Abstract
Purpose
A prognostic nomogram was applied to predict survival in osteosarcoma patients.
Patients and methods
Data collected from 2,195 osteosarcoma patients in the Surveillance, Epidemiology, and End Results (SEER) database between 1983 and 2014 were analyzed. Independent prognostic factors were identified via univariate and multivariate Cox analyses. These were incorporated into a nomogram to predict 3- and 5-year overall survival (OS) and cancer-specific survival (CSS) rates. Internal and external data were used for validation. Concordance indices (C-indices) were used to estimate nomogram accuracy.
Results
Patients were randomly assigned into a training cohort (n=1,098) or validation cohort (n=1,097). Age at diagnosis, tumor site, histology, tumor size, tumor stage, use of surgery, and tumor grade were identified as independent prognostic factors via univariate and multivariate Cox analyses (all P<0.05) and then included in the prognostic nomogram. C-indices for OS and CSS prediction in the training cohort were 0.763 (95% CI 0.761–0.764) and 0.764 (95% CI 0.762–0.765), respectively. C-indices for OS and CSS prediction in the external validation cohort were 0.739 (95% CI 0.737–0.740) and 0.740 (95% CI, 0.738–0.741), respectively. Calibration plots revealed excellent consistency between actual survival and nomogram prediction.
Conclusion
Nomograms were constructed to predict OS and CSS for osteosarcoma patients in the SEER database. They provide accurate and individualized survival prediction.
Introduction
Osteosarcoma, mainly originated from primitive malignant mesenchymal cells in bone,Citation1 is the most common primary malignant bone tumor, typically affecting adolescents under 24 years of age with an estimated incidence of 0.34/100,000 per year.Citation2 The metaphyses of long bones are the primary sites of most osteosarcomas, including distal femur, proximal humerus, and proximal tibia, with approximately 10% of osteosarcomas derived from the axial skeleton.Citation3 Local swelling, pain, and restricted joint movement are the most common symptoms. Before the 1970s, amputation was still the main therapeutic measure for high-grade osteosarcoma because of the lack of adjuvant chemotherapy,Citation4 which seriously affected patient quality of life and reduced the probability of survival. With the introduction of the adjuvant chemotherapy and limb salvage surgery, the survival rate rose from less than 20% to approximately 70%.Citation5 Currently, wide resection together with adjuvant chemotherapy and limb reconstruction have been widely applied to treat high-grade osteosarcoma.Citation6,Citation7 Nevertheless, these options are often insufficient for patients with metastatic and recurrent osteosarcoma.Citation1,Citation8 Better comprehension of the prognostic variables of osteosarcoma can provide more assistance to guide therapeutic intervention, which contributes to prolonging survival and enhancing quality of life.
Although previous studies focused on prognostic factors for osteosarcoma patients, including tumor size, response to chemotherapy, recurrence, and metastasis,Citation9–Citation11 these variables only served as a single index to evaluate prognosis, which limited their impact on a precise individualized survival prediction of osteosarcoma patients. Considering the limitation of the single factor, we sought to develop a novel prognostic model. In the present study, we constructed a nomogram, an efficient prognostic tool, to more precisely estimate an individual patient’s survival more precisely by integrating all prognostic factors for osteosarcoma patients. A prognostic nomogram is an ocular and effective tool based on statistical regression models.Citation12 It can provide a graphic calculating scales method that can be used to estimate the probability of patient survival.Citation13 A nomogram can improve the predictive accuracy of individual prognosis because of its strong robustness and better predictive accuracy.Citation12–Citation14 The Surveillance, Epidemiology, and End Results (SEER) dataset between 1983 and 2014 provided clinical information of osteosarcoma patients that allowed detailed analyses of survival of osteosarcoma. This cancer database covers approximately 30% of the overall US population.Citation12 It is composed of 18 registries that contain clinical information on patients with tumors in the US.Citation12 The purpose of current study was to construct effective prognostic nomograms to predict 3- and 5-year overall survival (OS) and cancer-specific survival (CSS) rates for osteosarcoma patients.
Patients and methods
Patient eligibility and variables
We identified all osteosarcoma patients listed in the SEER database, which collects anonymized clinical data from population-based cancer registries. Use of these clinical data does not require patients’ informed consent since no case-identifying information is provided.Citation15 No ethics approval was sought for this study as the data used were from the publicly available, de-identified SEER database.Citation16 All procedures were performed in accordance with the Helsinki Declaration (1964) and its later amendments or comparable ethical standards.Citation16 SEER*Stat software (version 8.3.5; NCI, Bethesda, MD, USA) was used to acquire patient information.
The inclusion criteria for osteosarcoma patients in the present study were as follows:
Diagnosed with osteosarcoma (International Classification of Diseases for Oncology [ICD-O]: 9180, 9181, 9182, 9183, 9184, 9185, 9186, 9187, 9192, 9193, 9194, or 9200) as a primary malignancy between 1983 and 2014.
Positive histological confirmation of osteosarcoma.
Site limited to extremity (long or short bones of the upper or lower extremities) or axial location (skull, pelvis, spine, or ribs).
Confirmation of histologic type of osteosarcoma.
Known cause of death and survival months after diagnosis.
The exclusion criteria for osteosarcoma patients in this study were:
Unknown use of surgery.
Unknown surgical stage.
Unknown tumor size.
Clinicopathological features including patient age, gender, histology, surgical stage, tumor size, tumor site, grade, marital status, race, use of surgery, and survival time were collected. The anatomic location of osteosarcoma was categorized as extremity (long or short bones of the upper or lower extremities) or axial (skull, pelvis, spine, or ribs). Low-grade tumors contained well- and moderately differentiated grades (ICD-O-3 Grades 1 and 2), and high-grade tumors contained poorly or undifferentiated grades (ICD-O-3 Grades 3 and 4). Cutoff values of age of diagnosis and tumor size were determined via X-tile software (Yale University, New Haven, CT, USA), which was previously shown to determine best cut-points of tumor variables.Citation17 The optimal cutoff values of tumor size were categorized as small (<2.9 cm), intermediate (2.9–10.0 cm), and large (>10.0 cm) (). The optimal age cutoffs were 25 and 51 years (), so patients were categorized into three age groups (0–24 years, 25–51 years, or >51 years). According to American Joint Committee on Cancer (AJCC) staging system for bone sarcomas, surgical stage was categorized as localized, regional, or distant.Citation18 Patients coded with “localized” disease were classified as disease confined to the periosteum, while those with “regional” disease had tumor extending beyond the periosteum but without distant metastasis. Patients with missing surgical stage data were excluded. Surgical resection was categorized as yes or no; data on the type of resection (eg, wide, marginal, or intralesional) could not be obtained from the SEER database. Race was categorized as white, black, or other (American Indian/Alaskan Native, Asian/Pacific Islander). In terms of chemotherapy and radiation, “No/Unknown” was used in the updated SEER dataset as a single option, impacting data completeness. These patients had no codes for radiation or chemotherapy in their medical records. Adding this information to the nomogram might have introduced relevant bias,Citation12 so use of chemotherapy and radiation was not included as a variable.
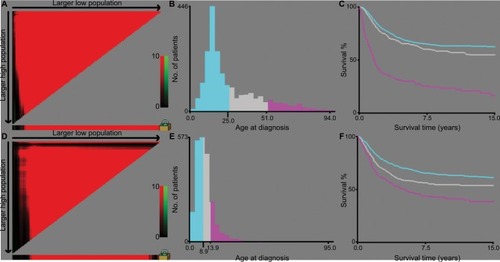
Figure 1 Identification of optimal cutoff values of age of diagnosis (A–C) and tumor size (D–F) via X-tile analysis.
Notes: Optimal cutoff values of age were identified as 29 and 51 years based on overall survival. Optimal cutoff values of tumor size were identified as 8.9 and 13.9 cm based on overall survival. Histogram and Kaplan–Meier analysis were developed based on these cutoff values.
Statistical analysis
Based on the abovementioned inclusion and exclusion criteria, osteosarcoma patients were randomly divided into a training cohort (n=1,098) or validation cohort (n=1,097) to construct and validate nomograms. Chi-squared tests were used to compare clinical characteristics between the cohorts.
Continuous and categorical variables are presented as the number of osteosarcoma patients with respective percentages. X-tile software was applied to calculate cutoff values for tumor size and age of diagnosis based on OS information (). The prognostic factors (age at diagnosis, gender, primary site, tumor size, histology, surgical stage, grade, marital status, race, use of surgery, etc) were further evaluated via univariate and multivariate Cox proportional hazards regression analyses. Hazard ratios and corresponding 95% CI of variables were also calculated. OS and CSS were the two primary endpoints. Survival times were calculated from the date of disease diagnosis to the date of death from any disease cause (OS) or death from osteosarcoma (CSS). Prognostic nomograms for 3- and 5-year OS and 3- and 5-year CSS were constructed according to the univariate and multivariate Cox analyses. Internal and external validations of the prognostic nomogram were performed. Harrell’s concordance-index (C-index) was applied to evaluate prognostic nomogram performance. This C-index was a useful evaluation value similar to calculating the area under the receiver operating characteristic curve.Citation19 C-indices range from 0.5 to 1.0, indicating total chance and perfect matching, respectively.Citation20 Calibration curves were constructed to compare consistency between predicted and observed survival. Chi-squared tests and univariate and multivariate Cox analyses were performed with SPSS 22.0 software (IBM Corp, Armonk, NY, USA). rms Package in R software (version 3.3.1) was used to construct and validate prognostic nomograms. Differences were considered significant at two-sided P<0.05.
Results
Patient baseline characteristics
The SEER database contained 2,195 osteosarcoma patients between 1983 and 2014, including 1,098 patients in the training cohort and 1,097 patients in the validation cohort. The training cohort was used to construct and internally validate the nomogram, and the validation cohort was used for external validation. In the training cohort, 363 patients died from osteosarcoma, and 32 patients died from other causes. In the validation cohort, 356 patients died from osteosarcoma, and 37 patients died from other causes.
The osteosarcoma patients’ characteristics are listed in . Of these patients, 981 (44.7%) patients were females and 1,214 (55.3%) patients were males. The most common primary location of these osteosarcoma patients was an extremity (80.8%), and 19.2% had a primary axial site. With regard to tumor stage, regional disease (48.0%) was most frequent, followed by localized disease (32.8%) and distant disease (19.1%). In both cohorts, the majority of patients were children or adolescents (<25 years; 63.2%). Most tumors were <8.9 cm (51.8%). Most of the patients in our study had received surgical treatment (91.4%). There were no significant differences between the training and validation cohorts.
Table 1 Baseline demographic and clinical characteristics of patients with osteosarcoma
Prognostic factors for OS and CSS
In the training cohort, data from 1,098 osteosarcoma patients were included in univariate and multivariate analyses to identify independent prognostic factors for OS and CSS. As is shown in and , gender, age at diagnosis, tumor site, histology, tumor size, tumor stage, use of surgery, tumor grade, and marital status were significantly associated with OS and CSS in the univariate analysis. These nine factors were further selected to conduct the multivariate Cox analysis in order to control for confounding variables. The multivariate Cox analysis revealed that seven factors including age at diagnosis, tumor site, histology, tumor size, tumor stage, use of surgery, and tumor grade were independent prognostic factors for OS and CSS.
Table 2 Univariate and multivariate analyses of overall survival in the training cohort
Table 3 Univariate and multivariate analyses of cancer-specific survival in the training cohort
Construction and validation of the OS and CSS nomograms
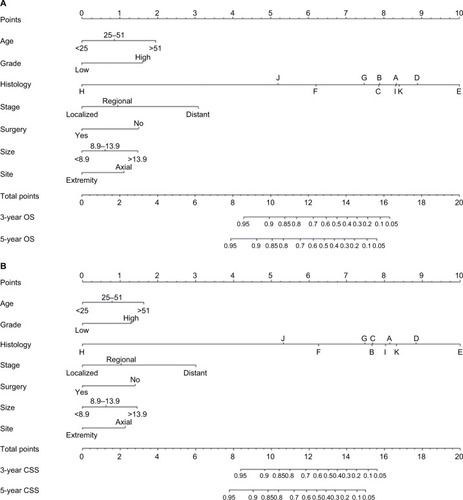
The significant independent factors of age at diagnosis, tumor site, histology, tumor size, tumor stage, use of surgery, and tumor grade were incorporated to create the prognostic nomograms for estimating the 3- and 5-year OS and CSS of osteosarcoma patients (). The nomogram gives every prognostic variable a score on the point scale (). By adding up these scores to the total on the bottom scale, the 3- and 5-year OS and CSS of osteosarcoma patients can be predicted.
Table 4 Detailed scores of prognostic factors in the overall and cancer-specific survival nomograms
Figure 2 Nomograms to predict 3- and 5-year overall survival (A) and cancer-specific survival (B) for osteosarcoma patients.
Notes: Vertical line between each variable and points scale can be drawn to acquire points of each variable. Predicted survival rate was calculated according to the total points by drawing a vertical line from Total Points scale to overall survival or cancer-specific survival scale. A, conventional osteosarcoma; B, chondroblastic osteosarcoma; C, fibroblastic osteosarcoma; D, telangiectatic osteosarcoma; E, osteosarcoma in Paget disease of bone; F, small cell osteosarcoma; G, central osteosarcoma; H, intraosseous well-differentiated osteosarcoma; I, parosteal osteosarcoma; J, periosteal osteosarcoma; K, high-grade surface osteosarcoma.
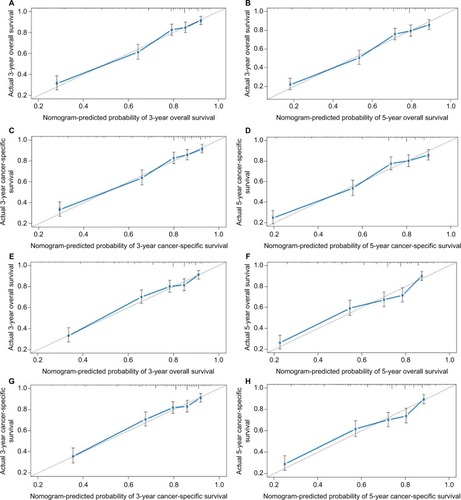
Prognostic nomogram validation was conducted both internally and externally (). Internal validation in the training cohort showed that the C-index values for nomogram predictions of OS and CSS were 0.763 (95% CI 0.761–0.764) and 0.764 (95% CI 0.762–0.765), respectively. Similarly, the corresponding C-index values in the external validation cohort were 0.739 (95% CI 0.737–0.740) and 0.740 (95% CI 0.738–0.741). These results confirm that our prognostic nomograms were reasonably accurate. The calibration plots () demonstrated excellent agreement between actual survival and nomogram prediction.
Figure 3 Internal calibration plots of 3-year (A) and 5-year (B) overall survival nomogram calibration curves; 3-year (C) and 5-year (D) cancer-specific survival nomogram calibration curves. External calibration plots of 3-year (E) and 5-year (F) overall survival nomogram calibration curves; 3-year (G) and 5-year (H) cancer-specific survival nomogram calibration curves.
Notes: The cohort was divided into five subgroups with the equal sample size for present internal validation. The dashed line represents an excellent match between actual survival outcome (Y-axis) and nomogram prediction (X-axis). Closer distances between dashed line and points indicated higher prediction accuracy.
In summary, we constructed and validated the nomogram to estimate 3- and 5-year OS and CSS for osteosarcoma patients. Based on an individual osteosarcoma patient’s prognostic factors, we can obtain a score associated with each prognostic factor on the nomogram point scale and calculate the total score. We can then evaluate 3- and 5-year survival probability by projecting the total points to the total score scale of the nomogram. As an example, an 18-year-old patient was diagnosed with an axial chondroblastic osteosarcoma with a primary tumor size of 10.0 cm that was high grade. This patient was found to have regional disease and underwent surgery. According to our nomograms, the patient has 12.1 and 11.7 points in OS and CSS, respectively. The 3-year OS and CSS rates of this osteosarcoma were 0.72 and 0.69, respectively, while the corresponding 5-year rates were 0.62 and 0.62.
Discussion
Multiple prognostic factors can affect osteosarcoma patient survival, but previous studies did not integrate overall prognostic factors. A single prognostic index may impose limitations on estimating an individual patient’s survival prognosis. The nomogram is a common statistical tool that can provide satisfactory accuracy and robustness to precisely predict an individual patient’s survival probability.Citation21 Kim et al constructed a prognostic nomogram for nonmetastatic osteosarcoma patients that could estimate and predict metastasis risk better than the AJCC staging system or tumor necrosis rate alone.Citation22 Xia et al also devised a nomogram to further predict the survival of osteosarcoma patients after surgical resection.Citation23 However, these studies were designed without validation, so their results might not be relevant in other populations due to potential bias. Kim et al developed a high-performance nomogram to predict the probability of metastasis in Enneking stage IIB extremity osteosarcoma using the medical records of 91 patients who had undergone surgery.Citation24 However, the small sample size was a significant limiting factor, and the generalizability of this nomogram should be validated in larger populations. In the present study, we constructed convenient and comprehensive prognostic nomograms using data from 2,195 osteosarcoma cases in the SEER dataset, which allowed us to calculate 3- and 5-year OS and CSS rates for osteosarcoma patients.
To accurately select the prognostic factors, we performed univariate log-rank and multivariate Cox analysis to identify independent prognostic factors. The results showed that age at diagnosis, tumor site, histology, tumor size, tumor stage, use of surgery, and tumor grade are independent prognostic factors for the survival of patients with osteosarcoma. In the current study, within the period from 1984 to 2014, year of diagnosis was not found to be independently associated with OS or CSS. One possible explanation is that progress made in clinical information has not been as successful for osteosarcoma. Similar approach has been taken in previous investigations.Citation25,Citation26 In previous studies, increasing patient age was associated with a statistically significant decrease in the survival prognosis of osteosarcoma patients.Citation26–Citation28 Ek et al reported that osteosarcoma patients older than 40 had worse survival outcomes.Citation29 Similarly, we identified increasing patient age as an independent negative prognostic factor for osteosarcoma patients. Our analysis used X-tile software to stratify the data of age based on status and survival time. It identifies the best cut-points of variables and was initially applied in breast malignancy. We determined that the optimal age cut-points of osteosarcoma patients were 25 and 51 years. Tumor size was also one of the key measures of survival prognosis of osteosarcoma patients. Several previous studies reported that patients with larger tumors had a poorer prognosis and decreased survival rate.Citation10,Citation30,Citation31 We also identified larger tumor size as an independent prognostic factor of shorter survival. To obtain the best cut-points for tumor size, we again used X-tile software for data stratification. The results showed that 8.9 and 13.9 cm were the optimal cutoff values. We also observed that adequate use of limb salvage surgery had a significant effect on osteosarcoma patient survival outcomes. Previous studies reported similar results.Citation9,Citation28,Citation32
In a previous study, the tumor site and stage were reported as the most significant prognostic factors for osteosarcoma patients.Citation32 These tumors appear mostly in the metaphyses of long bones, with approximately 10% of osteosarcomas occurring in the axial skeleton.Citation33 Seker et al reported that osteosarcoma patients with extremity primary tumors have better survival prognoses than those with non-extremity tumors.Citation32 Other groups also found that an axial primary site of osteosarcoma was associated with considerably worse survival outcomes.Citation33–Citation35 The present study also demonstrated that tumor site influences the survival of osteosarcoma patients. With regard to the tumor stage at diagnosis, several groups reported that osteosarcoma patients with metastases have a significantly worse survival prognosis.Citation1,Citation10,Citation36,Citation37 Patients with metastases may have better relative outcomes if they had only lung metastases and underwent curative metastasectomy.Citation8 Consistent with these findings, we showed that osteosarcoma patients with distant metastases had a higher risk of death. We also identified tumor grade and histology as independent prognostic for osteosarcoma patients, which is in line with previous studies.Citation38,Citation39 Jawad et alCitation26 demonstrated that Paget’s osteosarcoma had significantly worse prognosis compared with all other histological subtypes. Their analysis of different histological subtypes confirmed the results reported by Damron et al.Citation40 Jawad et alCitation26 also reported that fibroblastic osteosarcoma had significantly better prognosis compared with conventional osteosarcoma, which was similar to our results.
By integrating the abovementioned independent prognostic factors, we created prognostic nomograms that offer an effective and functional method to estimate 3- and 5-year OS and CSS for osteosarcoma patients. These nomograms can improve the accuracy of predicting individual survival outcomes of osteosarcoma patients at certain time points.
Although the prognostic nomograms in the present study showed good predictive ability, there are some limitations which should be taken into consideration. First, the data on radiotherapy and chemotherapy were limited in the SEER database, which might have led to incompleteness of several meaningful clinicopathological parameters and caused other relevant bias. For this reason, chemotherapy or radiation use was not incorporated in our study. Second, since our study was retrospective, it is inevitable that certain patient data were missing. This might have decreased the number of eligible cases. Third, our findings will be more reliable if the nomogram model is externally validated using another independent, large-scale dataset; this would verify whether our results are universally applicable. Despite these limitations, our prognostic nomogram is a significant and effective model for accurately predicting the individual survival outcomes of osteosarcoma patients.
Conclusion
The present study identified age at diagnosis, tumor site, histology, tumor size, tumor stage, use of surgery, and tumor grade as independent prognostic variables for both the OS and CSS rates of osteosarcoma patients. These independent prognostic variables were integrated to build a nomogram prognosis evaluation model for osteosarcoma patients. These offer a more reliable and accurate prediction of osteosarcoma patient survival. Utilizing our nomogram, the 3- and 5-year OS and CSS rates for osteosarcoma patients can be estimated, enabling surgeons to assess personalized survival probability and identify mortality risk.
Acknowledgments
This study was supported by Guangzhou Science and Technology Project of China (grant no. 201607010021) and Science and Technology Planning Project of Guangdong Province, People’s Republic of China (grant no. 2014A020212571).
Disclosure
The authors report no conflicts of interest in this work.
References
- RitterJBielackSSOsteosarcomaAnn Oncol201021Suppl 7vii320vii32520943636
- MirabelloLTroisiRJSavageSAInternational osteosarcoma incidence patterns in children and adolescents, middle ages and elderly personsInt J Cancer2009125122923419330840
- BielackSSKempf-BielackBDellingGPrognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocolsJ Clin Oncol200220377679011821461
- EilberFGiulianoAEckardtJPattersonKMoseleySGoodnightJAdjuvant chemotherapy for osteosarcoma: a randomized prospective trialJ Clin Oncol19875121263543236
- BacciGFerrariSBertoniFLong-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated reportJ Clin Oncol200018244016402711118462
- AyerzaMAMuscoloDLAponte-TinaoLAFarfalliGEffect of erroneous surgical procedures on recurrence and survival rates for patients with osteosarcomaClin Orthop Relat Res200645223123516906060
- HongAMMillingtonSAhernVLimb preservation surgery with extracorporeal irradiation in the management of malignant bone tumor: the oncological outcomes of 101 patientsAnn Oncol201324102676268023852310
- KagerLZoubekAPötschgerUPrimary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocolsJ Clin Oncol200321102011201812743156
- BernerKHallKSMongeORWeedon-FekjærHZaikovaOBrulandØSPrognostic factors and treatment results of high-grade osteosarcoma in Norway: a scope beyond the “classical” patientSarcoma201520155168431425784831
- ClarkJCDassCRChoongPFA review of clinical and molecular prognostic factors in osteosarcomaJ Cancer Res Clin Oncol2008134328129717965883
- WangWYangJWangYSurvival and prognostic factors in Chinese patients with osteosarcoma: 13-year experience in 365 patients treated at a single institutionPathol Res Pract2017213211912528040328
- DongFShenYGaoFNomograms to predict individual prognosis of patients with primary small cell carcinoma of the bladderJ Cancer2018971152116429675096
- ZhouHZhangYQiuZNomogram to predict cause-specific mortality in patients with surgically resected stage I non-small-cell lung cancer: a competing risk analysisClin Lung Cancer2018192e195e20329153966
- NaritaYKadowakiSOzeIEstablishment and validation of prognostic nomograms in first-line metastatic gastric cancer patientsJ Gastrointest Oncol201891526329564171
- LinZYanSZhangJPanQA Nomogram for distinction and potential prediction of liver metastasis in breast cancer patientsJ Cancer20189122098210629937928
- LiDZhongCTangXYuLDingKYuanYCompeting nomograms help in the selection of elderly patients with colon cancer for adjuvant chemotherapyJ Cancer Res Clin Oncol2018144590992329460089
- CampRLDolled-FilhartMRimmDLX-tile: a new bioinformatics tool for biomarker assessment and outcome-based cut-point optimizationClin Cancer Res200410217252725915534099
- GiuffridaAYBurguenoJEKoniarisLGGutierrezJCDuncanRScullySPChondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER databaseJ Bone Joint Surg Am20099151063107219411454
- HarrellFELeeKLMarkDBMultivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errorsStat Med19961543613878668867
- ValentiniVvan StiphoutRGLammeringGNomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trialsJ Clin Oncol201129233163317221747092
- BalachandranVPGonenMSmithJJDematteoRPNomograms in oncology: more than meets the eyeLancet Oncol2015164e173e18025846097
- KimMSLeeSYLeeTRPrognostic nomogram for predicting the 5-year probability of developing metastasis after neo-adjuvant chemotherapy and definitive surgery for AJCC stage II extremity osteosarcomaAnn Oncol200920595596019153123
- XiaWKLiuZLShenDLinQFSuJMaoWDPrognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcomaWorld J Surg Oncol20161412727125872
- KimSHShinKHKimHYPostoperative nomogram to predict the probability of metastasis in Enneking stage IIB extremity osteosarcomaBMC Cancer20141466625216622
- SongKSongJShiXDevelopment and validation of nomograms predicting overall and cancer-specific survival of spinal chondrosarcoma patientsSpine20181
- JawadMUCheungMCClarkeJKoniarisLGScullySPOsteosarcoma: improvement in survival limited to high-grade patients onlyJ Cancer Res Clin Oncol2011137459760720514491
- SongWSKongCBJeonDGPrognosis of extremity osteosarcoma in patients aged 40–60 years: a cohort/case controlled study at a single instituteEur J Surg Oncol201036548348820363585
- FaishamWIMat SaadAZAlsaighLNPrognostic factors and survival rate of osteosarcoma: a single-institution studyAsia Pac J Clin Oncol2017132e104e11025870979
- EkETOjaimiJKitagawaYChoongPFOutcome of patients with osteosarcoma over 40 years of age: is angiogenesis a marker of survival?Int Semin Surg Oncol20063716551370
- BielingPRehanNWinklerPTumor size and prognosis in aggressively treated osteosarcomaJ Clin Oncol19961438488588622033
- BacciGLonghiAVersariMMercuriMBriccoliAPicciPPrognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institutionCancer200610651154116116421923
- SekerMMSekerAAksoySOzdemirNUncuDZenginNClinicopathologic features and prognosis of osteosarcoma in Turkish adultsAsian Pac J Cancer Prev20141583537354024870753
- WangZXQiuMZJiangYMZhouZWLiGXXuRHComparison of prognostic nomograms based on different nodal staging systems in patients with resected gastric cancerJ Cancer20178695095828529606
- OzakiTFlegeSKevricMOsteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study GroupJ Clin Oncol200321233434112525527
- OzakiTFlegeSLiljenqvistUOsteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study GroupCancer20029441069107711920477
- BielackSSKempf-BielackBDellingGPrognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocolsJ Clin Oncol200220377679011821461
- JanewayKABarkauskasDAKrailoMDOutcome for adolescent and young adult patients with osteosarcoma: a report from the Children’s Oncology GroupCancer2012118184597460522252521
- WangZLiSLiYPrognostic factors for survival among patients with primary bone sarcomas of small bonesCancer Manag Res2018101191119929795990
- ArshiASharimJParkDYPrognostic determinants and treatment outcomes analysis of osteosarcoma and Ewing sarcoma of the spineSpine J201717564565527856382
- DamronTAWardWGStewartAOsteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base ReportClin Orthop Relat Res2007459404717414166
