Abstract
Background
Biological mechanism of prostate cancer (PCa) recurrence and progress is complex but many of the key elements are not fully understood. Polo-like kinases (Plks) represent a family of highly conserved serine–threonine kinases that play essential roles in cell cycle progression. Plk3 plays contradictory roles in different cancers. However, the roles of Plk3 in PCa remain largely unexplored.
Methods
Kaplan–Meier analysis and Cox regression analysis were performed to evaluate the relationship between Plk3 and prognosis of patients with PCa. Gene set enrichment analysis (GSEA) was conducted to evaluate proliferation and metastasis gene sets using The Cancer Genome Atlas Dataset. MTS assay, clone formation assay, cell migration, and wound healing assay were carried out to investigate biological functions of Plk3.
Results
We found that high Plk3 expression was closely correlated with poor prognosis. GSEA revealed that Plk3 was involved in proliferation and metastasis. Loss-of-function assays demonstrated that Plk3 promoted proliferation and metastasis in PCa cells in vitro.
Conclusion
We discovered that Plk3 plays a critical role in PCa, indicating that it may be a potential prognostic marker and help predict the progression, especially recurrence of PCa.
Background
Prostate cancer (PCa) is the second most common cancer and the third leading cause of cancer death among men worldwide.Citation1 For localized PCa, radical prostatectomy (RP) or radical radiotherapy is the mature treatment option. But there is still a certain risk of recurrence after treatment. About 16%–35% of the patients needed the second-line treatment within 5 years after primary treatment.Citation2 Prostate-specific antigen (PSA) recurrence (namely biochemical recurrence) is the sign of clinical recurrence (CR), which includes local and distant recurrences. However, only 34% of those with PSA recurrence subsequently had a CR according to Pound et al,Citation3 which was also confirmed by Boorjian et al.Citation4 Recently, Nini et alCitation5 reported that among patients experiencing PSA recurrence (n=370), CR occurred in 183 patients who experienced PSA recurrence after surgery (49.5%). Among patients who experienced CR, recurrence was local and/or nodal in 56 (30.6%), retroperitoneal in 25 (13.7%), skeletal in 77 (42.1%), and visceral in 25 (13.7%). There is defect in specificity of PSA to predict CR.
Physicians predict the CR according to prostate-specific antigen-doubling time, Gleason score, clinical stage, pathological stage, nodal and margin status besides PSA, and several predictive tools have been developed to estimate the risk of relapse following the main standard treatment options for localized PCa.Citation6 However, some patients with good prognostic features still relapsed and succumbed to the disease due to heterogeneities of PCa. We need to explore more sensitive and specific method.
Mechanistically, recurrence arises from local and/or disseminated residual cancer cells. Cancer dormancy can be separated into mechanisms that antagonize the expansion of a dividing tumor cell population (tumor mass dormancy) and mechanisms that result in tumor cell growth arrest (tumor cell dormancy or cellular dormancy).Citation7 Polo-like kinases (Plks) including Plk1, Plk2, Plk3, Plk4, and Plk5 represent a family of highly conserved serine-threonine kinases that play essential roles in cell cycle progressionCitation8,Citation9 and in the cellular response to different types of stress.Citation10–Citation12 Mitogenic stimulation of serum-starved quiescent cells with fetal calf serum resulted in a transient modification of murine Plk3, suggesting a functional change during the entry of cells into the cell cycle from quiescence.Citation13 Accumulating evidence has revealed that Plk3 plays mysterious roles in different cancers. Although reduced in cancers of head/neck, lung, and liver,Citation14–Citation16 Plk3 was overexpressed in ovarian and breast cancers.Citation17,Citation18 A bad prognosis was correlated with the downregulation of Plk3 in patients suffering from hepatocarcinoma. In contrast, bad prognosis was linked with overexpression of Plk3 in breast and ovarian cancers. To date, however, the role of Plk3 in the PCa remains largely unexplored.
Our previous study has proved that Plk3 is upregulated in PCa compared with the normal PCa tissues and was positively correlated with the progression of PCa.Citation19 To go deeper into the relationship between Plk3 and PCa, we studied if the expression of Plk3 correlated with prognosis of PCa according to the Kaplan–Meier method and Cox proportional hazard regression models. We also investigated the function of Plk3 in PCa cells. Our findings strongly suggest that Plk3 participates in PCa progression and may help predict the recurrence of PCa.
Methods
Cell lines and cell culture
Human PCa cell line DU145 was bought from Center of Experiment Animal of Sun Yat-sen University (Guangzhou, China) and cultured in a humidified CO2 incubator at 37°C. DU145 was cultured with RPMI 1640 (Thermo Fisher Scientific, Waltham, MA, USA) and supplemented with 10% FBS (Thermo Fisher Scientific) and 1% penicillin–streptomycin (Thermo Fisher Scientific).
siRNA transfection
siRNA oligos targeting human Plk3 (siRNA-2: GCAU-CAAGCAGGUUCACUATT, siRNA-4: GCAGAAA- GAAGAAGAGUAATT) or negative control siRNA were designed and synthesized by GenePharma (Shanghai, China). The siRNAs were transfected with RNAiMAX (Thermo Fisher Scientific) for 6–8 hours. Mock cells were cultured in Opti-MEM for 6–8 hours, but without siRNA.
Western blot
Cell lysates were prepared and subjected to immunoblot analysis of Plk3 protein. Cells (about 1×107 cells) washed twice with ice-cold PBS were lysed with RIPA lysis buffer (no. P0013B; Beyotime Biotechnology, Shanghai, China) and complete protease inhibitor (cocktail, no. B14001a; Selleck, Shanghai, China) for 30 minutes on ice and then cleared by centrifugation at 12,000 rpm at 4°C for another 30 minutes. The total protein concentration in the extracts was measured utilizing a bicinchoninic acid protein assay kit (Beyotime Biotechnology). Equal amounts of protein were separated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% BSA or non-fat dry milk in a mixture tris-buffered saline (TBS) and Tween 20 for 1 hour and then probed with antibodies against Plk3 (D14F12, Rabbit mAb, 1:1,000; Cell Signaling Technology Danvers, MA, USA), β-tubulin (abs830032, 1:1,000; Absin, Shanghai, China), GAPDH (abs130609, 1:1,000; Absin), SLUG (sc-166476, 1:500; Santa Cruz Biotechnology Inc., Dallas, TX, USA), Claudin-1 (D3H7C, 1:1,000; Cell Signaling Technology and D3H7C, American) and MMP2 (sc-13594, 1:500; Santa Cruz Biotechnology Inc.). Western blotting was performed as previously described.Citation20
Cell proliferation assay
The MTS colorimetric assay was used to screen for cell viability. PCa cells transfected with siRNA for 48 hours, reseeded in 96-well plates at 2,000 cells per well for a final volume of 100 mL and then incubated for 6 days. Cell viability was determined with the CellTiter 96 non-radioactive cell proliferation assay (MTS; Promega Corporation, Madison, WI, USA). Colony formation assay was performed as described previously.Citation21
Cell migration assay
Cells were suspended in serum-free RPMI-1640 medium, and 5×104 cells were seeded into the upper chambers of a Tran-swell well (Corning, NY, USA) 48 hours after transfection with siRNA. The lower chamber of each well was incubated with 500 µL of RPMI-1640 medium with 10% FBS at 37°C for 24 hours. Cells were fixed and stained, and non-migratory cells in the upper chamber were removed. Migrated cells were counted in 10 random high-power fields.
Wound-healing assay
Wound-healing assays were performed to investigate migration under siRNA treatment. PCa cells were transfected with siRNAs for 48 hours. When cell density in six-well plates was ~90%, a sterile 10 µL pipette tip was used to make a linear wound. Cells were washed to remove superfluous floating cells and debris, then incubated in serum-free RPMI-1640 for an additional 72 hours. Wound healing was photographed at 0, 24, 48, and 72 hours using a microscope. The width of the wound was measured by ImageJ software (National Institutes of Health, Bethesda, MD, USA). The wound-healing ratio (%) = (wound area at 0 hour − wound area at 24, 48, and 72 hours)/wound area at 0 hour ×100%.
Apoptosis assay
PCa cells were transfected with siRNAs for 48 hours. After harvesting and washing twice with PBS, the cells were stained with annexin-V–fluorescein isothiocyanate and propidium iodide (PI) according to the manufacturer’s directions. The stained cells (106 cells) were then analyzed immediately using a FACSVerse flow cytometer (BD, Franklin Lakes, NJ, USA), and the results were expressed as a percentage of living (AnnV− [RayBiotech, Peachtree Corners, GA, USA], PI− [MP Biomedicals, Santa Ana, CA, USA]), early apoptotic (AnnV+, PI−) and late apoptotic/dead cells (AnnV+, PI+). Apoptotic rates were reported as the percentage of apoptotic cells (including early apoptotic cells and late apoptotic cells) among total cells.
Gene Set Enrichment Analysis (GSEA)
GSEA software was downloaded from Broad Institute (http://www.broadinstitute.org/gsea/index.jsp). The high group and low group were classified according to the average of mRNA expression of Plk3 because the mRNA expression obeys the normal distribution for its large sample. Significantly enriched gene sets were identified, which produced FDR q-value <0.05.
The Cancer Genome Atlas (TCGA) data mining
Patients’ clinical profiles in the TCGA prostate adenocarcinoma cohortCitation22 are available at TCGA or cBioPortal (TCGA).Citation23,Citation24
Statistical analysis
SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Student’s t-tests were used to analyze the association of Plk3 mRNA expression with clinicopathological characteristics. Disease-free survivals were analyzed using the Kaplan–Meier method, and differences were assessed using the log-rank test. Univariate analysis comparisons and multivariate survival comparisons were performed using Cox proportional hazard regression models. The RRs of death were expressed as adjusted HRs and corresponding 95% CIs. One-way ANOVA was used to analyze the difference between the control and treatment group. Differences were statistically significant when P<0.05.
Results
Plk3 serves as an independent prognostic factor for the disease-free survival of PCa patients
The association of Plk3 expression with the disease-free survival time of PCa patients was analyzed by Kaplan–Meier plots using the TCGA database. As shown in and , the disease-free survival of PCa patients (all patients) with high Plk3 expression was significantly shorter than that with low Plk3 expression (P=0.035), whereas it is not significant in non-metastatic patients (P=0.054). Then, we carried out univariate analysis using age, Gleason score, tumor stage, lymph node stage, distant metastasis, PSA, and Plk3 expression. The univariate analysis indicated that Gleason score, tumor stage, lymph node stage, PSA, and Plk3 expression (HR 1.855, 95% CI 1.048–3.285; P=0.034) were significant prognostic factors for disease-free survival in patients with PCa (). Multivariate analysis using Cox proportional hazards model revealed that high Plk3 expression and tumor stage were significant independent prognostic factors in PCa (P=0.025, P<0.001, respectively) ().
Figure 1 The disease-free survival rates of all patients and non-metastatic patients.
Notes: (A) All patients (low Plk3 expression [N=324, 65%], high Plk3 expression [N=167, 33%]) and (B) non-metastatic patients (low Plk3 expression [N=292, 58%], high Plk3 expression [N=158, 32%]) with prostate cancer were compared according to low- and high-Plk3 status. Statistical significance was determined using the log-rank test.
Abbreviation: Plk3, polo-like kinase 3.
![Figure 1 The disease-free survival rates of all patients and non-metastatic patients.Notes: (A) All patients (low Plk3 expression [N=324, 65%], high Plk3 expression [N=167, 33%]) and (B) non-metastatic patients (low Plk3 expression [N=292, 58%], high Plk3 expression [N=158, 32%]) with prostate cancer were compared according to low- and high-Plk3 status. Statistical significance was determined using the log-rank test.Abbreviation: Plk3, polo-like kinase 3.](/cms/asset/59968f33-7453-4790-b0bf-6489765b679a/dcmr_a_12185970_f0001_c.jpg)
Table 1 Prognostic value of Plk3 expression for the disease-free survival by Cox proportional hazards model
Downregulation of Plk3 reduces proliferation and metastasis of PCa
We suppressed Plk3 in PCa cells via siRNA transfection to study the role of Plk3 in PCa. We designed four siRNAs and screened their effectiveness with Western blotting. As shown in , Plk3 was remarkably reduced in DU145 cells transfected with the Plk3 siRNA2 and siRNA4 as compared with those transfected with control siRNA (). We initially classified gene expression profiles of PCa obtained from TCGA (498 PCa tissues) into 2 groups according to the Plk3 expression level (high and low) using the average as the cutoff to detect biological function of Plk3 using GSEA. The list of genes expressed in Plk3 high expression groups was highly enriched in genes involved in proliferation when assessed through GSEA (). The ten top differential expression genes (according to rank metric score) are shown in . To validate the bioinformatics analysis result, we performed MTS assay and clone formation assay to determine the biological functions of Plk3. Plk3 knockdown remarkably reduced DU145 proliferation as compared with control (). Consistently, Plk3 knockdown cells formed obviously fewer colonies than control (). Collectively, these results indicate that Plk3 plays a key role in proliferation of PCa. Given that a previous publication has shown the implication of Plk3 in the induction of apoptosis in PCa cell line LNCap,Citation25 we assessed the effect of Plk3 knockdown on apoptosis of DU145 according to FCM. We found that Plk3 knockdown does not affect the apoptosis of DU145 obviously ().
Figure 2 Plk3 knockdown reduces prostate cancer cell proliferation.
Notes: (A) Western blotting verification of si-Plk3 knockdown efficiency of DU145 cells. (B) GSEA of proliferation-associated gene set in Plk3 high (H) and low (L) expression groups. The samples were classified into low (mRNA expression <136) or high (mRNA expression >136) based on the average. (C) MTS assay evaluation of influence of Plk3 knockdown on DU145 viability. (D) Colony formation assay determining the effect of Plk3 knockdown in DU145 cells. (E) FCM assay evaluation of influence of Plk3 knockdown on DU145 apoptosis. The data are presented as mean ± SD. *P<0.05 and **P<0.01.
Abbreviations: Ctrl, control; FCM, flow cytometry; GSEA, Gene Set Enrichment Analysis; n.s., no significance; Plk3, polo-like kinase 3.
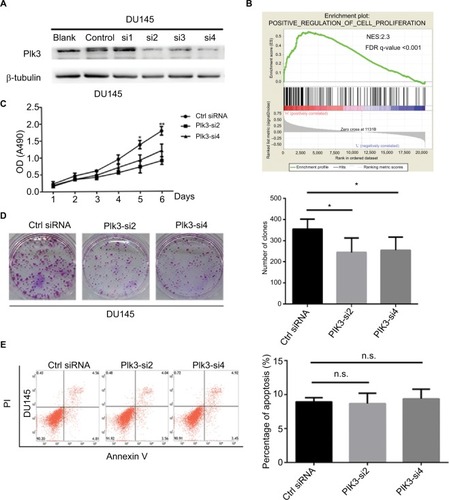
Table 2 GeneSet: POSITIVE_REGULATION_OF_CELL_PROLIFERATION
Given that high expression of Plk3 was correlated with tumor invasion, lymph node metastasis, and distant metastasis in our previous work, initially, metastasis gene sets were significantly enriched in the Plk3 high expression group when performing GSEA, which indicated that Plk3 was involved in metastasis of PCa (). The ten top differential expression genes (according to rank metric score) in each GSEA are shown in and . Additionally, we carried out migration and wound healing assay. Compared with control, Plk3 depletion significantly decreased migration of DU145 ( and ). Furthermore, we detected the expression of metastasis-associated protein. We found that Plk3 knockdown reduced expression of SLUG and MMP2 ().
Figure 3 Plk3 knockdown inhibits prostate cancer cell migration
Notes: (A) GSEA of metastasis-associated gene sets in the Plk3 high (H) and low (L) expression groups. The samples were as low (mRNA expression <136) or high (mRNA expression <136) based on the average. (B) Migration assay measurement of influence of Plk3 knockdown on DU145 mobility. (C) Wound healing assay measurement of influence of Plk3 knockdown on DU145 mobility. (D) Western blot evaluation of influence of Plk3 knockdown on metastasis-associated protein. The results are presented as mean ± SD. *P<0.05,**P<0.01, and ***P<0.001.
Abbreviations: Ctrl, control; GSEA, Gene Set Enrichment Analysis; h, hours; Plk3, polo-like kinase 3.
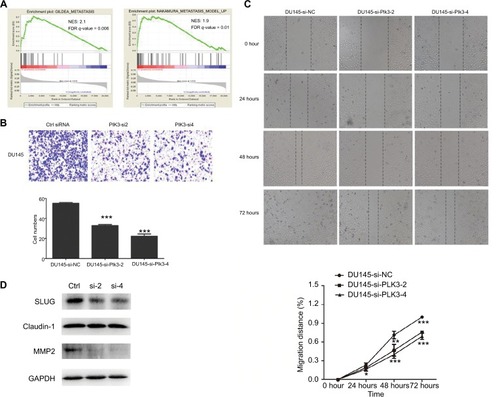
Table 3 GeneSet: NAKAMURA_METASTASIS_MODEL_UP
Table 4 GeneSet: GILDEA_METASTASIS
Discussion
Primary curative procedures such as RP are well-established therapeutic options in the management of localized PCa. Jeong et alCitation26 reported that for patients undergoing RP with pT2 and pT3a PCa, the 10-year overall survival (OS) was over 90.0%. For patients with pT3b and lymph node-positive disease, the 10-year OS was 82.8% and 76.2%, respectively. Despite technical improvements, there is still a significant risk of cancer recurrence after therapy. Not all patients with PSA recurrence after RP develop CR. More sensitive and specific markers are needed to predict the recurrence of PCa. Previously, we discovered that Plk3, upregulated in PCa tissues, was significantly associated with aggressive clinicopathological parameters of PCa. The results show that the overexpression of Plk3 is significantly associated with advanced pathological grade, clinical stage, lymph node metastasis, and distant metastasis. As opposed to cancers of lung, head/neck, and liver,Citation14–Citation16 Plk3 was upregulated in cancers of ovary and breast.Citation17,Citation18 We hypothesized that whether Plk3 plays an oncogenic or tumor suppressive role is associated with sex hormone.
Plk3 participates in multiple biological processes crucial for PCa development including proliferation, apoptosis, and angiogenesis.Citation27 The disease-free survival of PCa patients (all patients) with high Plk3 expression was significantly shorter than those with low Plk3 expression (P=0.035), whereas it is not significant in non-metastatic patients (P=0.054). It implied that Plk3 expression in PCa may be related to metastasis of PCa. GSEA proved that Plk3 correlated with the expression of proliferation and metastasis genes. In this study, we proved that Plk3 knockdown reduced proliferation of PCa cell in vitro. It may inhibit the proliferation of Plk3 but did not affect the apoptosis of PCa cell obviously. Supporting out findings, Zimmerman et al reported that Plk3 is required for entry into the S phase,Citation28 and Chase et alCitation13 showed that Plk3 may participate in the G2/M phase. It is noted that Plk3 knockdown can promote proliferation in human hepatocellular carcinoma with downregulated Plk3 as we said earlier.Citation16 According to the clinicopathological parameter, correlation analysis and biological function, we found that Plk3 depletion impeded the metastasis of DU145. It is the first time to report that Plk3 knockdown decreased the mobility of cancer cell. However, Juntermanns et alCitation29 found that Plk3 is associated with decreased tumor cell migration in cholangiocarcinoma, suggesting that the effect of Plk3 on migration differs among cancers.
Conclusion
It is our novel discovery that Plk3 serves as an independent prognostic factor for the disease-free survival of PCa patients. Moreover, Plk3 augments PCa cell proliferation and metastasis. Therefore, Plk3 may be the potential biomarker for PCa and may help predict the recurrence of PCa or gain better effect when combined with PSA.
Acknowledgments
In this study, the work of Hai Huang was supported by the National Natural Science Foundation of China (No: 81472382 and 81672550); the National Natural Science Foundation of China for Young Scientists Grant (No: 81101947); the Guangdong Province Natural Science Foundation (No: 2014A030313079); the Fundamental Research Funds for the Central Universities (No: 14ykpy19); Guangdong Province Science and Technology for Social Development Project (No: 2013B021800107 and 2017A020215018); Guangzhou City in 2015 scientific research projects (201510010298); International Science and technology cooperation project of Guangdong province science and technology plan (No: 2016A050502020); and Guangzhou International Science and Technology Cooperation Program (No: 201807010087). Also, the work of Zhenghui Guo was supported by National Natural Science Foundation of China (No: 81772733); Guangdong Province Science and Technology for Social Development Project (No: 2014A020212018, 2016A020215011); and Guangzhou science and technology plan (No: 201707010371). Also, the work of Yiming Lai was supported by the National Natural Science Foundation of China for Young Scientists Grant (No: 81802527). All these study sponsors have no roles in the study design, collection, analysis, and interpretation of data.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- BottSRManagement of recurrent disease after radical prostatectomyProstate Cancer Prostatic Dis20047321121615278094
- PoundCRPartinAWEisenbergerMAChanDWPearsonJDWalshPCNatural history of progression after PSA elevation following radical prostatectomyJAMA1999281171591159710235151
- BoorjianSAThompsonRHTollefsonMKRangelLJBergstralhEJBluteMLKarnesRJLong-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrenceEur Urol201159689389921388736
- NiniAGandagliaGFossatiNPatterns of clinical recurrence of node-positive prostate cancer and impact on long-term survivalEur Urol201568577778425959166
- ShariatSFKattanMWVickersAJKarakiewiczPIScardinoPTCritical review of prostate cancer predictive toolsFuture Oncol20095101555158420001796
- Aguirre-GhisoJAModels, mechanisms and clinical evidence for cancer dormancyNat Rev Cancer200771183484617957189
- BruinsmaWApreliaMGarcía-SantistebanIKoolJXuYJMedemaRHInhibition of Polo-like kinase 1 during the DNA damage response is mediated through loss of Aurora A recruitment by BoraOncogene201736131840184827721411
- McCoyRCDemkoZRyanACommon variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryosScience2015348623123523825859044
- XuDDaiWLiCPolo-like kinase 3, hypoxic responses, and tumorigenesisCell Cycle201716212032203628857653
- DengSWangHJiaCMicroRNA-146a induces lineage-negative bone marrow cell apoptosis and senescence by targeting polo-like kinase 2 expressionArterioscler Thromb Vasc Biol201737228029027908889
- AndrysikZBernsteinWZDengLThe novel mouse polo-like kinase 5 responds to DNA damage and localizes in the nucleolusNucleic Acids Res20103892931294320100802
- ChaseDFengYHanshewBWinklesJALongoDLFerrisDKExpression and phosphorylation of fibroblast-growth-factor-inducible kinase (Fnk) during cell-cycle progressionBiochem J1998333Pt 36556609677325
- LiBOuyangBPanHPrk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomasJ Biol Chem19962713219402194088702627
- DaiWLiYOuyangBPRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancerGenes Chromosomes Cancer200027333233610679924
- PellegrinoRCalvisiDFLaduSOncogenic and tumor suppressive roles of polo-like kinases in human hepatocellular carcinomaHepatology201051385786820112253
- WeichertWDenkertCSchmidtMPolo-like kinase isoform expression is a prognostic factor in ovarian carcinomaBr J Cancer200490481582114970859
- WeichertWKristiansenGWinzerKJPolo-like kinase isoforms in breast cancer: expression patterns and prognostic implicationsVirchows Arch2005446444245015785925
- LinCLaiYPengSExpression and clinical significance of Polo-like kinase 3 in prostate cancerLingnan Modern Clinics in Surgery201818133136
- ChenXXieWGuPUpregulated WDR5 promotes proliferation, self-renewal and chemoresistance in bladder cancer via mediating H3K4 trimethylationSci Rep20155829325656485
- ChenXGuPXieRHeterogeneous nuclear ribonucleo-protein K is associated with poor prognosis and regulates proliferation and apoptosis in bladder cancerJ Cell Mol Med20172171266127927862976
- Cancer Genome Atlas Research NetworkThe molecular taxonomy of primary prostate cancerCell201516341011102526544944
- GaoJAksoyBADogrusozUIntegrative analysis of complex cancer genomics and clinical profiles using the cBioPortalSci Signal20136269pl123550210
- CeramiEGaoJDogrusozUThe cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics dataCancer Discov20122540140422588877
- ThangavelCBoopathiECimentSThe retinoblastoma tumor suppressor modulates DNA repair and radioresponsivenessClin Cancer Res201420215468548225165096
- JeongBCChalfinHJLeeSBThe relationship between the extent of extraprostatic extension and survival following radical prostatectomyEur Urol201567234234624968968
- HelmkeCBeckerSStrebhardtKThe role of Plk3 in oncogenesisOncogene201635213514725915845
- ZimmermanWCEriksonRLPolo-like kinase 3 is required for entry into S phaseProc Natl Acad Sci U S A200710461847185217264206
- JuntermannsBSydorSKaiserGMPolo-like kinase 3 is associated with improved overall survival in cholangiocarcinomaLiver Int201535112448245725818805
