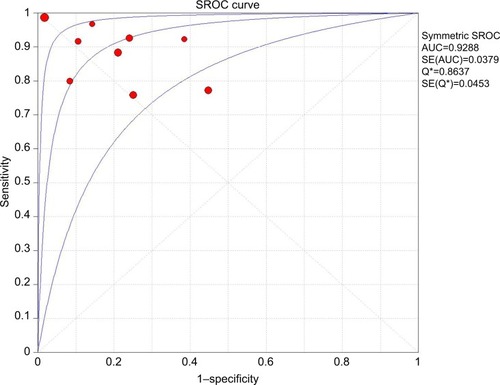
The Spearman correlation coefficient between the log of sensitivity and the log of 1 – specificity was −0.467 (P=0.205), indicating that there was no significant threshold effect. The meta-regression analysis was performed to explore the potential sources of heterogeneity. shows the relationship between the characteristics of studies and the diagnostic OR. These data suggested that there was no relationship between the characteristics of studies and the diagnostic OR. The forest plots (–) showed that the studies by Zenk et alCitation27 and Moritz et alCitation32 were outliers. Therefore, subgroup analysis excluding outliers was performed, which implied that the heterogeneities were almost eliminated in pooled estimates (). The SROC AUC was 0.8950 (SE =0.0318).
Table 3 Meta-regression analysis of the possible sources of heterogeneity
Table 4 Subgroup analysis excluding outliers
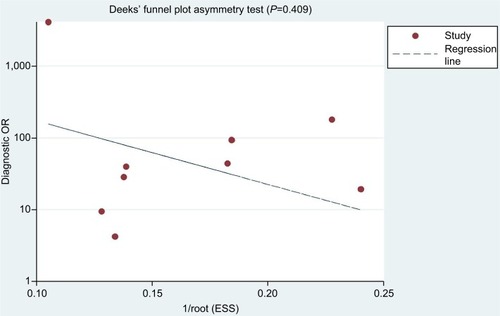
Deeks’ funnel plot of diagnostic log OR vs 1/sqrt (effective sample size) did not show significant asymmetry (P=0.409), indicating that there was no significant publication bias in this meta-analysis ().
Discussion
The histological status of LNs is a very powerful prognostic indicator for patients with carcinoma and lymphoma. US has proven to be a valuable technique for evaluating superficial LNs. US is superior to CT and MRI in the view of assessing the morphology and internal structure of LNsCitation17 because it can allow accurate LN characterization including morphology, margin, and internal structure, while color/power Doppler imaging provides further information about vascularization.Citation6,Citation8,Citation21,Citation38 Until now, the most commonly used approaches to detect LNs metastases were CT and MRI due to their lower interobserver variation, less time consumption, and ease of detection of deeply located LNs. However, imaging alone is not accurate enough to guide clinical staging and treatment decisions for patients with carcinoma and lymphoma. In this context, CEUS is a simple approach which can allow evalua tion of tissue perfusion in real time and extends US examination by a few minutes. This tool has also been shown to be very useful in the differential diagnosis of lymphadenopathy.Citation13–Citation15
Our meta-analysis demonstrated that CEUS, as a diagnostic imaging technique for superficial metastatic LNs, has a high pooled sensitivity (88%) with a fair pooled specificity (80%). No significant publication bias was found using Deeks’ asymmetry test based on the funnel plot. Therefore, we performed meta-regression analysis to find the potential source of heterogeneity among the studies. Our results revealed that there was no relationship between the characteristics of studies and the diagnostic OR. The forest plots (–) showed that the studies by Zenk et alCitation27 and Moritz et alCitation32 were outliers. Therefore, subgroup analysis excluding outliers was performed, which revealed that the heterogeneities were almost eliminated in pooled estimates ().
CEUS could be performed by color/power Doppler and by contrast harmonic-enhanced US. US contrast agents were first used to accentuate color/power Doppler signals as reported by Moritz et al, obtaining 100% sensitivity and 98% specificity in differentiation of benign from malignant LNs.Citation32 Nevertheless, Doppler imaging has some limitations, especially in cases of LNs situated nearby pulsating tissues such as arterial vessels. Contrast harmonic-enhanced US imaging is a relatively new technique that can detect the microcirculation and parenchymal perfusion of lesions with a very low mechanical index, thus allowing minimum bubble destruction and a prolonged time of microvascularization assessment. Over recent years, contrast harmonic-enhanced US has been most commonly used for diagnosis of LNs.
The enhanced microvascular pattern is a visualized diagnostic standard. Generally, malignant LNs are inhomogeneous, with early impregnation, perfusion defects, peripheral perfusion, or mixed patterns with heterogenic hyperperfusion. These characteristics of microvascular pattern seem to be reliable features of malignant LNs.Citation1,Citation14,Citation15,Citation18,Citation19,Citation25,Citation32,Citation39 Yet, these observations largely depend on the individual skills and experience of the radiologist. Although the objective CEUS parameters concerning the kinetics of contrast agent are significant, their predictive ability is not yet reliable enough for routine clinical use. In addition, Scheipers et al have reported that the computer-guided self-learning analysis systems may help to identify investigator-independent classification characteristics.Citation40 Automated quantitative software of US signal intensity was used to analyze the difference between maximum and minimum signal intensity.Citation25,Citation28 It demonstrated that the value of SImax–SImin ranging from 24 to 31 had the highest accuracy for differentiation between benign and malignant LNs with a sensitivity of 92% and a specificity of 89%. Consequently, further random, blinded, and multicenter studies should be performed to confirm the optimal CEUS parameters and cutoffs to differentiate malignant LNs from benign LNs before this approach can be applicable in practice. Perfusion software with higher accuracy should be developed to optimize measurement of functional parameters and to improve the accuracy of CEUS in diagnosing superficial metastatic LNs.
Patients with lymphoma, which have a more variable CEUS appearance, are regarded as a special group because the microvascular pattern of lymphoma partially overlaps with reactive and metastatic LNs.Citation39,Citation41,Citation42 Yu et al have demonstrated that T-cell lymphomas have intense homogeneous mass, while B-cell lymphomas show perfusion defects.Citation39 Controversially, the most often reported pattern is intense homogeneous enhancement, which causes difficulties in distinguishing lymphomatous nodes from reactive LNs.Citation1,Citation43,Citation44 Thus, we excluded the studies which recruited patients with lymphoma from the present meta-analysis to avoid heterogeneities. Additionally, since there are very few published studies that are in general based on a very limited sample, the CEUS microvascular pattern for lymphomatous nodes still remains controversial. Therefore, further random, blinded, large sample, and multicenter studies are required to assess the CEUS pattern of lymphoma and to assess the ultimate benefit of CEUS in diagnosing patients with lymphoma before this approach can be applied in practice.
The present study has some limitations. Firstly, various diagnostic standards for CEUS were used in the selected studies, as well as different study designs, contrast agents, and contrast modes. Accordingly, it is very plausible that many variations in characteristics of these studies may lead to the heterogeneity. Secondly, we did not include articles written in other languages besides English. Thirdly, some studies were also excluded because of insufficient data; although we tried to contact the authors to obtain additional data, unfortunately the data were still not sufficient.
Conclusion
CEUS, as a novel imaging modality for the characterization of superficial LNs, showed a high sensitivity and specificity in diagnosing superficial metastatic LNs. The different diagnostic standards, contrast agents, and contrast modes contributed to a considerable level of heterogeneity among the studies. A uniform standard for distinguishing benign from metastatic LNs is needed for further clinical application.
Disclosure
The authors report no conflicts of interest in this work.
References
- SlaisovaRBendaKJarkovskyJPetrasovaHSzturzPValekVContrast-enhanced ultrasonography compared to gray-scale and power doppler in the diagnosis of peripheral lymphadenopathyEur J Radiol201382469369823298797
- VassalloPWerneckeKRoosNPetersPEDifferentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution USRadiology199218312152201549675
- LiaoLJLoWCHsuWLWangCTLaiMSDetection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalitiesBMC Cancer20121223622691269
- de BondtRBNelemansPJHofmanPADetection of lymph node metastases in head and neck cancer: a meta-analysis comparing US, USgFNAC, CT and MR imagingEur J Radiol200764226627217391885
- YingMAhujaABrookFSonographic appearances of cervical lymph nodes: variations by age and sexJ Clin Ultrasound200230111111807848
- YingMAhujaASonography of neck lymph nodes. Part I: normal lymph nodesClin Radiol200358535135812727162
- van den BrekelMWStelHVCastelijnsJACervical lymph node metastasis: assessment of radiologic criteriaRadiology199017723793842217772
- DudeaSMLenghelMBotar-JidCVasilescuDDumaMUltrasonography of superficial lymph nodes: benign vs. malignantMed Ultrason201214429430623243643
- HalpernEJRosenbergMGomellaLGProstate cancer: contrast-enhanced us for detectionRadiology2001219121922511274560
- Ladam-MarcusVMacGJobLPiot-VeronSMarcusCHoeffelCPerformance de l’échographie decontraste en pathologie hépatique: synthèse de la littérature [Contrast-enhanced ultrasound and liver imaging: review of the literature]J Radiol2009901 Pt 29310619212278
- HirookaYItohAKawashimaHContrast-enhanced endoscopic ultrasonography in digestive diseasesJ Gastroenterol201247101063107223001249
- CoxKSeverAJonesSValidation of a technique using micro-bubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patients with a normal grey-scale axillary ultrasoundEur J Surg Oncol201339776076523632319
- PoantaLSerbanOPascuIPopSCosgareaMFodorDThe place of CEUS in distinguishing benign from malignant cervical lymph nodes: a prospective studyMed Ultrason201416171424567918
- RubaltelliLBeltrameVScaglioriEPotential use of contrast-enhanced ultrasound (CEUS) in the detection of metastatic superficial lymph nodes in melanoma patientsUltraschall Med2014351677123860858
- DudauCHameedSGibsonDCan contrast-enhanced ultrasound distinguish malignant from reactive lymph nodes in patients with head and neck cancers?Ultrasound Med Biol201440474775424462154
- LiuHXiaoJFlow imaging of residual neck lymph nodes in nasopharyngeal carcinoma by contrast-enhanced ultrasonography after radiotherapyZhong Nan Da Xue Xue Bao Yi Xue Ban2012358861863
- SumiMOhkiMNakamuraTComparison of sonography and CT for differentiating benign from malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neckAJR Am J Roentgenol200117641019102411264102
- RubaltelliLBeltrameVTregnaghiAScaglioriEFrigoACStramareRContrast-enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanomaAJR Am J Roentgenol20111961W8W1221178038
- PoantaLSerbanOPascuIPopSCosgareaMFodorDThe place of CEUS in distinguishing benign from malignant cervical lymph nodes: a prospective studyMed Ultrason201416171424567918
- Schulte-AltedorneburgGDemharterJLinnéRDrosteDWBohndorfKBückleinWDoes ultrasound contrast agent improve the diagnostic value of colour and power Doppler sonography in superficial lymph node enlargement?Eur J Radiol200348325225714652142
- ChongWKPapadopoulouVDaytonPAImaging with ultrasound contrast agents: current status and futureAbdom Radiol2018434762772
- CuiQLYinSSFanZHYangWWangSYanKDiagnostic Value of Contrast-Enhanced Ultrasonography and Time-Intensity Curve in Differential Diagnosis of Cervical Metastatic and Tuberculous Lymph NodesJ Ultrasound Med2018371839228691324
- RubaltelliLBeltrameVScaglioriEPotential use of contrast-enhanced ultrasound (CEUS) in the detection of metastatic superficial lymph nodes in melanoma patientsUltraschall Med2014351677123860858
- PodkrajsekMHocevarMThe role of contrast enchanced axillary ultrasonography in early breast cancer patientsColl Antropol2011351333721661352
- OuyangQChenLZhaoHXuRLinQDetecting metastasis of lymph nodes and predicting aggressiveness in patients with breast carcinomasJ Ultrasound Med201029334335220194931
- de GiorgiVGoriAGrazziniMContrast-enhanced ultrasound: a filter role in AJCC stage I/II melanoma patientsOncology2010795–637037521430406
- ZenkJBozzatoAHornungJNeck lymph nodes: prediction by computer-assisted contrast medium analysis?Ultrasound Med Biol200733224625317306695
- RubaltelliLCorradinSDorigoAAutomated quantitative evaluation of lymph node perfusion on contrast-enhanced sonographyAJR Am J Roentgenol2007188497798317377033
- Schmid-WendtnerMHDill-MüllerDBaumertJLymph node metastases in patients with cutaneous melanoma: improvements in diagnosis by signal-enhanced color Doppler sonographyMelanoma Res200414426927615305157
- Schmid-WendtnerMHPartschtKKortingHCVolkenandtMImproved differentiation of benign and malignant lymphadenopathy in patients with cutaneous melanoma by contrast-enhanced color Doppler sonographyArch Dermatol2002138449149711939811
- YangWTMetreweliCLamPKChangJBenign and malignant breast masses and axillary nodes: evaluation with echo-enhanced color power Doppler USRadiology2001220379580211526284
- MoritzJDLudwigAOestmannJWContrast-enhanced color Doppler sonography for evaluation of enlarged cervical lymph nodes in head and neck tumorsAJR Am J Roentgenol200017451279128410789776
- WhitingPFRutjesAWWestwoodMEQUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studiesAnn Intern Med2011155852953622007046
- DeeksJJSystematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening testsBMJ2001323730515716211463691
- MosesLEShapiroDLittenbergBCombining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerationsStat Med19931214129313168210827
- HanleyJAMcNeilBJThe meaning and use of the area under a receiver operating characteristic (ROC) curveRadiology1982143129367063747
- DeeksJJMacaskillPIrwigLThe performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessedJ Clin Epidemiol200558988289316085191
- AhujaAYingMSonography of neck lymph nodes. Part II: abnormal lymph nodesClin Radiol200358535936612727163
- YuMLiuQSongHPClinical application of contrast-enhanced ultrasonography in diagnosis of superficial lymphadenopathyJ Ultrasound Med201029573574020427785
- ScheipersUSiebersSGottwaldFSonohistology for the computerized differentiation of parotid gland tumorsUltrasound Med Biol200531101287129616223631
- SidhuPSCantisaniVDietrichCFThe EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version)Ultraschall Med201839215418029510440
- JiangWXueHWangQZhangXWangZZhaoCValue of contrast-enhanced ultrasound and PET/CT in assessment of extramedullary lymphomaEur J Radiol201899889329362156
- NakaseKYamamotoKHiasaATawaraIYamaguchiMShikuHContrast-enhanced ultrasound examination of lymph nodes in different types of lymphomaCancer Detect Prev200630218819116632242
- CuiXWJenssenCSaftoiuAIgneeADietrichCFNew ultrasound techniques for lymph node evaluationWorld J Gastroenterol20131930485023946589