Abstract
Purpose
By examining and identifying circulating tumor cell (CTC) counts and subtypes of peripheral blood in osteosarcoma patients, we evaluated the relationship between CTCs and characteristics of osteosarcoma patients, as well as CTC changes after neoadjuvant chemotherapy and surgery.
Methods
CanPatrol™ CTC technology was used to detect CTCs in peripheral blood before and after treatment in 32 osteosarcoma patients. Peripheral blood samples from 10 healthy volunteers were included as controls and examined for the presence of CTCs.
Results
Of the 32 osteosarcoma patients, CTCs were detected in 30 patients before treatment, and the average CTC count was 14.06±9.08. No CTCs were detected in the 10 healthy volunteers. The detected CTCs were divided into epithelial CTCs, mesenchymal CTCs (M-CTCs), and biophenotypic epithelial/mesenchymal CTCs. The average number of pretreatment CTCs was higher in stage III patients than in stage IIB patients (P=0.012). Twenty-eight patients were screened for changes in CTC count at 1 week after neoadjuvant chemotherapy and at 4 weeks after surgery. We divided these 28 patients into two groups according to the changes in the percentage of M-CTCs before and after treatment, and the results showed that the disease-free survival (DFS) was significantly shorter in the M-CTC percentage-increased group than in the M-CTC percentage-decreased or no-change group (P=0.032). Five patients with stage II osteosarcoma were examined for CTCs at the appearance of lung metastases, and the total number of CTCs was found to be higher at the appearance of lung metastases than before treatment in these patients.
Conclusion
The rate of presence of CTCs in the peripheral blood of osteosarcoma patients is high, and patients with an increased percentage of M-CTCs after treatment have a shorter DFS. The dynamic monitoring of changes in CTC counts after treatment has clinical significance for the timely detection of recurrence or metastasis.
Introduction
Osteosarcoma is a rapidly growing malignant tumor and accounts for about 60% of primary malignant bone tumors in adolescents.Citation1 Local recurrence and distant metastasis are the most important factors causing death in patients with osteosarcoma. The local recurrence rate of osteosarcoma after surgery is approximately 10%–20%. Once the tumor recurs, the survival rate is significantly reduced.Citation2 About 10%–20% of patients have distant metastases at the first visit, of which 90% are lung metastases, and about 50% of patients receiving regular treatment have lung metastases. Once patients with osteosarcoma develop distant metastases, their 5-year survival rate drops to 20%–30%.Citation3 However, since the advent of combined neoadjuvant chemotherapyCitation4 and extensive tumor resection,Citation5 the 5-year survival rate has increased from 20% to 70%, and the limb salvage rate has increased from <20% to >80%.
Circulating tumor cells (CTCs) are a subset of cells derived from a primary tumor and are released into the peripheral blood circulation under specific conditions. They possess the biological characteristics of the tumor and become the seeds of metastases in vital distant organs. Hence, CTCs are considered to be one of the important causes of tumor recurrence and distant metastasis.Citation6 CTCs include five subtypes according to the epithelial-to-mesenchymal transition (EMT) markers, namely epithelial CTCs (E-CTCs), mesenchymal CTCs (M-CTCs), and biophenotypic epithelial/mesenchymal CTCs (E<M-CTCs, E>M-CTCs, and E≈M-CTCs), which are closely related to tumor progression.
The number of CTCs in the peripheral blood is extremely small. In recent years, with the rapid development of immunomagnetic beads, biological cell-sorting chips, microporous membranes, and multicolor fluorescent labels, the enrichment, separation, and detection of CTCs have become a reality. Detection of CTCs in the peripheral blood of patients with cancer is of great significance for the diagnosis and prognosis evaluation. Detection of CTCs can indicate recurrence and micrometastasis of tumor cells earlier than imaging methods and can be used to monitor the development status of tumors, as well as to evaluate the prognosis of patients.Citation7
In 2004, the Food and Drug Administration approved the CellSearch® system for the clinical detection of CTCs, which is a representative product for the positive enrichment of CTCs based on immunomagnetic bead assays and has been widely verified by many studies.Citation8,Citation9 EpCAM is expressed exclusively in epithelia and epithelial-derived neoplasms.Citation10 The CellSearch® system specifically enriches the E-CTCs through an EpCAM antibody. However, CTCs act as a bridge between primary tumors and metastases, undergoing EMT during metastasis and giving them greater metastatic potential. Tumor cells that have acquired the mesenchymal phenotype after EMT are more likely to enter the circulatory system. Therefore, the limitation of using the CellSearch® detection system is that it cannot enrich the M-CTCs.Citation11,Citation12
CanPatrol™ CTC second-generation capture technology was used to isolate and characterize CTCs from the peripheral blood of cancer patients. The red blood cells in the peripheral blood are first lysed, and then the CTCs are separated and enriched by nanotechnology according to the difference in the size of CTCs and white blood cells. Subsequently, EMT-related markers and RNA in situ hybridization (RNA-ISH) were used to identify the five subtypes of CTCs, including M-CTCs, which make up for the shortcomings of the Cell-Search® system.Citation13,Citation14
In this study, CanPatrol™ CTC second-generation capture technology was used to detect the phenotypes of CTCs in the peripheral blood of patients with osteosarcoma at different stages. We evaluated the relationship between CTCs and characteristics of osteosarcoma patients, as well as CTC changes after neoadjuvant chemotherapy and surgery.
Methods
Patients and blood samples
This study was approved by the Committee on Reasoning at the Affiliated Tumor Hospital of Guangxi Medical University. All subjects signed an informed consent form, and written informed consents were signed by a parent or legal guardian for participants under the age of 18 years. For the study, 32 osteosarcoma patients were recruited and their diagnosis was confirmed by pathological examination at the Affiliated Tumor Hospital of Guangxi Medical University from January 2015 to December 2017. None of these patients had undergone any prior treatment. Ten healthy volunteers from Guangxi Medical University were included in the study as controls. The following information was collected from participants: age, gender, Enneking stage, tumor location, tumor size, and clinicopathological type. All enrolled patients received four cycles of neoadjuvant chemotherapy after the diagnosis of osteosarcoma, followed by surgery 4 weeks later and chemotherapy 4 weeks after surgery.
Blood samples were collected at the following three time points: before neoadjuvant chemotherapy, 1 week after neo-adjuvant chemotherapy, and 4 weeks after surgery. To avoid cell contamination due to venipuncture of the skin, the first 2 mL of the collected peripheral blood was discarded, and then 7.5 mL of blood was collected into a 10 mL tube containing 2.5 mL of EDTA anticoagulant. All blood samples were tested within 4 hours of collection using the CanPatrol™ System (SurExam Biotech, Guangzhou, People’s Republic of China).Citation13
Enneking staging system
Musculoskeletal tumors are staged according to the Enneking system,Citation15 which includes two staging systems for benign and malignant tumors. The Enneking system for benign musculoskeletal tumors is based on radiographic characteristics of the tumor host margin: latent, active, and aggressive. The Enneking surgical staging system for primary malignant mesenchymal tumors is based on the tumor grade (G, a histologic evaluation of cellular atypia and relates to the risk of metastasis), anatomical location (T), and metastasis (M), and classifies tumors into three stages ().Citation15,Citation16
Table 1 Enneking staging system for primary malignant mesenchymal tumors
Isolation of CTCs from peripheral blood
We used the previously reported CanPatrol™ CTC enrichment techniqueCitation14 to identify CTCs. Briefly, red blood cell lysis buffer was used to remove erythrocytes from the blood sample, and a size-based filtration system with an 8 µm pore filter membrane was used to remove leukocytes. RNA-ISH, as described below, and EMT markers were used to identify CTC subpopulations according to differential expressions of EpCAM, vimentin, and the leukocyte marker CD45. E-CTCs are only positive for epithelial-related markers (EpCAM and cytokeratins 8/18/19), M-CTCs are only positive for mesenchymal markers (vimentin and twist), and biophenotypic epithelial/mesenchymal CTCs simultaneously express epithelial and mesenchymal genes.
RNA-ISH assay
The detailed RNA-ISH assay procedure has been previously published.Citation13 Briefly, enriched cells from the CanPatrol™ technique were permeabilized and digested with protease (Qiagen, Hilden, Germany). The digested cell suspension was subjected to a series of hybridization reactions with captured probes specific for EpCAM, vimentin, and CD45. Finally, DAPI was used to stain the cell nucleus. The samples were analyzed with a fluorescent microscope.
Statistical analysis
The data were analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Data were recorded as median and range (or mean and SD) for continuous variables or as frequencies and percentages for categorical variables. The correlations of CTC counts with clinical parameters were evaluated using a two-sided chi-squared test or one-way ANOVA. A P-value of <0.05 was considered statistically significant.
Results
Patient characteristics and classification of CTCs
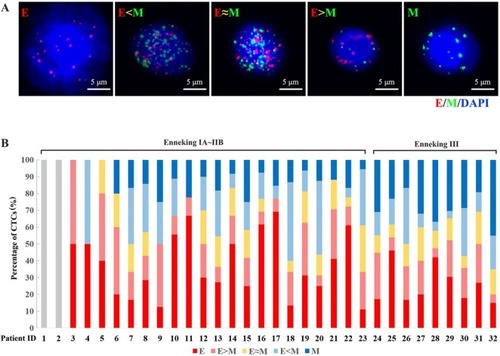
This study enrolled 32 patients with osteosarcoma (mean age: 23 years, range: 10–56 years) which included 25 patients (78%) with intramedullary osteosarcoma and seven patients (22%) with intracortical osteosarcoma. According to the Enneking staging system, two cases (6%) were classified as IB, two cases (6%) were classified as IIA, 19 cases (60%) were classified as IIB, and nine cases (28%) were classified as III. The detected CTCs were divided into E-CTCs, E>M-CTCs, E≈M-CTCs, E<M-CTCs, and M-CTCs (). Of the 32 patients with osteosarcoma, CTCs were detected in the peripheral blood of 30 patients (94%) before treatment, and the average CTC count was 14.06±9.08 (). Two patients (stage IB) did not have any CTCs. No CTCs were present in the peripheral blood of the 10 healthy volunteers. Furthermore, the average number of pretreatment CTCs was significantly related to the Enneking stage and was higher in stage III patients than in stage IIB patients (P=0.012). There was no significant relationship between the number of CTCs and clinicopathological variables of osteosarcoma, including age, gender, tumor location, tumor size, and clinicopathological type ().
Table 2 Relationship between characteristics of osteosarcoma patients and number of CTCs before treatment
Figure 1 (A) Five subtypes of CTCs (E-CTCs, E>M-CTCs, E≈M-CTCs, E<M-CTCs, and M-CTCs) were detected from the peripheral blood sample of osteosarcoma patients based on the expression of EMT-related markers and RNA-ISH. (B) The pretreatment CTC detection in 32 osteosarcoma patients and the percentage distribution of the five subtypes in each patient.
Abbreviations: CTC, circulating tumor cell; E, epithelial; M, mesenchymal; EMT, epithelial-to-mesenchymal transition; RNA-ISH, RNA in situ hybridization.
CTCs and therapeutic response
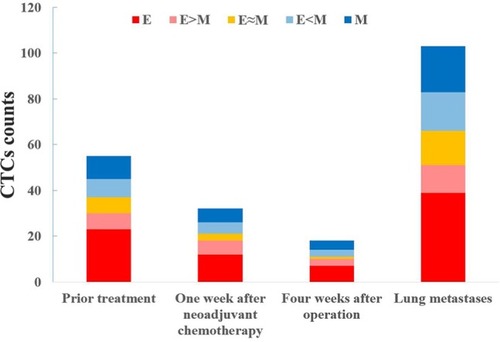
Twenty-eight patients with osteosarcoma (19 in stage IIB and nine in stage III) were screened for changes in CTC counts at 1 week after neoadjuvant chemotherapy and at 4 weeks after surgery, which included limb salvage surgery and amputations. Statistical results showed that at both of these time points, the total number of CTCs, the percentage of E-CTCs, and the percentage of mixed CTCs were all decreased in 28 patients, but the percentage of M-CTCs was increased (). Furthermore, the average number of CTCs was higher in stage III patients than in stage IIB patients both at 1 week after neoadjuvant chemotherapy (P=0.026) and at 4 weeks after surgery (P=0.010) (). We divided the 28 patients into two groups according to the changes in the total number of CTCs before treatment and 4 weeks after surgery, and the Kaplan–Meier method was used to analyze the differences in the disease-free survival (DFS) between the two groups. The results showed that there was no statistical difference in the DFS between the two groups (P=0.315). We then divided the 28 patients into two groups according to the changes in the percentage of the M-CTCs before treatment and 4 weeks after surgery. The results showed that the DFS was significantly shorter in the M-CTC percentage-increased group than in the M-CTC percentage-decreased or no-change group (P=0.032) (). All included patients completed four cycles of neoadjuvant chemotherapy, limb salvage surgery or amputation, and an average of 12.5±3.3 cycles of postoperative chemotherapy. Five of these patients with stage II osteosarcoma were examined for CTCs at the appearance of lung metastases (median 2.1 years after surgery) on computed tomography. The total number of CTCs was higher at the appearance of lung metastases than before treatment in these five patients ().
Figure 2 The changes in CTCs were measured in 32 patients with osteosarcoma after neoadjuvant chemotherapy and after surgery, including (A) the total number of CTCs and (B) the percentage of each subtype.
Abbreviations: CTC, circulating tumor cell; E, epithelial; M, mesenchymal.
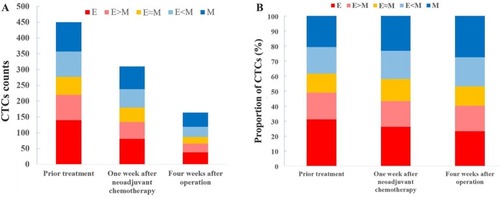
Figure 3 At pretreatment (P=0.012), 1 week after neoadjuvant chemotherapy (P=0.026), and 4 weeks after surgery (P=0.010), the average number of CTCs was higher in Enneking stage III patients than in stage IIB patients.
Abbreviation: CTC, circulating tumor cell.
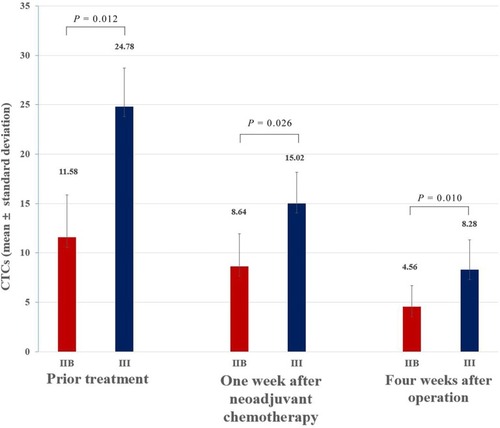
Figure 4 (A) The disease-free survival curve of CTC count-decreased or no-change group and CTC count-increased group. The results showed that there was no statistical difference in the disease-free survival between the two groups (P=0.315). (B) The disease-free survival curve of M-CTC percentage-decreased or no-change group and M-CTC percentage-increased group. The results showed that there was statistical difference in the disease-free survival between the two groups (P=0.032).
Abbreviations: CTC, circulating tumor cell; M, mesenchymal.
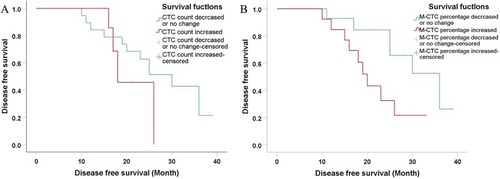
Figure 5 Five patients with stage II osteosarcoma were examined for CTCs at the appearance of lung metastases (median 2.1 years after surgery) on CT.
Note: The total number of CTCs was higher at the appearance of lung metastases than before treatment in these five patients.
Abbreviations: CTC, circulating tumor cell; CT, computed tomography; E, epithelial; M, mesenchymal.
Discussion
Compared with traditional tissue biopsy, CTC detection and characterization have the advantages of simplicity, noninvasiveness, and real-time monitoring, and can obtain a large amount of tumor-related information, earning it the nickname “liquid biopsy”. Current CTC capture techniques involve the use of physical properties and employing cell surface antigens for separation. In the current study, the CanPatrol™ CTC detection and phenotyping system, which includes CTC separation and typing identification, was tested on 32 osteosarcoma patients. In the peripheral blood, the CTCs were separated from the lysed red blood cells and enriched by nanotechnology according to the difference in the size of CTCs and white blood cells. RNA-ISH and EMT markers were then used to identify five different subtypes of CTCs, including the “M-CTCs”, which is not possible using CellSearch®.
CTCs were detected in 30 of 32 osteosarcoma patients before treatment, and the rate of presence of CTCs was as high as 94%. This study also revealed that the total number of CTCs and the percentage of M-CTCs were significantly higher in stage III patients than in stage IIB patients. This suggests that CTCs, particularly M-CTCs, may play a crucial role in the metastasis of osteosarcoma. The results of thisstudy are consistent with those reported by Zhong et al;Citation17 the CTC counts correlated significantly with the Enneking stage (P<0.001), and the ratio of M-CTCs correlated with the distant metastases (P<0.001).
Studies have shown that changes in CTCs can reflect the tumor’s growth activity and sensitivity to chemotherapy, and can be used as a reference to evaluate the effect of individualized tumor therapy.Citation18 In this study, 28 patients with osteosarcoma were retested for CTCs after treatment (including neoadjuvant chemotherapy and surgical treatment). In these 28 patients, the total number of CTCs after treatment decreased compared with that before treatment, but the total percentage of M-CTCs increased compared with that before treatment. Subsequent subgroup analysis revealed no significant difference in DFS between the CTC count-decreased and CTC count-increased groups. Patients with an increased percentage of M-CTCs after surgery had a shorter DFS. The specific reason for this outcome is not yet clear, but may be related to the strong metastatic invasion and the antiapoptotic ability of M-CTCs.Citation19
After a primary tumor has been surgically resected, an increase in the total number of CTCs in the peripheral blood may indicate that the primary tumor has undergone coloni-zation and metastasis in some target organs. This important indicator of metastasis may not be detected by traditional test methods.Citation20 In this study, five patients with osteosarcoma who suffered lung metastasis after treatment were tested for peripheral blood CTCs, and it was found that the total number of CTCs was higher at the appearance of lung metastases than before treatment. The results suggest that changes in CTCs can be detected on a regular basis, and that metastases may be discovered in time for successful treatment.
Nevertheless, the current study has some limitations. First, additional CTC testing methods should be investigated to determine whether our results are affected by the detection method. Second, the number of patients in our study is small, and the results should be interpreted with caution.
In conclusion, the rate of presence of CTCs in the peripheral blood of osteosarcoma patients is high, and patients with an increased percentage of M-CTCs after treatment have a shorter DFS. The dynamic monitoring of changes in CTC levels and types after treatment has clinical significance for the timely detection of recurrence or metastasis.
Acknowledgments
The authors would like to thank SurExam Biotech, Guangzhou, People’s Republic of China, for technical support. This work was supported, in part, by the Guangxi Traditional
Author contributions
Z-JW and Z-CY were involved in the concept and design of this manuscript. XQ and J-CT were involved in the acquisition of subjects and data. Z-JW, XQ, J-CT, and BL were involved in the collection of CTC data. Z-JW, XQ, and J-CT were involved in data analysis. All authors contributed toward drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- MirabelloLTroisiRJSavageSAOsteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results ProgramCancer200911571531154319197972
- BacciGForniCLonghiALocal recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institutionJ Surg Oncol200796211812317577221
- BriccoliARoccaMSaloneMGuzzardellaGABalladelliABacciGHigh grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005Surg Oncol201019419319919515554
- CollinsMWilhelmMConyersRBenefits and adverse events in younger versus older patients receiving neoadjuvant chemotherapy for osteosarcoma: findings from a meta-analysisJ Clin Oncol201331182303231223669227
- AksnesLHBauerHCJebsenNLLimb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patientsJ Bone Joint Surg Br200890678679418539673
- KimMYOskarssonTAcharyyaSTumor self-seeding by circulating cancer cellsCell200913971315132620064377
- HellerGMccormackRKheohTCirculating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical TrialsJ Clin Oncol201836657258029272162
- CristofanilliMCirculating tumor cells, disease progression, and survival in metastatic breast cancerSemin Oncol2006333 Suppl 9914
- RiethdorfSFritscheHMüllerVDetection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch systemClin Cancer Res200713392092817289886
- ArmstrongAEckSLEpCAM: A new therapeutic target for an old cancer antigenCancer Biol Ther20032432032514508099
- KangYPantelKTumor cell dissemination: emerging biological insights from animal models and cancer patientsCancer Cell201323557358123680145
- PlaksVKoopmanCDWerbZCancerWZCancer. Circulating tumor cellsScience201334161511186118824031008
- WuSLiuSLiuZClassification of circulating tumor cells by epithelial-mesenchymal transition markersPLoS One2015104e012397625909322
- WuSLiuZLiuSLinLYangWXuJEnrichment and enumeration of circulating tumor cells by efficient depletion of leukocyte fractionsClin Chem Lab Med201553233725568985
- EnnekingWFSpanierSSGoodmanMAA system for the surgical staging of musculoskeletal sarcomaClin Orthop Relat Res1980153106120
- JawadMUScullySPIn brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal systemClin Orthop Relat Res201046872000200220333492
- ZhongGXFengSDShenRWuZYChenFZhuXThe clinical significance of the Ezrin gene and circulating tumor cells in osteosarcomaOnco Targets Ther20171052753328223819
- CristofanilliMBuddGTEllisMJCirculating tumor cells, disease progression, and survival in metastatic breast cancerN Engl J Med2004351878179115317891
- YuMBardiaAWittnerBSCirculating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal compositionScience2013339611958058423372014
- KleinCAParallel progression of primary tumours and metastasesNat Rev Cancer20099430231219308069
