Abstract
Objective
Hepatocellular carcinoma (HCC) recurrence is a clinical challenge. An accurate prediction system for patients with HCC is needed, since the choice of HCC treatment strategies is very important.
Patients and methods
A total of 804 patients with HCC who underwent curative resection at Sun Yat-sen University Cancer Center were included in this study. Demographics, clinicopathological data, and follow-up information were collected.
Results
A logistic regression analysis was conducted to investigate the relationships between clinical features and HCC recurrence. Tumor size (OR=1.454, 95% CI: 1.047–2.020, P=0.026) and TNM stage (OR=1.360, 95% CI: 1.021–1.813, P=0.036) were independent predictors of HCC recurrence after curative resection. Therefore, the following equation was established to predict HCC recurrence: 0.308×TNM+0.374×tumor size–0.639. The equation score was 0.53±0.23 in patients who experienced HCC recurrence compared with 0.47±0.24 in other patients. A similar trend was observed in patients who survived after the last follow-up, compared with those who did not, with scores of 0.37±0.26 vs 0.52±0.22, respectively (P<0.001). The Kaplan–Meier analysis showed that patients with HCC with equation values >0.5 had significantly worse outcomes than those with equation values ≤0.5 (P<0.001) for overall survival (OS) and recurrence (P=0.043). Multivariate Cox analyses showed that tumor multiplicity (P=0.039), involucrum (P=0.029), vascular invasion (P<0.001), and equation value (P<0.001) were independent prognostic variables for OS, whereas tumor multiplicity (P=0.01), tumor differentiation (P=0.007), vascular invasion (P<0.001), involucrum (P=0.01), and equation value (P<0.001) were independent prognostic variables for HCC recurrence.
Conclusion
We established a novel and effective equation for predicting the probability of recurrence and OS after curative resection. Patients with a high recurrence score, based on this equation, should undergo additional high-end imaging examinations.
Introduction
Hepatocellular carcinoma (HCC) remains the third most common malignancy in the world due to the increased incidence of nonalcoholic fatty liver disease and the high infection rate of hepatitis virus. The prognosis of HCC depends on tumor expansion.Citation1 One of the few opportunities for patients with HCC to achieve a cure is surgical resection.Citation2,Citation3 However, it is well known that HCC recurrence after hepatectomy is a major risk factor that affects survival.Citation4 A proportion of patients with HCC experience HCC recurrence after complete HCC resection.Citation4–Citation6 Therefore, HCC recurrence is a main clinical challenge of HCC treatment. An accurate prediction system for patients with HCC is needed, since the choice of HCC treatment strategy is very important.
Identifying patients with high or low risks of recurrence after hepatectomy for HCC will help determine other therapy and management strategies.Citation7,Citation8 Recently, several prognostic staging systems have been reported, such as the Japanese General Stage score, the cancer of the liver Italian program (CLIP) score, and the Barcelona Clinical Liver Cancer staging system.Citation9–Citation12 Although these staging systems help divide patients into different groups with different outcomes, they are not suitable for use in predicting recurrence after HCC resection. Therefore, an accurate model is needed to predict the likelihood of HCC recurrence after curative resection.
Although some clinicopathological data, such as tumor multiplicity and serum α-fetoprotein (AFP) levels, have been established as poor prognostic indicators and risk factors for HCC recurrence,Citation13–Citation16 such clinicopathological data have limited prognostic value when used alone. Combining indicators provides an effective method for improving the prognostic value. Therefore, the objective of this study was to construct an equation for distinguishing the risk of recurrence based on routine markers in patients with HCC who had undergone an HCC curative resection.
Patients and methods
Patients
A total of 804 patients with HCC who underwent curative resection at the Sun Yat-sen University Cancer Center were included in this study. The inclusion criteria were as follows: 1) pathological diagnosis of HCC (by an experienced pathologist), 2) patients with complete clinicopathological and follow-up data, and 3) patients who did not receive any chemotherapy or radiotherapy prior to the surgery. The study protocol was approved by the Clinical Research Ethics Committee of the Sun Yat-sen University Cancer Center. All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Declaration of Helsinki (1975), as revised in 2008. Written informed consent was obtained from all patients prior to inclusion in this study.
Demographic and clinicopathological data collection
Demographic and clinicopathological data, including age, sex, hepatitis B surface antigen (HBsAg), serum AFP level, liver cirrhosis nodule, tumor size, tumor multiplicity, tumor encapsulation, tumor differentiation, TNM stage, and microvascular invasion, were collected. The TNM stage in this study was defined according to the American Joint Committee on Cancer TNM Staging for Liver Tumors as follows:Citation17 primary tumor (T): (TX) Primary tumor cannot be assessed; (T0) no evidence of primary tumor; (T1) solitary tumor without vascular invasion; (T2) solitary tumor with vascular invasion or multiple tumors less than 5 cm in size; (T3a) multiple tumors more than 5 cm in size; (T3b) single tumor or multiple tumors of any size, involving a major branch of the portal vein or hepatic vein; and (T4) tumor(s) with direct invasion of adjacent organs, other than the gallbladder, or with perforation of visceral peritoneum. Regional lymph nodes (N): (NX) regional lymph nodes cannot be assessed; (N0) no regional lymph node metastasis and (N1) regional lymph node metastasis. Distant metastasis (M): (M0) no distant metastasis and (M1) distant metastasis.
Follow-up
After receiving curative hepatectomies, patients with HCC underwent follow-ups and received serological and imaging examinations, including serum AFP level analysis, abdomen ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI), once every 1–6 months. For patients without evidence of an event, the last follow-up date was obtained from the medical record.
Statistical analyses
Statistical analyses were performed using SPSS (version 16.0, SPSS Inc., Chicago, IL, USA). A Student’s t-test and Pearson’s chi-squared test, or Fisher’s exact test, were chosen for examining the correlations between HCC recurrence and clinical and pathological variables. Survival curves were constructed using the Kaplan–Meier method (log-rank test). A logistic regression was performed to construct the recurrence prediction equation. A multivariate Cox proportional hazards regression model was used to evaluate the independence of the equation in predicting outcomes. Differences with P-values less than 0.05 were defined as significant.
Results
Associations between clinical features and HCC recurrence
The associations between HCC recurrence and clinical features are shown in . Significantly more patients experienced HCC recurrences among patients with larger tumor size (P=0.008), poor-undifferentiated tumor differentiation (P=0.042), III–IV tumor TNM stage (P=0.011), and positive lymph node metastasis (P=0.026).
Table 1 Association between clinical features and hepatocellular carcinoma recurrence
Construction and performance of the HCC recurrence equation
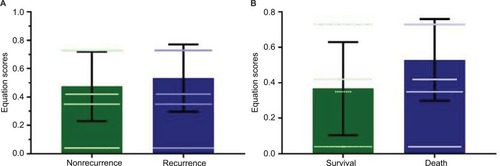
A logistic regression was conducted to analyze the relationships between clinical features and HCC recurrence (). Tumor size (OR=1.544, 95% CI: 1.118–2.131, P=0.008), tumor differentiation (OR=1.667, 95% CI: 1.014–2.743, P=0.044), and TNM stage (OR=1.439, 95% CI: 1.085–1.908, P=0.012) were predictors of HCC recurrence after curative resection. However, only tumor size (OR=1.454, 95% CI: 1.047–2.020, P=0.026) and TNM stage (OR=1.360, 95% CI: 1.021–1.813, P=0.036) were independent predictors of HCC recurrence after curative resection. Therefore, the following equation was established to predict HCC recurrence: 0.308×TNM+0.374×tumor size–0.639. To validate the equation in the prediction of HCC recurrence after curative resection, we compared the equation value among patients with different prognoses, as shown in . The equation score was 0.53±0.23 in patients who experienced an HCC recurrence, compared with 0.47±0.24 for other patients. Moreover, a similar trend was observed in patients who survived after the last follow-up, compared with those who did not (0.37±0.26 vs 0.52±0.22 [P<0.001]).
Table 2 Logistic regression of prognostic variables for hepatocellular carcinoma recurrence
Figure 1 Equation scores in patients with different prognoses.
Notes: (A) The equation score was significantly higher for patients who experienced hepatocellular carcinoma recurrence than for patients without recurrence (0.53±0.23 vs 0.47±0.24, P=0.001). (B) Similarly, the equation score was 0.37±0.26 in patients who achieved survival, which was significantly lower than that of patients who did not, 0.53±0.23.
Performance of the HCC recurrence equation in patient prognosis
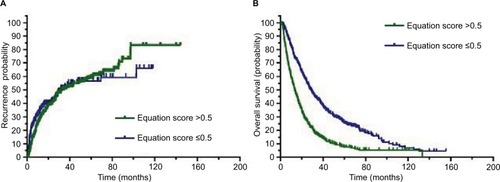
To determine the prognostic impact of the equation on patients with HCC, we conducted a Kaplan–Meier survival analysis using data from the 804 patients with HCC who were enrolled in this study. Based on the mean equation values, we divided the 804 patients into two groups: those with equation values >0.5 and those with equation values ≤0.5. In the equation value >0.5 cohort, the Kaplan–Meier analysis revealed that these patients with HCC had significantly worse recurrence outcomes than those in the other cohort (P=0.043). Similar trends were observed for overall survival (OS), which showed that patients with HCC with equation values >0.5 had significantly worse outcomes than those with equation values ≤0.5 (P<0.001), as shown in .
Figure 2 Higher equation score correlates with an unfavorable prognosis for patients with HCC.
Notes: (A) The Kaplan–Meier analysis showed significant differences in recurrence probabilities between HCC patients with equation scores >0.5 and ≤0.5 (P=0.043). (B) In addition, a significant difference was observed in overall survival for patients with HCC with equation scores >0.5 and ≤0.5 (P<0.001).
Abbreviation: HCC, hepatocellular carcinoma.
To further explore the relationship between the equation and clinical features, patients with HCC with equation values >0.5 were compared to those with equation values ≤0.5 (). The patients with HCC with equation values >0.5 exhibited the majority of poor HCC clinical features.
Table 3 Association between clinical features and equation scores in hepatocellular carcinoma
Univariate and multivariate Cox analyses of HCC prognostic variables
To evaluate whether equation value was an independent risk factor for HCC outcomes, both univariate and multivariate Cox analyses were conducted. Age, serum AFP level, tumor size, tumor multiplicity, tumor differentiation, TNM stage, vascular invasion, involucrum, and equation value were all found to be prognostic variables for OS in patients with HCC. In the multivariate analysis, only tumor multiplicity (P=0.039), involucrum (P=0.029), vascular invasion (P<0.001), and equation value (P<0.001) were independent prognostic variables that were associated with OS (). Similarly, after conducting univariate and multivariate Cox analyses, tumor multiplicity (P=0.01), tumor differentiation (P=0.007), vascular invasion (P<0.001), involucrum (P=0.01), and equation value (P<0.001) were found to be independent prognostic variables for HCC recurrence ().
Table 4 Univariate and multivariate analyses of prognostic variables for overall survival
Table 5 Univariate and multivariate analyses of prognostic variables for recurrence
Subgroup analyses of equation values in patients with HCC
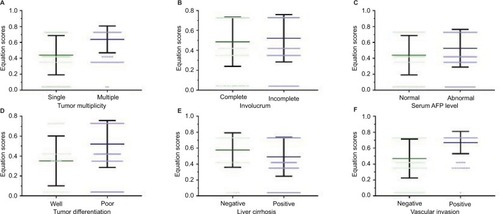
A stratified survival analysis was also conducted to further reveal the significance of equation values among patients with HCC. The equation score was significantly higher in patients with multiple tumors than in those with single tumors (0.63±0.17 vs 0.44±0.24, P<0.001). Additionally, the equation score in patients with incomplete involucrum was 0.52±0.23, compared to 0.48±0.24 in patients with complete involucrum (P=0.042). The equation score was 0.52±0.23 and 0.44±0.24, respectively, in patients with abnormal and normal serum AFP levels (P<0.001). Among patients with well-differentiated tumors, the equation score was 0.35±0.25, compared with 0.52±0.23 in patients with poorly differentiated tumors (P<0.001). For patients with and without liver cirrhosis, the equation scores were 0.49±0.24 and 0.57±0.22 (P<0.001), respectively, whereas in patients with positive and negative vascular invasion, the scores were 0.67±0.14 and 0.47±0.24, respectively (P<0.001), as shown in .
Figure 3 Equation scores in HCC subpopulations.
Notes: (A) The equation score was significantly higher in patients with multiple tumors than in those with a single tumor (0.63±0.17 vs 0.44±0.24, P<0.001). (B) In patients with incomplete involucrum, the equation value was 0.52±0.23, compared to 0.48±0.24 in patients with complete involucrum (P=0.042). (C) The equation score was 0.52±0.23 and 0.44±0.24, respectively, in patients with abnormal and normal serum AFP levels (P<0.001). (D) Among patients with well-differentiated tumors, the equation score was 0.35±0.25, compared to 0.52±0.23 in patients with poorly differentiated tumors (P<0.001). (E) For patients with and without liver cirrhosis, the equation scores were 0.49±0.24 and 0.57±0.22 (P<0.001), respectively, (F) whereas in patients with positive and negative vascular invasion, the scores were 0.67±0.14 and 0.47±0.24, respectively (P<0.001).
Abbreviations: AFP, α-fetoprotein; HCC, hepatocellular carcinoma.
Discussion
In this study, we explored the risk factors for recurrence in patients with HCC who underwent curative resection. Moreover, we established a novel, effective, and valid equation for predicting the probability of recurrence and OS after curative resection. TNM stage and tumor size were integrated into the equation, and the Kaplan–Meier survival analysis showed that patients with HCC with higher equation values displayed worse outcomes.
The etiology of HCC is variable and includes chronic virus infection, nonalcoholic liver disease, aflatoxin, and other complications.Citation18 Due to the increased incidence of obesity in the Western countries and the chronic virus infections in Asia, the incidence of HCC has remained high.Citation19–Citation21 Many studies have demonstrated the prognostic value of TNM stage, tumor burden, and impaired liver function (liver fibrosis, liver cirrhosis, and decompensated liver function) in HCC.Citation22–Citation27 Other studies have also reported that for HCC caused by chronic hepatitis B virus infection, many HBV- related indicators, including hepatitis B virus DNA load, HBsAg, and antiviral treatment, also affect the prognosis of patients with HCC.Citation21,Citation28–Citation33 However, these prognostic indicators have limited value when used alone. How to combine data to improve prognostic value is a clinically practical issue. The CLIP score was the first system that took liver function and tumor characteristics into consideration for the classification of HCC treatment. However, the prognostic performance was reported to be poor because ~80% of the patients were classified with scores of 0–2.Citation34,Citation35 In this study, we combined TNM stage and tumor size into an equation. We have conducted a multivariable logistic regression analysis and included age, sex, HBsAg status, AFP level, tumor multiplicity, and other variables to determine which variables are most related to HCC recurrence and constructed a prediction equation. Our results suggest that TNM stage and tumor size are most relevant to HCC recurrence. Therefore, other variables were not included in our equation, and the Kaplan–Meier survival analysis shows that the equation value could effectively predict the outcomes of the HCC population.
Combining several markers into one equation allows an analysis of patients with HCC with more comprehensive information and provides individualized risk assessments. Similar to the results from a previous study, our results indicate that tumor size is an independent risk factor for recurrence in patients with HCC.Citation36 Tumor size is one of the most important tumor burden parameters. The results of this and previous studies indicate that advanced TNM stage closely associates with the development of HCC because of its positive correlation with tumor size and poor OS.Citation37,Citation38 Our equation exhibited superior discrimination of HCC recurrence in patients. Hence, the equation could be used to guide routine follow-up for patients. Especially, patients with HCC with high recurrence scores should undergo examinations more frequently, such as MRI or CT examinations, even if the most recent examination after curative resection indicates no cause for concern.
AFP and tumor multiplicity have been reported as prognostic markers in HCC.Citation39 AFP is a serum HCC marker that has been previously used to monitor HCC recurrence. However, AFP levels can also be elevated in some diseases, such as liver cirrhosis and female reproductive system tumors.Citation40,Citation41 Although high preoperative AFP levels are associated with poorer HCC outcomes, there is a certain proportion of patients with HCC with AFP levels that are within the upper limit of normal.Citation42 For those AFP-negative patients with HCC, AFP is not a suitable prognostic marker. In this study, patients with HCC with abnormal or normal AFP levels could be effectively stratified using this equation. Tumor multiplicity is another prognostic marker that has been reported in HCC.Citation43 According to this study, equation scores significantly differ between patients with a single HCC and those with multiple HCCs. No matter whether patients with HCC have a single tumor or multiple tumors, our equation can further risk stratify these subpopulations with HCC.
Whether or not the equation can improve the prognosis of patients with HCC remains an interesting and important question. The main reason why HCC is difficult to treat is its high recurrence rate. Moreover, the clinical symptoms of HCC are not obvious. When the typical symptoms occur, HCC has typically progressed to a state that makes treatment difficult. Therefore, the method for screening patients with HCC after surgery to facilitate early recurrence detection is a clinically critical problem. The early detection of HCC recurrence and early intervention can improve patient prognosis. However, high-frequency screening of all patients is not a cost-effective strategy. According to the results of this study, high-frequency follow-up and screening for high-risk patients, early detection of HCC recurrence, and early interventions may ultimately improve the prognosis of patients. However, this requires further prospective studies to confirm.
Conclusion
Here we established a novel and effective equation for predicting the probability of recurrence and OS after curative resection. Patients with a high recurrence score, based on this equation, should undergo additional high-end imaging examinations. Although our equation showed good performance, several limitations need to be addressed. First, the equation was derived from data collected at a single institution. Second, the etiology of HCC in China is mostly due to chronic hepatitis B. Third, because this study was a retrospective study, the results may be biased. For future performance algorithms, prospective cohort studies are needed.
Acknowledgments
This study was supported by the Guangdong Provincial Medical Research Fund (A2018003).
Disclosure
The authors declare that they have no financial or personal relationships with other people or organizations that could inappropriately influence this work. The authors report no other conflicts of interest in this work.
References
- FornerAReigMBruixJHepatocellular carcinomaLancet2018391101271301131429307467
- GranitoABolondiLNon-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosisLancet Oncol2017182e101e11228214411
- CaiSHLuSXLiuLLZhangCZYunJPIncreased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinomaTherap Adv Gastroenterol20171010761771
- ChangK-VChenJ-DWuW-THuangK-CHsuC-THanD-SAssociation between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysisLiver Cancer2018719010329662836
- FornerALlovetJMBruixJHepatocellular carcinomaLancet201237998221245125522353262
- HirokawaFHayashiMMiyamotoYOutcomes and predictors of microvascular invasion of solitary hepatocellular carcinomaHepatol Res201444884685323834279
- XiaoYLiWWanHTanYWuHCentral hepatectomy versus major hepatectomy for patients with centrally located hepatocellular carcinoma: a meta-analysisInt J Surg20185229730229530828
- ButtASSharifFAbidSImpact of direct acting antivirals on occurrence and recurrence of hepatocellular carcinoma: biologically plausible or an epiphenomenon?World J Hepatol201810226727629527262
- ToyodaHHiraokaATadaTCharacteristics and prognosis of hepatocellular carcinoma in Japanese patients undergoing dialysisTher Apher Dial201721546547228880488
- SatoTTakahashiYImaiMIsokawaOHepatic arterial infusion chemotherapy using a reservoir for advanced hepatocellular carcinomaGan To Kagaku Ryoho2016431737726809529
- BorzioMDionigiERossiniAExternal validation of the ITA. LI.CA prognostic system for patients with hepatocellular carcinoma: a multicenter cohort studyHepatology2017
- YangZYePXuQElevation of serum GGT and LDH levels, together with higher BCLC staging are associated with poor overall survival from hepatocellular carcinoma: a retrospective analysisDiscov Med20151910740941826175398
- YuSJKwonJHKimWInitial alpha-fetoprotein response predicts prognosis in hepatitis B-related solitary HCC patients after radiofrequency ablationJ Clin Gastroenterol2018523e18e2628795996
- LeeKLeeKBYiNJSuhKSJangJJPrognosis of hepatocellular carcinoma after liver transplantation: comparative analysis with partial hepatectomyJ Pathol Transl Med2017511798628013531
- KohKCLeeHChoiMSClinicopathologic features and prognosis of combined hepatocellular cholangiocarcinomaAm J Surg2005189112012515701504
- IkedaKSaitohSTsubotaARisk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinomaCancer199371119258380116
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- CaiSCaoJYuTXiaMPengJEffectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre- treated with interferon compared with de novo therapy with entecavir and telbivudineMedicine20179622e702128562554
- CaiSOuZLiuDRisk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndromeUnited European Gastroenterol J201864558566
- OuHCaiSLiuYXiaMPengJA noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis BTherap Adv Gastroenterol2017102207217
- XueXCaiSOuHZhengCWuXHealth-related quality of life in patients with chronic hepatitis B during antiviral treatment and off- treatmentPatient Prefer Adherence201711859328138226
- HoSYLiuPHHsuCYPrognostic performance of ten liver function models in patients with hepatocellular carcinoma undergoing radiofrequency ablationSci Rep20188184329339752
- NaSKYimSYSuhSJALBI versus Child-Pugh grading systems for liver function in patients with hepatocellular carcinomaJ Surg Oncol2018117591292129448306
- ChangKVChenJDWuWTHuangKCHsuCTHanDSAssociation between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysisLiver Cancer2018719010329662836
- ShenJLiuJLiCWenTYanLYangJThe prognostic significance of serum HBeAg on the recurrence and long-term survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysisJ Viral Hepat2018
- KomorowskiALHsuCCJulkaKDAFP role in predicting recurrence of hepatocellular carcinoma after living donor liver transplantation in HCV patientsNeoplasma201865345546029788730
- XueXCaiSComment on “Assessment of liver stiffness in pediatric fontan patients using transient elastography”Can J Gastroenterol Hepatol201620169343960228025635
- HuangGLiPPLauWYAntiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: a randomized controlled trialAnn Surg20181
- YangXGaoJYWangJChengJThe impact of anti-HBV treatment on the occurrence and recurrence of hepatocellular carcinoma: focus on Asian studiesDiscov Med201519103899925725223
- ZhangLXieXYChenYHepatitis B surface antigen predicts recurrence after radiofrequency ablation in patients with low hepatitis B virus loadsMedicine20179652e937729384914
- ZengJCaiSLiuJXueXWuXZhengCDynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis BJ Ultrasound Med201736226126827914175
- CaiSYuTJiangYZhangYLvFPengJComparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week resultClin Exp Med201616342943626164128
- CaiSLiZYuTXiaMPengJSerum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogsInfect Drug Resist20181146947729662321
- GanWHuangJLZhangMXNew nomogram predicts the recurrence of hepatocellular carcinoma in patients with negative preoperative serum AFP subjected to curative resectionJ Surg Oncol201811771540154729572833
- TokumitsuYSakamotoKTokuhisaYA new prognostic model for hepatocellular carcinoma recurrence after curative hepatectomyOncol Lett20181544411442229556288
- McpeakeJRO’GradyJGZamanSLiver transplantation for primary hepatocellular carcinoma: tumor size and number determine outcomeJ Hepatol19931822262347691926
- GaoQWangXYQiuSJTumor stroma reaction-related gene signature predicts clinical outcome in human hepatocellular carcinomaCancer Sci201110281522153121564420
- ZimmermanMATrotterJFWachsMPredictors of long-term outcome following liver transplantation for hepatocellular carcinoma: a single-center experienceTranspl Int200720974775317565579
- NotarpaoloALayeseRMagistriPValidation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCCJ Hepatol201766355255927899297
- LiDSatomuraSBiomarkers for hepatocellular Carcinoma (HCC): an updateAdv Exp Med Biol201586717919326530367
- ToyodaHKumadaTTadaTSoneYKaneokaYMaedaATumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCCLiver Cancer20154212613626020034
- BaigJAAlamJMMahmoodSRHepatocellular carcinoma (HCC) and diagnostic significance of A-fetoprotein (AFP)J Ayub Med Coll Abbottabad20092117275
- DuZGWeiYGChenKFLiBRisk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution’s experience with 398 consecutive patientsHepatobiliary Pancreat Dis Int2014131536124686542
