Abstract
Purpose
DKK1 is an antagonist of the Wnt signaling pathway that has various roles in human physiology. Notably, aberrant DKK1 expression is observed in several cancers. In this retrospective study, we assessed the association between DKK1 expression levels and head and neck squamous cell carcinoma (HNSCC) and its prognostic value.
Materials and methods
Using RNA-seq data from HNSCC tumors (N=520) and adjacent normal tissue (N=44) in The Cancer Genome Atlas, we evaluated DKK1 expression levels. Additionally, we evaluated the association of DKK1 expression levels and pathophysiological features of patients with HNSCC and the value of DKK1 expression for prediction of overall survival (OS). We also explored the correlation between DKK1 expression and methylation of its promoter in HNSCC.
Results
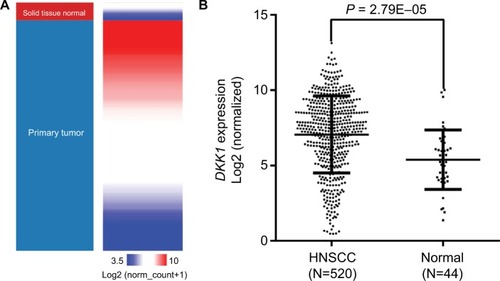
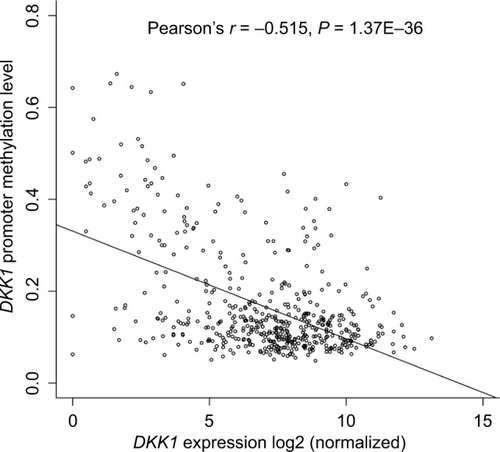
DKK1 expression was significantly upregulated in HNSCC compared with normal tissues. Moreover, DKK1 expression was significantly associated with smoking, alcohol abuse, sex, human papillomavirus status, tumor site, tumor invasion, and pathologic stage in HNSCC patients. Kaplan–Meier curves showed that high DKK1 expression was correlated with inferior OS. In addition, univariate and multivariate analyses showed that elevated DKK1 expression was an independent prognostic factor for poor OS (HR: 1.85, 95% CI: 1.31–2.62, P<0.001). Regression analysis identified a strong negative correlation between DKK1 expression and methylation of its promoter.
Conclusion
These findings support the hypothesis that elevated DKK1 expression is modulated via methylation of its promoter and indicate that DKK1 expression is a highly informative prognostic biomarker for patients with HNSCC.
Introduction
Head and neck cancer is the sixth most common malignancy and fifth leading cause of cancer-related death worldwide, and mainly comprises head and neck cancer squamous cell carcinomas (HNSCCs) arising from the mucosal surfaces of the upper-aerodigestive tract (oral and nasal cavity, oropharynx, hypopharynx, and larynx).Citation1 According to recent epidemiological data from the American Cancer Society, HNSCC incidence and mortality rates have both increased in recent years.Citation2,Citation3 Despite aggressive multimodal treatment approaches, including surgical resection, chemotherapy, targeted therapy, and radiotherapy, the 5-year survival rate of patients with HNSCC is <50%,Citation4 because the majority are diagnosed with advanced-stage diseaseCitation5 and there is a high risk of local recurrence and/or distant metastatic treatment failure.Citation6 Therefore, identification of potential markers to predict prognosis or optimize therapy is a matter of clinical urgency for patients with HNSCC.
DKK1, a member of the DKK family, is an antagonist of Wnt signaling, which regulates diverse cellular and biological processes, including cell proliferation, migration, and apoptosis, through both β-catenin-dependent and -independent mechanisms, in various tissues and numerous cancers.Citation7–Citation9 Abnormal expression of DKK1 is observed in numerous tumors and contributes to malignant transformation. In human renal cell carcinoma, DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation.Citation10 Pang et alCitation11 demonstrated that upregulation of DKK1 in SBC-3 cells enhanced their proliferation, colony formation, cell migration, and invasion in vitro, as well as bone metastasis in vivo. In prostate cancer, levels of DKK1 were found to increase during cancer development and were associated with inferior patient survival.Citation12 A recent study showed that DKK1 expression is also elevated in the Hep-2 cell line and laryngeal squamous cell carcinoma samples and has potential as an independent unfavorable predictor of overall survival (OS) in patients with this type of cancer;Citation13 however, the small sample size limited the statistical power of the study and the association of DKK1 with HNSCC remains insufficiently investigated.
The Cancer Genome Atlas (TCGA), supervised by the National Cancer Institute’s Center for Cancer Genomics and the National Human Genome Research Institute, is an open data-rich resource for biological discovery that has collated information from more than 10,000 patient samples, together with clinicopathological data, across over 30 types of human cancers.Citation14 Genomic and phenotypic data from 528 HNSCC and normal tissues are recorded in the HNSCC-specific section of the database, TCGA-HNSC. In this retrospective study, using data from TCGA-HNSC, we explored the DKK1 expression levels in HNSCC, their association with disease clinicopathological characteristics, and their prognostic value.
DNA methylation is a common epigenetic modification, and aberrant promoter methylation at CpG islands in gene promoters leads to transcriptional inactivation, which is critical in cancer initiation, progression, invasion, and metastasis.Citation15–Citation17 Accumulating evidence demonstrates that transcriptional silencing of DKK1 by methylation of its promoter is observed in hepatocellular carcinoma,Citation18 non-small-cell lung cancer,Citation19 colorectal cancer,Citation20 and oral and oropharyngeal cancer.Citation21 In the current study, we also explored the association between DKK1 expression and methylation of its promoter using Illumina Human Methylation 450K BeadChip data (Illumina Inc., San Diego, CA, USA) from TCGA-HNSC.
Materials and methods
Available TCGA data
TCGA-HNSCC cohort data (Project Id: TCGA-HNSC) were downloaded from the University of California Santa Cruz Xena browser (https://xenabrowser.net/). Gene expression data, measured by RNA-seq, were available for 520 primary HNSCC tumor and 44 adjacent normal tissue samples; 517 primary HNSCC cases had both RNA-seq and OS data recorded. Details of the clinicopathological characteristics of patients, including age at initial pathologic diagnosis, sex, smoking history, alcohol history, histologic grade, tumor site, human papillomavirus (HPV) status, pathologic tumor category, pathologic nodal category, nodal pathologic stage, margin status, recurrence status, and OS data, were downloaded for secondary analysis. DNA methylation profiles (Illumina Human Methylation 450K BeadChip Kit) were also downloaded to explore the potential mechanisms of DKK1 dysregulation in HNSCC.
Statistical analyses
Statistical analyses were performed using SPSS version 20.0 software (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between groups were conducted using independent Student’s t-tests and one-way analysis of variance. For dichotomization of DKK1 expression values, the 517 primary HNSCC patients with integrated OS data were divided into high and low DKK1 expression groups, according to median gene expression. Survival analyses were conducted by Kaplan–Meier and Cox proportional hazard regression. The average of the β values (methylation ratios) of seven Illumina Human Methylation 450K BeadChip probes (cg25454948, cg27411220, cg04932230, cg11931116, cg09445939, cg07684796, and cg27591349) on the DKK1 promoter region was calculated and used for subsequent analysis. Correlation between DKK1 expression and methylation was tested using the Spearman’s rank correlation coefficient. P-values lower than 0.05 were considered significant.
Results
High DKK1 expression levels in HNSCC tumor samples
In the current study, DKK1 RNA-seq data from 520 patients with histologically confirmed HNSCC and 44 adjacent normal tissues were available from TCGA database. Comparison of DKK1 expression, as determined by RNA-seq, demonstrated that DKK1 levels were significantly elevated in HNSCC tissues compared with the adjacent normal samples (P<0.001; ).
Association of DKK1 expression with clinicopathological parameters
We also explored the association of DKK1 expression with the clinicopathological parameters in patients with HNSCC (). Significantly elevated DKK1 expression levels were found in patients with histories of smoking (P=0.020) and alcohol consumption (P=0.024) in the TCGA-HNSCC cohort. We also identified a difference in DKK1 expression between patients who were HPV– and those who were HPV+, with the latter group having significantly lower DKK1 expression levels (P<0.001). Additionally, we found that hypopharynx and larynx cancers presented with significantly higher DKK1 expression levels compared with oral cavity and oropharynx cancers (P=0.045). Advanced tumor (T) category (P=0.006) and pathologic stage (P=0.048) were also associated with significantly higher DKK1 expression levels compared with early-stage disease. No differences in DKK1 expression were observed according to sex, age, histologic grade, nodal (N) category, surgical margin status, or recurrence status.
Table 1 Association of DKK1 expression with clinicopathological characteristics of patients with HNSCC
High DKK1 expression level as an independent prognostic factor for unfavorable OS
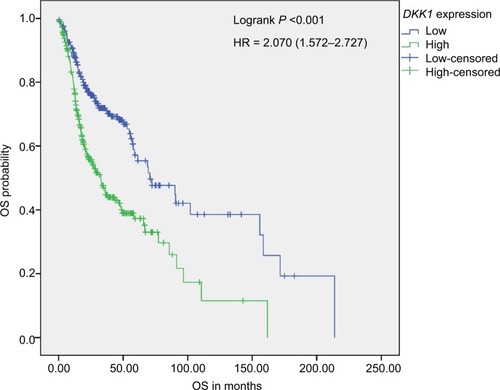
We constructed survival curves to investigate whether DKK1 expression can act as a prognostic biomarker for HNSCC (). The median OS for both the DKK1 high and DKK1 low expression groups were 32.83 and 70.67 months, respectively. Kaplan–Meier analysis and log-rank testing demonstrated that the group with high DKK1 expression had significantly inferior outcome (log-rank P<0.001).
Figure 2 Kaplan–Meier survival analysis.
Notes: Kaplan–Meier analysis of OS in 517 HNSCC patients stratified according to median DKK1 expression. OS in patients with high DKK1 expression was significantly reduced compared with that of patients with low DKK1 expression.
Abbreviations: HNSCC, head and neck squamous cell carcinoma; OS, overall survival.
In univariate Cox proportional hazards analysis (), we found that age ≥60 years, female, advanced T, lymphatic metastasis, advanced stage, positive surgical margin, and increased DKK1 expression were significantly associated with inferior OS of patients with HNSCC. To avoid overfitting of the results, univariate Cox regression analysis was also performed using DKK1 expression as a continuous variable. The result confirmed that there was a significantly increased risk of death for patients with high DKK1 expression. Multivariate Cox proportional hazard analysis was conducted including only variables that showed significance in univariate analysis (). The results confirmed that advanced T, lymphatic metastasis, positive surgical margin, and increased DKK1 expression were independent prognostic indicators of unfavorable OS for patients with HNSCC.
Table 2 Univariate and multivariate analyses of overall survival in HNSCC patients
DKK1 expression was negatively correlated with methylation of its promoter in HNSCC
We examined the association of DKK1 expression with methylation of its promoter using data from 520 patients with HNSCC from TCGA. Regression analysis revealed a strong negative correlation (Pearson’s r=−0.515, P<0.001 between DKK1 expression and methylation of its promoter) ().
Discussion
The Wnt signaling pathway is a well-conserved and critical regulatory cascade that contributes to the development and homeostasis of the human body. DKK1 is a canonical Wnt signaling pathway inhibitor that has various functions in numerous human diseases, particularly cancers.Citation22–Citation24 A previous study showed that DKK1 expression is elevated in laryngeal squamous cell carcinoma, which is the second most common malignant HNSCC neoplasm;Citation13 however, the relationship between DKK1 and HNSCC remains unclear. In the current study, through characterization of DKK1 expression based on data from TCGA-HNSC, we confirmed that DKK1 expression is significantly upregulated in HNSCC.
Subsequently, we also determined the association between DKK1 expression and the clinicopathological characteristics of patients with HNSCC. The etiologic risk of developing HNSCC is associated with multiple factors, including geographical region, dietary habits, and genetic background, with cigarette smoking and abusive consumption of alcohol representing the most important risk factors for HNSCC development and exhibiting a synergistic effect.Citation25 By analysis of TCGA data, we found that DKK1 expression was significantly elevated in HNSCC patients with smoking and alcohol abuse, suggesting that smoking and alcohol consumption may be involved in the progression of HNSCC via regulation of DKK1. Over the past decade, clear molecular and epidemiologic evidence has emerged supporting a vital role for HPV in a subset of HNSCC tumors, particularly oral and oropharynx squamous cell carcinomas.Citation26,Citation27 Also, consistent with prior reports, our analysis showed that DKK1 expression significantly decreased in the HPV associated tumors in TCGA-HNSCC, which may be due to the fact that integration of HPV into the genome results in altered mRNA transcript abundance and splicing.Citation28 Tumor invasion and TNM stage are vital factors for assessment of prognosis in patients with cancer and are among the most common tools used for that purpose.Citation29–Citation31 Our analyses revealed that DKK1 expression was higher in HNSCC patients with advanced tumor and clinical stage compared with those with early-stage disease, supporting a possible role for DKK1 in the development of HNSCC.
HNSCC is a common type of cancer with a dismal prognosis, and the rates of local or distant recurrence are up to 30% and 25%, respectively.Citation32 TNM staging remains a vital tool for predicting cancer prognosis;Citation33,Citation34 however, the latest edition of the TNM classification is unable to absolutely justify clinical application, due to the heterogeneous molecular mechanisms and clinical behaviors of HNSCC. Consequently, reliable prognostic biomarkers are urgently needed to aid the risk stratification of patients and inform personalized clinical treatment decision-making processes and subsequent surveillance for HNSCC.
DKK1 upregulation has prognostic value in various cancers, including chondrosarcoma,Citation35 bladder cancer,Citation36 pancreatic cancer,Citation37 and laryngeal squamous cell carcinoma.Citation13 In this study, using the large TCGA dataset, Kaplan–Meier survival analysis demonstrated that patients with high DKK1 expression had remarkably shorter OS. In addition, our univariate and multivariate analyses showed that elevated DKK1 expression was an independent prognostic factor for poor OS (HR: 1.85, 95% CI: 1.31–2.62, P<0.001), after adjustment for age, sex, tumor invasion, lymphatic metastasis, pathologic stage, and surgical margin status. Therefore, we infer that DKK1 expression is a potential biomarker of unfavorable OS in HNSCC patients and that implementation of DKK1 expression screening into prospective clinical trials would be highly desirable to provide further support for these findings.
Development of HNSCC is a multistep process that includes various molecular alterations during carcinogenesis. Both genetic and epigenetic alterations can influence gene expression and modulate cancer cell behavior. As one of the most important epigenetic alterations, aberrant DNA methylation in the promoter region can directly affect gene transcription in numerous cancers, including HNSCC.Citation15,Citation16 Regression analysis confirmed a significant negative correlation between DKK1 expression and methylation of its promoter, suggesting that DNA methylation may be an important mechanism influencing DKK1 expression in HNSCC.
Conclusion
In the current study, we took advantage of public data and identified that high DKK1 expression may be mediated by methylation of its promoter and that this gene is a highly informative prognostic biomarker for patients with HNSCC. However, a well-designed prospective large sample size study and further cell experiments are urgently needed to ascertain our results.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 81670920 and No. 81541039), Natural Science Foundation of Zhejiang Province (No. LY15H130003), Medical and Health Science Research Foundation of Zhejiang Province (No. 2016KYB272), Huimin Technology Research and Development Projects of Ningbo (No. 2015C50026), and Ningbo Health Branding Subject Fund (PPXK2018-02).
Disclosure
The authors report no conflicts of interest in this work.
References
- VigneswaranNWilliamsMDEpidemiologic trends in head and neck cancer and aids in diagnosisOral Maxillofac Surg Clin North Am201426212314124794262
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- SiegelRLMillerKDJemalACancer statistics, 2017CA: A Cancer J Clin2017671730
- ShieldKDFerlayJJemalAThe global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012CA Cancer J Clin2017671516428076666
- SeiwertTYCohenEEState-of-the-art management of locally advanced head and neck cancerBr J Cancer20059281341134815846296
- MarurSForastiereAAHead and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and TreatmentMayo Clin Proc201691338639626944243
- OlsenJJPohlSÖDeshmukhAThe Role of Wnt Signalling in AngiogenesisClin Biochem Rev201738313114229332977
- AnastasJNMoonRTWNT signalling pathways as therapeutic targets in cancerNat Rev Cancer2013131112623258168
- ChoiHJParkHLeeHWKwonYGThe Wnt pathway and the roles for its antagonists, DKKS, in angiogenesisIUBMB Life201264972473122807036
- HirataHHinodaYNakajimaKWnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinomaInt J Cancer201112881793180320549706
- PangHMaNShenWEffects of DKK1 overexpression on bone metastasis of SBC-3 cellsOncol Lett20181556739674429731859
- HallCLDaignaultSDShahRBPientaKJKellerETDickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasisProstate200868131396140418561248
- ShiYGongHLZhouLTianJWangYDickkopf-1 is a novel prognostic biomarker for laryngeal squamous cell carcinomaActa Otolaryngol2014134775375924834937
- Cancer Genome Atlas Research NetworkWeinsteinJNCollissonEAThe Cancer Genome Atlas Pan-Cancer analysis projectNat Genet201345101113112024071849
- ZhouCLiJLiQCDKN2A methylation in esophageal cancer: a meta-analysisOncotarget2017830500715008328637022
- ShenZZhouCLiJSHISA3 Promoter Methylation Is a Potential Diagnostic and Prognostic Biomarker for Laryngeal Squamous Cell CarcinomaBiomed Res Int20172017905874928299336
- LinBZhouXLinSEpigenetic silencing of PRSS3 provides growth and metastasis advantage for human hepatocellular carcinomaJ Mol Med (Berl)201795111237124928844099
- LiangLHeHLvRPreliminary mechanism on the methylation modification of Dkk-1 and Dkk-3 in hepatocellular carcinomaTumour Biol20153621245125025344678
- NaYLeeSMKimDSParkJYPromoter methylation of Wnt antagonist DKK1 gene and prognostic value in Korean patients with non-small cell lung cancersCancer Biomark2012122737923396252
- GalambOKalmárAPéterfiaBAberrant DNA methylation of WNT pathway genes in the development and progression of CIMP-negative colorectal cancerEpigenetics201611858860227245242
- PaluszczakJSarbakJKostrzewska-PoczekajMThe negative regulators of Wnt pathway-DACH1, DKK1, and WIF1 are methylated in oral and oropharyngeal cancer and WIF1 methylation predicts shorter survivalTumour Biol20153642855286125487617
- LuoJChenMLiuYNature-derived lignan compound VB-1 exerts hair growth-promoting effects by augmenting Wnt/β-catenin signaling in human dermal papilla cellsPeerJ20186e473729761053
- HongSAYooSHLeeHHPrognostic value of Dickkopf-1 and ß-catenin expression in advanced gastric cancerBMC Cancer201818150629720122
- FatimaSLukJMPoonRTLeeNPDysregulated expression of dick-kopfs for potential detection of hepatocellular carcinomaExpert Rev Mol Diagn201414553554824809435
- BlotWJMcLaughlinJKWinnDMSmoking and drinking in relation to oral and pharyngeal cancerCancer Res19884811328232873365707
- SpenceTBruceJYipKWLiuFFHPV Associated Head and Neck CancerCancers (Basel)20168875
- JosephAWD’SouzaGEpidemiology of human papillomavirus-related head and neck cancerOtolaryngol Clin North Am201245473976422793850
- ParfenovMPedamalluCSGehlenborgNCancer Genome Atlas NetworkCharacterization of HPV and host genome interactions in primary head and neck cancersProc Natl Acad Sci USA201411143155441554925313082
- TalmiYPTakesRPAlonEEPrognostic value of lymph node ratio in head and neck squamous cell carcinomaHead Neck20184051082109029394461
- HuangSHXuWWaldronJRefining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomasJ Clin Oncol201533883684525667292
- FengMWangWFanZTumor volume is an independent prognostic indicator of local control in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapyRadiat Oncol2013820824007375
- CooperJSPajakTFForastiereAARadiation Therapy Oncology Group 9501/IntergroupPostoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neckN Engl J Med2004350191937194415128893
- SchlitterAMJesinghausMJägerCpT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinomaEur J Cancer20178412112928802189
- ChenKChenHYangFSuiXLiXWangJValidation of the Eighth Edition of the TNM Staging System for Lung Cancer in 2043 Surgically Treated Patients With Non-small-cell Lung CancerClin Lung Cancer2017186e457e46628539214
- ChenCZhouHZhangXMaXLiuZLiuXElevated levels of Dickkopf-1 are associated with β-catenin accumulation and poor prognosis in patients with chondrosarcomaPLoS One201498e10541425144498
- SunDKWangLWangJMZhangPSerum Dickkopf-1 levels as a clinical and prognostic factor in patients with bladder cancerGenet Mol Res2015144181811818726782465
- HanSXZhouXSuiXSerum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancerOncotarget2015623199071991726101916