Abstract
Background
The survival advantage of radiotherapy for patients with extensive-disease small-cell lung cancer (ED-SCLC) has not been adequately evaluated.
Methods
We analyzed stage IV SCLC patients enrolled from the Surveillance, Epidemiology, and End Results (SEER) registry through January 2010 and December 2012. Propensity score analysis with 1:1 matching was performed to ensure well-balanced characteristics of all comparison groups. Kaplan–Meier and Cox proportional hazardous model were used to evaluate the overall survival (OS), cancer-specific survival (CSS), and corresponding 95% CI.
Results
Overall, for all metastatic ED-SCLC, receiving radiotherapy was associated with both improved OS and CSS. Radiotherapy for thoracic lesion and any metastatic sites could significantly improve the OS and CSS, except for brain metastasis. For M1a-SCLC patient, radiotherapy, most likely to the primary site, significantly improved the survival (P<0.001). Furthermore, for those ED-SCLC patients with ≥ 2 metastatic sites, that is, polymetastatic ED-SCLC patients, radiation also significantly improved the median OS from 6.0 to 8.0 months (P=0.015) and the median CSS from 7.0 to 8.0 months (P=0.020).
Conclusion
The large SEER results support that radiotherapy in addition to chemotherapy might improve the survival of patients with metastatic ED-SCLC.
Background
Small-cell lung cancer (SCLC) is usually present with a bulky intrathoracic mass, rapid growth, early dissemination, and high chemosensitivity, but with a dismal prognosis.Citation1 Extensive-disease small-cell lung cancer (ED-SCLC), or stage IV disease, accounts for 60%–70% of SCLC cases, and the expected median survival is only approximately 10 months.Citation1
Though controversial, combined chemotherapy and prophylactic cranial irradiation (PCI) are now considered the standard of care in many guidelines for ED-SCLC.Citation2 Although it is highly sensitive to chemotherapy, relapse of ED-SCLC is almost a rule.Citation1,Citation3 Among the consequences of relapse, thoracic tumor progression is a major cause of morbidity in ED-SCLC. Even after chemotherapy, 75%–90% of patients have residual intrathoracic disease or develop intrathoracic progression during the first year after diagnosis.Citation4 In addition, most ED-SCLCs develop distant metastasis outside the thorax. The role of radiotherapy (RT) at intra- and extrathoracic sites is not well established and is thus an ongoing study topic.
Given the radiosensitive nature of SCLC, RT has been used to control locoregional and/or metastatic disease to improve overall survival (OS) by Jeremic et al and others over the past two decades.Citation5–Citation12 However, controversy remains regarding the role of consolidative RT in ED-SCLC because of scant prospective randomized controlled trial (RCT) data. Although a recent Phase II study did not find a survival benefit at 1 year when thoracic radiotherapy (TRT) was added to induction chemotherapy for patients with stage IV disease,Citation13 two Phase III prospective RCTs observed an improved OS for ED-SCLC patients who responded to chemotherapy.Citation5,Citation10 Palma et al systematically reviewed these two trials and concluded that TRT increased OS and progression-free survival in patients with ED-SCLC who responded to the initial chemotherapy, with only a small increase in the risk of esophageal toxicity.Citation14 Another small trial showed that postchemotherapy consolidation chest RT for ED-SCLC patients was well tolerated.Citation7 Even patients with distance metastatic ED-SCLC or polymetastatic ED-SCLC were found to benefit from RT.Citation6,Citation10 Moreover, it was reported that an aggressive palliative radiation dose delivered to the primary tumor was associated with better OS and local control in patients with stage IV non-small cell lung cancer (NSCLC).Citation15–Citation17 Furthermore, the advancement of RT techniques may reduce its toxicity to the normal tissue, allow an increased tumor dose, and further improve the efficacy.
Thus, using RT to control the primary and metastatic tumors should be explored as an approach to improve the dismal outcome of ED-SCLC. Therefore, we analyzed a large set of data from the Surveillance, Epidemiology, and End Results (SEER) registry, which is representative of the entire US patient population, through conventional methods and a propensity score matching (PSM) approach.
Methods
Study population and data sources
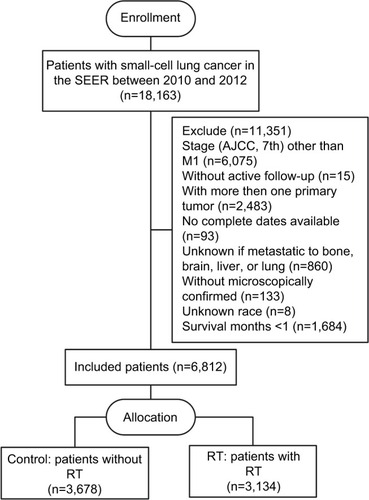
The SEER database encompasses population-based cancer registries covering ~28% of the US population and records basic demographics and some clinical characteristics.Citation18 Eligible participants who were diagnosed as lung and bronchus cancer cases with a pathologic report of small cell carcinoma from January 2010 to December 2012 were identified from the SEER database. The SEER*stat software (version 8.3.4) was used to select patients (n=18,163). Included in our study were participants with an American Joint Committee on Cancer seventh edition stage M1; with one primary tumor only; with complete data on age, race, gender, tumor size, radiation recode, metastases of the bone, liver, brain, lung at diagnosis; with active follow-up; and with more than 30 days of survival. As a result, 6,812 patients were recruited in this study, including both those who received RT (cases, n=3,134) and those who did not (controls, n=3,678) ().
Propensity score matching
PSM is a tool for reducing selection bias in nonrandomized studies. Propensity 1:1 nearest-neighbor matching was employed to reduce possible bias.Citation19 Chi-squared tests were used to examine the covariate balance. Significant parameters were then entered into the multivariate logistic regression model. Parameters that were significant in the final model were selected for PSM. Multivariate logistic regression models were used to calculate propensity scores for each patient in the group with RT and without RT.
Definition of oligometastases and polymetastases
There is no specific definition for ED-SCLC in SEER; thus, we used M1 SCLC, which can be obtained according to the SEER option “CS mets at dx”. We selected all M1 SCLC classified into the following three groups in SEER: 1) M1a with effusion (CS mets at dx: 1–22, indicating M1a SCLC with malignant pleural or pericardial effusion); 2) M1a without effusion (CS mets at dx: 23–28); and 3) M1b (CS mets at dx: 29–75). We could not obtain detailed information about metastatic sites to define oligometastases (fewer than five sites). We defined ED-SCLC polymetastases as more than or equal to two organ metastases and oligometastases as less than one organ metastasis. This SCLC categorization method was also described recently by Jeremic et al and Luo et al.Citation3,Citation20
Statistical analysis
General linear models or Mantel–Haenszel chi-squared tests were used to compare the distribution of demographic characteristics. The Kaplan–Meier method was used to analyze the primary outcomes of OS and cancer-specific survival (CSS). The multivariate Cox proportional hazardous model was performed to evaluate the HR and 95% CI. Variables selected for multivariate analysis included age (≤65, 66–68, and >68 years), sex, race (white, other), marital status (married, unmarried), median annual family income (categorized by tertile of study participants as <$61,830, $61,830–71,030, and >$71,030), grade (1, 2, 3, 4, and unknown), tumor size (categorized by tertiles of study participants as ≤2.8, 2.9–4.3, >4.3 cm, diffuse, and unknown), metastatic status (M1a with pleural effusion, M1a without pleural effusion, and M1b), sites of distant metastasis (brain, bone, liver, and lung), number of distant metastatic sites, chemotherapy (yes/no), surgery (yes/no), and RT (yes/no). P-values for linear trends were derived from regression models treating target categories (excluding unknowns) as continuous covariates. P-values for the interaction were derived from the coefficients for the interaction term. P-values≤0.05 (two-sided) were considered statistically significant. All analyses were conducted using SPSS 23 (IBM Corp, Armonk, NY, USA).
Ethical approval and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors. This study used publicly available, de-identified data sources; thus, informed consent is not applicable.
Results
The distributions of the characteristics are presented in for the study groups that were categorized by receiving or not receiving RT. Nearly half of the patients received RT. Compared to the controls, cases were more likely to be younger and married and to have well-defined tumor sizes and a lower family income before PSM. The distributions of most demographic and clinical factors were well balanced between the cases and controls after PSM.
Table 1 Selected baseline characteristics for study population by study groups, the SEER database, 2010–2012
All the baseline characteristics and selected variables were included in the univariate analyses between the cases and controls in relation to both OS and CSS. shows all the significant variables in the univariate analysis and the results when all the variables were entered into a multivariate model. As expected, older age, male, being unmarried, and having a lower family income, larger tumor size, and more distant metastasis were associated with poorer OS and CSS. Receiving chemotherapy and RT was strongly associated with better survival (P<0.001). Receiving surgery was also associated with better survival (P<0.005). However, histology grade and T stage were not found to be associated with better survival. The facts that all SCLC cases can be considered high grade and that the cases included in this study were all M1 disease could be a possible explanation.
Table 2 Univariate and multivariate analyses of OS and CSS before PSM
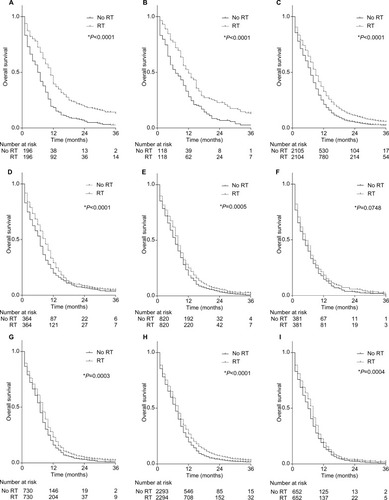
shows the results of subgroup analyses of OS, which were significantly improved by RT. In general, RT could significantly improve the OS of M1 disease of ED-SCLC, regardless of M1a or M1b status. RT was found to significantly improve the survival of ED-SCLC patients with metastases to sites of the bone (HR=0.85; 95% CI: 0.72–0.99), liver (HR=0.68; 95% CI: 0.56–0.81), and lung (HR=0.61; 95% CI: 0.49–0.76) but not in brain (HR=0.91; 95% CI: 0.74–1.12) after PSM. However, RT improved the survival of ED-SCLC patients who received chemotherapy but not that of those who did not receive chemotherapy (Pinteraction<0.01, data not shown). Thus, the survival benefit of RT for ED-SCLC patients was conditional upon chemotherapy.
Table 3 Subtype analysis of effect to OS by radiotherapy to different groups before and after PSMTable Footnotea
and further show the OS and CSS for different subgroups of ED-SCLC patients. The median OS was 9.0 months for all M1 who received RT, but it was only 7.0 months for those who had not received RT. Receiving RT could bring the median OS close to 12.0 months for M1a disease and to ~8.0 months for M1b disease. For those patients who only had one site of metastasis and had received RT, the worst median OS was observed for patients with only brain metastasis. However, the best OS was observed in those with only lung metastasis and, presumably, those who received TRT. Furthermore, RT could significantly improve the OS and CSS of ED-SCLC patients with polymetastases, that is, patients with metastases at more than two sites. The median OS in patients with polymetastases was 8.0 months for those who received RT and 6.0 months for those who did not (P<0.05).
Table 4 Median survival month (s) of all the subgroups after PSMTable Footnotea
Figure 2 The effect of RT on the OS for metastatic SCLC patients after PSMa. (A) The effect of RT on the OS of SCLC with pleural effusion and (or) pericardial effusion; (B) the effect of RT on the OS of other M1a SCLC without pleural effusion and (or) pericardial effusion; (C) the effect of RT on the OS of all M1 SCLC; (D) the effect of RT on the OS of SCLC with lung metastasis; (E) the effect of RT on the OS of SCLC with bone metastasis; (F) the effect of RT on the OS of SCLC with brain metastasis; (G) the effect of RT on the OS of SCLC with liver metastasis; (H) the effect of RT on the OS of M1b SCLC; (I) the effect of RT on the OS of SCLC with more than one metastasis.
Notes: *Using Gehan–Breslow–Wilcoxon test; aEntered in PSM were variables including age, gender, marital status, median family income, N stage, T stage, tumor size, surgery or not, and chemotherapy recode.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; PSM, propensity score matching; RT, radiotherapy.
Discussion
To our knowledge, the present study is the first population-based analysis to use PSM to assess the role of RT in treating ED-SCLC, particularly in metastatic SCLC. In this study, we found that RT, in general, could bring a survival benefit for metastatic SCLC in both multivariate regression and PSM analyses. The beneficial effect of the survival observed from the SEER database highlighted the importance of RT in the management of ED-SCLC.
The role of TRT for LD-SCLC in improving survival has been well established.Citation21 However, for ED-SCLC, the effectiveness of TRT is yet to be defined. This is largely due to ambiguous reports in the past about its role in controlling the primary tumor in the thorax and the metastatic nature of the disease.Citation22–Citation27 The earliest studies showed that RT appeared feasible and effective in ED-SCLC, with acceptable risks and benefits.Citation22,Citation23 However, the results of subsequent early studies were conflicting.Citation24–Citation26 Moreover, those findings may not be extrapolated directly into current practice due to the old chemotherapy regimens, outdated diagnostic/staging tools, and two-dimensional radiotherapy (2D-RT) techniques, which might negatively influence the usefulness of RT for ED-SCLC. In 1999, Jeremic et al published a randomized phase III trial of modern treatment for ED-SCLC.Citation5 This was the first study to show that TRT played an indispensable role in treating patients with ED-SCLC after initial chemotherapy. Since then, modern RT techniques, such as 3D planning, intensity-modulated radiation therapy (IMRT), and adaptive radiation therapy, as well as platinum-based chemotherapy have been widely introduced into the management of ED-SCLC. More recent clinical studies have demonstrated that consolidative TRT in addition to platinum-based chemotherapy improved the 2-year OS from 13% to 38%.Citation5–Citation12 The current study also showed that consolidative RT in addition to chemotherapy improved the median OS for all metastatic SCLC from 7.0 to 9.0 months (P<0.001). We observed the best OS in patients with only lung metastasis who, presumably, had also received TRT, with a median OS of 12.0 months. Moreover, we found that the survival benefit of RT was conditioned upon chemotherapy (Pinteraction<0.01). Therefore, modern studies generally supported the usefulness of consolidative TRT after chemotherapy in the treatment of ED-SCLC patients.
For stage IV NSCLC, local control of the primary tumor could reduce pulmonary symptoms, the intrathoracic disease burden, and bronchial/vascular compression, all of which are associated with better OS.Citation15–Citation17 The control of metastatic tumors for stage IV NSCLC has also been found to be related to better survival for NSCLC patients with one to five metastases, metachronous or synchronous metastases.Citation17,Citation28–Citation30 Similarly, we expect that controlling both primary and metastatic tumors may also prolong the survival of ED-SCLC patients. Jeremic et al and Slotman et al found that consolidative TRT in patients with —one to two metastases led to improved survival.Citation5,Citation10,Citation31 Secondary analysis of the CREST trial did not find that receiving TRT prolonged PFS for patients with more than two distant metastases. The recent RTOG 0937 trial evaluated TRT in treating patients with one to four metastatic sites. It demonstrated a delay in progression but no improvement in the 1-year OS with the addition of extracranial irradiation.Citation13 Studies have rarely reported the different roles of RT in oligometastatic and polymetastatic SCLC patients. Therefore, the metastatic tumor burden should be considered because patients with ≥2 metastases had a significantly worse outcome.Citation3,Citation12,Citation32
In the current SEER database analysis, we found that radiation, including but not limited to TRT, for metastases of two or more organs, defined as polymetastatic disease, could still lead to a 2-month improvement in the median OS (P<0.001). Receiving RT was associated with improved OS and CSS for all ED-SCLC, regardless of limited extensive disease (M1a) or extensive disease (M1b). We specified the ED-SCLC oligometastases and polymetastases as recently described by Xu et al and Jeremic et al.Citation5,Citation12 However, our results were consistent with those of Xu et al, who found that RT, including but not limited to TRT, improved survival in both oligometastatic (HR=2.9) and polymetastatic ED-SCLC (HR=1.7).Citation12 However, consistent with our results, Xu et al found that the improvement in survival was decreased in magnitude as the metastasis became more extensive.Citation12 Due to this decreased magnitude of survival benefit, previous studies have demonstrated a significant prolonged survival for oligometastatic patients but not polymetastatic SCLC receiving RT due to a relatively inadequate sample size.Citation5–Citation12,Citation31 We demonstrated the survival advantage of RT for oligometastatic patients. This finding is novel. And our SEER analysis provides very meaningful information to complement the current results of RCTs by Jeremic et al and others. Further, we demonstrated the survival advantage of RT for polymeta-static patients. This is even more novel since the current RCTs did not directly address the role of RT in this setting. Thus, our results indicated that, overall, RT might have more than only a palliative role in extensive metastatic SCLC. This is interesting, although the novel findings should be confirmed in future Phase III RCTs. Therefore, future trials should focus on polymetastatic SCLC and try to find suitable patients for RT. Because RT was mostly used in the palliation of thoracic lesions for ED-SCLC, the doses used were also usually palliative. It was also indicatedCitation6 and suggestedCitation3,Citation32 that a higher TRT dose was associated with better survival for ED-SCLC. High-dose radiation was found to be safe even in the 2D-RT era.Citation5 Modern RT techniques such as IMRT and stereotactic ablative RT have reduced the radiation to the normal tissues and increased the doses to tumors.Citation33 Simultaneous RT to >5 sites has been shown to be feasible, tolerable, and effective.Citation34 Because of the remarkable physical and biophysical advantages, radiation modalities such as protonCitation35 and heavy ion therapyCitation36 could be developed to treat ED-SCLC. Furthermore, as systemic therapies such as immunotherapies continue to improve, consolidative local therapy will likely become more relevant.Citation37
The lack of benefit of radiation in the case of brain metastases is an interesting finding in the current study. This finding corresponds to the results obtained in two trials by Postmus concerning the lack of benefit of adding RT to chemotherapy in brain metastases from SCLC.Citation38,Citation39 The lack of benefit of whole brain radiotherapy (WBRT) in patients who developed brain metastases might be owing to 1) brain metastasis in SCLC indicating a systematic disease with very dismal prognosis as shown in two Postmus’ trials and the current study. WBRT is a local therapy and thus might not be adequate to control this systematic disease; 2) WBRT is usually conducted at 10×3.0 Gy, and the dose might not be enough to control the metastatic lesions. Stereotactic ablative RT with a higher dose might be better; however, WBRT is usually recommended in this situation in guidelines because brain metastasis was considered as a systematic disease. Nevertheless, the prognosis of the patients in the European Organisation for Research and Treatment of Cancer (EORTC) trials and the SEER database who were treated with radiation alone was very dismal. Clinically, RT in the absence of chemotherapy was probably given to the most unfavorable patients. For example, PCI can only prolong the survival of ED-SCLC patients when the tumor responds well to chemotherapy. In fact, we found that RT without chemotherapy did not improve OS/CSS, although RT alone might be effectively palliative. Thus, we found that the survival benefit of any RT was conditioned upon chemotherapy.
We could not exclude PCI given for ED-SCLC because PCI was not listed separately in the SEER database. Based on the Slotman trial considering the benefit of PCI in ED-SCLC, published in 2007, and because we included patients from the SEER database from January 2010 to December 2012, many of the ED-SCLC patients in this analysis were given PCI.Citation4 Analyses from the National Cancer Database (NCDB, a large cancer database, and most of its information overlaps with that in the SEER database) showed that 30.7% of SCLC patients in the database who were administered RT accepted PCI.Citation40 We might also speculate that approximately one-third of the patients in our current SEER analysis received PCI.Citation40 In our research, patients with RT obtained an additional 2–3 months of survival time; this survival benefit seemed larger than that reported in the Slotman trial. Thus, we expected that most of this larger survival benefit was derived from RT other than PCI.
Unlike RCTs, the SEER database usually has high completeness and is representative of the real-world patient population. RCTs are prone to selection bias by recruiting a specific group of patients of interest, thus limiting the generalizability of the findings. Jeremic et al and Slotman et al as well as the recent RTOG 0937 trials investigated ED-SCLC patients who responded to chemotherapy.Citation5,Citation10,Citation13 However, the survival benefit is generally not addressed for patients who do not respond to chemotherapy. Trials usually set age limits or select patient groups with a favorable outcome. For example, the EORTC study used an inclusive age limit of 75 years.Citation4 We did not set an age limit to the study cohort, and we did not refine the results to include chemotherapy responders, many of whom might experience progression after chemotherapy, to make the current findings more applicable to real-world settings.
We acknowledge several limitations to this study. First, as with any observational studies, the possibility of bias is a concern. We used the PSM method, which might reduce the bias caused by the imbalanced distribution of the measured covariates. However, bias from unmeasured factors is unavoidable. Second, although our results might be applicable for real-world settings, we acknowledge that differences in the radiation dose timing, intent, methods, side effects, and second-line chemotherapy may all have contributed to study bias. However, RT in the SEER database is defined as using RT during the first course of cancer-directed therapy, with no information on the dose and intended target. Third, metastatic sites other than bone, brain, lung, and liver were not coded in SEER, which might lead to underestimate the number of metastatic sites. Although similar definitions have been used in other SEER/NCDB studies,Citation41 it is still likely that it never eliminates selection biases. In other words, we sought to demonstrate the survival advantage of RT in general for ED-SCLC patients, especially for polymetastatic patients. Our study was conducted from a qualitative, rather than quantitative, perspective. In this regard, we believe that the currently available data in the SEER database service the aim of this manuscript very well. In the current analysis, we did not intend to demonstrate the types, timing, dosage, intent, methods, or sites of RT that should be used in ED-SCLC. In addition, the SEER database does not provide any data on the risk factors of SCLC, such as smoking, which may have influenced the survival. Nevertheless, the study participants were recruited through a representative national database, thus reducing the possible selection bias. Both multivariable and PSM analyses were performed. The OS and CSS results did not change appreciably and, thus, seemed stable and valid.
Conclusion
The present study based on the large SEER database supports that radiation therapy in addition to chemotherapy might be beneficial for the survival of patients with ED-SCLC, particularly metastatic SCLC. Although well-designed Phase III RCTs are warranted to ascertain the value of RT in this setting, it is prudent to routinely select suitable patients for radiation therapy to the primary and metastatic sites in ED-SCLC based on chemotherapy.
Acknowledgments
This study was partially supported by grants 81472971 and 81773555 from the National Science Foundation of China (NSFC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NSFC. The abstract of this paper was presented at the 8th edition European Lung Cancer Congress (ELCC), which took place on 11–14 April, in Geneva, Switzerland, as Poster presentation (display) with interim findings. The abstract was published in Journal of Thoracic Oncology (https://doi.org/10.1016/S1556-0864(18)30358-7).
Author contributions
ZF designed and directed the study. RZ and ZF analyzed the data. RZ, PL, and ZF drafted the manuscript. QS and ZF supervised the study. PL, YQ, and QL helped with the statistical analysis and data cleaning. QS, TX, and PR provided clinical insights and did the literature review and help with the drafting of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- van MeerbeeckJPFennellDAde RuysscherDKSmall-cell lung cancerLancet201137898041741175521565397
- FrühMde RuysscherDPopatSSmall-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and followupAnn Oncol201324Suppl 6vi99vi10523813929
- JeremicBGomez-CaamanoADubinskyPCihoricNCasasFFilipovicNRadiation therapy in extensive stage small cell lung cancerFront Oncol2017716928848708
- SlotmanBFaivre-FinnCKramerGProphylactic cranial irradiation in extensive small-cell lung cancerN Engl J Med2007357766467217699816
- JeremicBShibamotoYNikolicNRole of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized studyJ Clin Oncol1999177209210561263
- ZhuHZhouZWangYThoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasisCancer2011117235423543121563176
- YeeDButtsCReimanAClinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancerRadiother Oncol2012102223423821930323
- WuCWangTWangJQuBWangHHuYEffect of radiotherapy on the treatment of patients with extensive stage small cell lung cancerGenet Mol Res20141348577858524615077
- NosakiKSetoTThe role of radiotherapy in the treatment of small-cell lung cancerCurr Treat Options Oncol201516125626467930
- SlotmanBJvan TinterenHPraagJOUse of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trialLancet20153859962364225230595
- AnCJingWZhangYThoracic radiation therapy could give survival benefit to elderly patients with extensive-stage small-cell lung cancerFuture Oncol201713131149115828326834
- XuLMChengCKangMThoracic radiotherapy (TRT) improved survival in both oligo- and polymetastatic extensive stage small cell lung cancerSci Rep201771925528835666
- GoreEMHuCSunAYRandomized phase II study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extracranial irradiation for extensive-disease small cell lung cancer (ED SCLC): NRG Oncology RTOG 0937J Thorac Oncol201712101561157028648948
- PalmaDAWarnerALouieAVSenanSSlotmanBRodriguesGBThoracic radiotherapy for extensive stage small-cell lung cancer: a meta-analysisClin Lung Cancer201617423924426498503
- GomezDRBlumenscheinGRLeeJJLocal consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 studyLancet Oncol201617121672168227789196
- FairchildAHarrisKBarnesEPalliative thoracic radiotherapy for lung cancer: a systematic reviewJ Clin Oncol200826244001401118711191
- LiDZhuXWangHQiuMLiNShould aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysisJ Thorac Dis20179231031728275479
- HankeyBFRiesLAEdwardsBKThe surveillance, epidemiology, and end results program: a national resourceCancer Epidemiol Biomarkers Prev19998121117112110613347
- RosenbaumPRRubinDBThe central role of the propensity score in observational studies for causal effectsBiometrika19837014155
- LuoJXuLZhaoLTiming of thoracic radiotherapy in the treatment of extensive-stage small-cell lung cancer: important or not?Radiat Oncol20171214228245874
- PignonJPArriagadaRIhdeDCA meta-analysis of thoracic radiotherapy for small-cell lung cancerN Engl J Med199232723161816241331787
- LivingstonRBMooreTNHeilbrunLSmall-cell carcinoma of the lung: combined chemotherapy and radiation: a Southwest Oncology Group studyAnn Intern Med1978882194199204239
- CoxJDByhardtRKomakiRWilsonJFLibnochJAHansenRInteraction of thoracic irradiation and chemotherapy on local control and survival in small cell carcinoma of the lungCancer Treat Rep197963812511255225027
- DillmanROTaetleRSeagrenSRoystonIKoziolJMendelsohnJExtensive disease small cell carcinoma of the lung: trial of non-cross resistant chemotherapy and consolidation radiotherapyCancer19824910200320086280831
- MasonBARichterMPCatalanoRBCreechRBUpper hemibody and local chest irradiation as consolidation following response to high-dose induction chemotherapy for small cell bronchogenic carcinoma: a pilot studyCancer Treat Rep1982668160916126286119
- NõuEBrodinOBerghJA randomized study of radiation treatment in small cell bronchial carcinoma treated with two types of four-drug chemotherapy regimensCancer1988626107910902457422
- BeithJMClarkeSJWoodsRLBellDRLeviJALong-term follow-up of a randomised trial of combined chemoradiotherapy induction treatment, with and without maintenance chemotherapy in patients with small cell carcinoma of the lungEur J Cancer199632A34384438814688
- AshworthARodriguesGBoldtGPalmaDIs there an oligometastatic state in non-small cell lung cancer? A systematic review of the literatureLung Cancer201382219720324051084
- AshworthABSenanSPalmaDAAn individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancerClin Lung Cancer201415534635524894943
- FleckensteinJPetroffASchäfersHJWehlerTSchöpeJRübeCLong-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancerBMC Cancer20161634827255302
- SlotmanBJFaivre-FinnCvan TinterenHWhich patients with ES-SCLC are most likely to benefit from more aggressive radiotherapy: a secondary analysis of the Phase III CREST trialLung Cancer201710815015328625628
- ZhangXYuJZhuHConsolidative thoracic radiotherapy for extensive stage small cell lung cancerOncotarget2017813222512226128118612
- BergsmaDPSalamaJKSinghDPChmuraSJMilanoMTThe evolving role of radiotherapy in treatment of oligometastatic NSCLCExpert Rev Anticancer Ther201515121459147126536370
- LeeCKLeeSRChoJMYangKAKimSHTherapeutic effect of gamma knife radiosurgery for multiple brain metastasesJ Korean Neurosurg Soc201150317918422102945
- SimoneCBRenganRThe use of proton therapy in the treatment of lung cancersCancer J201420642743225415690
- DuranteMOrecchiaRLoefflerJSCharged-particle therapy in cancer: clinical uses and future perspectivesNat Rev Clin Oncol201714848349528290489
- BartonMKLocal consolidative therapy may be beneficial in patients with oligometastatic non-small cell lung cancerCA Cancer J Clin2017672899028094844
- PostmusPEHaaxma-ReicheHGregorABrain-only metastases of small cell lung cancer: efficacy of whole brain radiotherapy. An EORTC phase II studyRadiother Oncol199846129329488124
- PostmusPEHaaxma-ReicheHSmitEFTreatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy: a phase III study of the European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative GroupJ Clin Oncol200018193400340811013281
- SharmaSMcmillanMTDoucetteAEffect of prophylactic cranial irradiation on overall survival in metastatic small-cell lung cancer: a propensity score-matched analysisClin Lung Cancer2018193260.e3269.e329358031
- YangJZhangYSunXThe prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based studyJ Cancer Res Clin Oncol Epub2018712