Abstract
Background
The purpose of this study was to analyze the incidence and prognostic factors of patients with breast cancer liver metastases (BCLM) at initial diagnosis.
Methods
We utilized the Surveillance, Epidemiology, and End Results database to extract data on patients with primary invasive breast cancer from 2010 to 2014. Multivariate logistic regression was conducted to determine factors associated with the presence of liver metastases upon initial diagnosis of breast cancer. Univariate and multivariate Cox regression analyses were performed to identify the prognostic factors in these patients.
Results
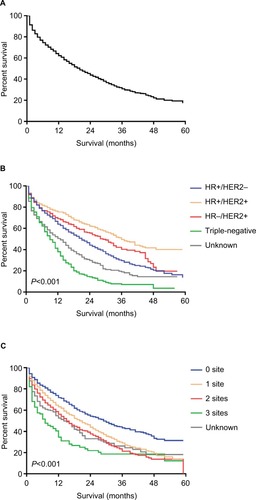
In total, 3,276 patients with liver metastases were identified upon initial diagnosis of breast cancer. Patients with hormone receptor-negative (HR−), human epidermal growth factor receptor 2-positive (HER2+) breast cancer had the highest incidence (4.6% among the entire population, 46.5% among the metastatic subgroup). Age, gender, race, pathological grade, extrahepatic metastases, tumor subtype, and marital status were identified as factors associated with the presence of liver metastases upon initial diagnosis of breast cancer. The median overall survival among the entire population with BCLM was 20.0 months. Patients with HR+/HER2+ breast cancer had the longest median survival of 36.0 months. The survival analyses indicated that older age, higher pathological grade, extrahepatic metastases, triple-negative subtype, unmarried status, and uninsured status were independent prognostic factors for a poorer prognosis.
Conclusion
The study provides insight into the incidence and prognostic factors for patients with BCLM at initial diagnosis, which is important clinical information for risk evaluation and prognostic assessment.
Introduction
According to the latest overview of cancer statistics, breast cancer is the most frequently diagnosed cancer and is the leading cause of cancer death among women worldwide.Citation1 The survival of breast cancer patients is strongly stage dependent. Specifically, the 5-year relative survival of patients with localized tumors can be as high as 98.6%, compared with 83.8% for patients with regional tumors and 23.3% for patients with distant metastases.Citation2 In addition, ~6% of patients present with metastatic disease at initial diagnosis, while ~30% of patients first diagnosed with early-stage disease eventually develop metastatic disease.Citation3,Citation4
It is widely recognized that breast cancer is a heterogeneous disease and can be categorized into several distinct molecular subtypes based on the presence of estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 (HER2).Citation5,Citation6 Recent studies imply that the distinct subtypes have different prognoses and anticancer therapy responses.Citation7,Citation8 Additionally, there is growing evidence indicating that different molecular subtypes have their own specific sites of distant metastases. Hormone receptor-positive (HR+) breast cancer preferentially metastasizes to bone, while the HER2-positive (HER2+) subtype and triple-negative breast cancer tend to metastasize to visceral organs such as the brain, liver, and lung.Citation9–Citation13
Notably, relative to the bone, lung, and brain, liver is one of the most common breast cancer metastatic sites, with clinical and autopsy incidence of 40%–50% and 50%–62%, respectively, among all metastatic breast cancers.Citation14–Citation17 Liver metastases may present asymptomatically or with abdominal discomfort, ascites, jaundice, abnormal function tests, hepa-tomegaly, or abdominal pain.Citation18–Citation20 Patients with breast cancer liver metastases (BCLM) can experience refractory complications including sudden hepatic failure, refractory ascites, esophageal varices, portal vein thrombosis, and nutritional compromise.Citation20 Earlier studies reported that the involvement of visceral metastases, especially liver metastases, is a sign of poor survival.Citation21,Citation22 The median survival time of patients with BCLM is only 4–8 months without treatment.Citation23 Research on metastasis mechanisms and organotropism may assist in improving the outcome of patients with BCLM. Furthermore, circulating tumor cells and Flammer syndrome had been studied to select and stratify potentially predisposed to liver metastases among patients with breast cancer.Citation24
In the present study, we utilized the Surveillance, Epidemiology, and End Results (SEER) database to study patients with BCLM at initial diagnosis stratified by breast cancer subtype. The goals of this study were: 1) to evaluate the clinicopathological characteristics of patients with BCLM, 2) to calculate the relative incidence of patients with BCLM, and 3) to determine the factors associated with the presence of liver metastases and the survival of patients with BCLM at initial diagnosis.
Methods
Database
SEER database consisting of 18 population-based cancer registries includes information on cancer incidence, patient characteristics, primary tumor site, tumor morphology, treatment, and survival of ~30% of the US population. The datasets of the current study are available from SEER Program (https://seer.cancer.gov/) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (1973–2014 varying), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
Study population
Because SEER began collecting sites of metastasis at initial diagnosis in 2010, we set the starting point of our study as 2010. We extracted 228,300 patients 18 years or older who were diagnosed with primary and histologically validated malignant breast cancer as the only primary malignancy between 2010 and 2014. Patients with carcinoma in situ and an unknown state of liver metastases were excluded from this cohort, leaving 224,449 patients in the final cohort for incidence analysis. Of these, 3,281 patients had liver metastases when first diagnosed with breast cancer. We subsequently excluded patients who were diagnosed by autopsy or death certificate, as well as patients who had an unknown follow-up or whose survival record presented with 0 months for the survival analysis, leaving 213,945 patients, among whom there were 2,804 patients diagnosed with liver metastases.
Ethics statement
Our study was approved by an independent ethical committee/institutional review board at Fudan University Shanghai Cancer Center (Shanghai Cancer Center Ethical Committee). The data released by the SEER database do not require informed patient consent for cancer is a reportable disease in every state in the USA.
Statistical analysis
Study variables, including age at diagnosis, year of diagnosis, sex, race, histology, pathological grade, type of breast cancer surgery, site of metastases, marital status, and insurance status, were enrolled into the descriptive statistics to evaluate the patient characteristics stratified by breast cancer subtype, which were classified into HR-positive/HER2-negative (HR+/HER2−), HR-positive/HER2-positive (HR+/HER2+), HR-negative/HER2-positive (HR−/HER2+), and triple-negative. In the SEER database, pathological grades were categorized into I (well differentiated), II (moderately differentiated), III (poorly differentiated), and IV (undifferentiated or anaplastic). Chi-square or Fisher’s exact tests were used as appropriate.
Incidence was defined as the number of patients with liver metastases divided by the total number of patients with breast cancer. We calculated the incidence of patients with liver metastases among the entire cohort and metastatic subgroup stratified by breast cancer subtype.
We used multivariable logistic regression to determine whether age at diagnosis, sex, race, marital status, and insurance status were associated with the presence of liver metastases at diagnosis; other variables, namely, histology, pathological grade, breast cancer subtype, and number of extrahepatic metastatic sites, including the lung, bone, and brain, were also enrolled into this model. ORs and 95% CIs from the logistic regression model are reported.
Overall survival (OS) was defined as the date of diagnosis to the date of death regardless of whether the death was caused by breast cancer, and breast cancer-specific survival (BCSS) was calculated from the date of diagnosis to the date of death due to breast cancer. We utilized the Kaplan–Meier method to obtain survival estimates and generate survival curves within subsets and analyzed the differences using log-rank tests. Univariable and multivariable Cox proportional hazards models were constructed to assess the association of the variables as described in the logistic regression model with increased all-cause mortality and breast cancer-specific mortality. We calculated hazard ratios and 95% CIs in the Cox regression model.
All the statistical analyses were performed using SPSS statistical software version 22.0 package (IBM Corporation, Armonk, NY, USA). All reported P-values were two-sided, and a P-value of 0.05 or less was considered as statistically significant.
Results
Patient characteristics
A total of 224,449 patients were diagnosed with breast cancer from 2010 to 2014 in the USA and were enrolled in the incidence analysis. Among the entire population, 3,276 patients had liver metastases at the initial diagnosis of breast cancer, and summarizes their clinicopathological characteristics according to breast cancer subtype. Patients with HR+/HER2−, HR+/HER2+, HR−/HER2+, triple-negative, and unknown subtype, respectively, accounted for 38.9%, 20.5%, 14%, 13.2%, and 13.4%, and only 851 patients had metastatic disease confined to the liver (26.0%). Compared with other patients, HR+/HER2− breast cancer patients with liver metastases were older (P<0.001), had a higher rate of invasive lobular carcinoma pathology (P<0.001), had a lower pathological grade (P<0.001), and had a higher rate of no surgery for breast cancer (P<0.001)). In contrast, triple-negative breast cancer patients with liver metastases had a higher rate of pathological grade III/IV (P<0.001), had a higher rate of breast cancer surgery (P<0.001), and presented with more extrahepatic metastatic sites (P<0.001).
Table 1 Clinicopathological characteristics of patients with liver metastases according to breast cancer subtype
Incidence
In the total of 224,449 patients, 11,997 patients were diagnosed with metastatic disease. displays the result of incidence of liver metastases stratified by breast cancer subtype among the entire population and the metastatic subgroup. A total of 3,276 patients diagnosed with liver metastases accounted for 1.5% of the entire population and 27.3% of the metastatic subgroup. Patients with HR−/HER2+ (4.6% of the entire population, 46.5% of the metastatic subgroup) and patients with HR+/HER2+ (2.9% of the entire population, 37.5% of the metastatic subgroup) had the highest incidence of liver metastases, while patients with HR+/HER2− (0.8% of the entire population, 20.6% of the metastatic subgroup) had the lowest incidence.
Table 2 Incidence and median OS of patients with liver metastases at initial diagnosis of breast cancer stratified by breast cancer subtype
The multivariable logistic regression was performed among the entire population and the metastatic subgroup (). Among the entire population, female sex (vs male sex, P=0.001); black race (vs white race, P=0.025); pathological grade II (vs grade I, P<0.001) and grade III/IV (vs grade I, P<0.001); metastatic diseases to one extrahepatic site (vs 0 extrahepatic site, P<0.001), two extrahepatic sites (vs 0 extrahepatic site, P<0.001), and three extrahepatic sites (vs 0 extrahepatic site, P<0.001); and HR+/HER2+ (vs HR+/HER2− subtype, P<0.001), HR−/HER2+ (vs HR+/HER2− subtype, P<0.001), and triple-negative subtypes (vs HR+/HER2− subtype, P<0.001) were significantly associated with an increased risk of having liver metastases at diagnosis. Age 41–60 years (vs age 18–40 years, P=0.001), age 61–80 years (vs age 18–40 years, P<0.001), age >80 years (vs age 18–40 years, P<0.001), and married status (vs unmarried, P=0.005) were significantly associated with a lower risk of having liver metastases at diagnosis. Neither histology nor insurance status was associated with the risk of having liver metastases at initial diagnosis of breast cancer in this model. Among the metastatic subgroup, age, sex, histology, pathological grade, extrahepatic metastatic sites, and tumor subtype were associated with the presence of liver metastases at diagnosis of breast cancer (P<0.05).
Table 3 Multivariate logistic regression for the presence of liver metastases at initial diagnosis of breast cancer
Survival analysis
A total of 2,804 patients with complete follow-up time were included in the survival analysis. The median OS among patients with BCLM was 20.0 months (). OS estimates stratified by breast cancer subtype is displayed in (log-rank test, P<0.001). Patients with HR+/HER2+ subtype had the longest median survival (36.0 months), while patients with triple-negative subtype had the shortest median survival (9.0 months). Significant difference was shown when stratified by the extent of extrahepatic metastatic disease (; log-rank test, P<0.001). Patients with more number of extrahepatic metastatic sites had worse prognosis.
Figure 1 Kaplan–Meier curves for OS among patients with liver metastases upon initial diagnosis of breast cancer.
Notes: (A) OS. (B) OS stratified by the breast cancer subtype. (C) OS stratified by the extent of extrahepatic metastatic diseases.
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; + denotes positive; - denotes negative; OS: overall survival.
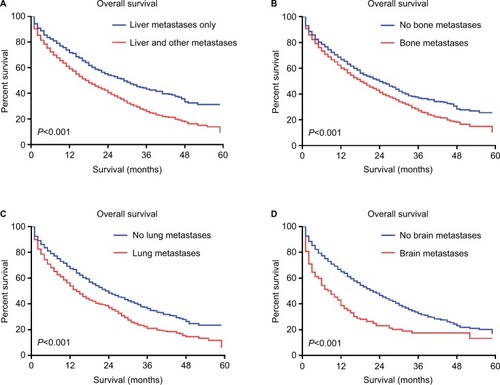
The impact of the presence of extrahepatic metastases on OS among the patients with BCLM is graphically displayed in . Patients with liver and other metastases had significantly shorter survival as compared with patients with liver metastases only (; log-rank test, P<0.001). Individually, patients with bone metastases had significantly shorter survival than those without bone metastases (; log-rank test, P<0.001). Similar findings were seen for patients with lung metastases vs no lung metastases (; log-rank test, P<0.001) and patients with brain metastases vs no brain metastases (; log-rank test, P<0.001).
Figure 2 Kaplan–Meier curves for overall survival among patients with liver metastases according to individual metastases.
Notes: (A) Patients with only liver metastases vs those with liver and other metastases. (B) Patients with bone metastases vs those without bone metastases. (C) Patients with lung metastases vs those without lung metastases. (D) Patients with brain metastases vs those without brain metastases.
Among the entire population, there were 8,442 patients with extrahepatic metastatic disease. The impact of the presence of liver metastases on the median survival of these patients stratified by the extent of extrahepatic metastatic sites in different breast cancer subtypes is provided in . Generally, patients with no baseline liver metastases had a longer median survival than patients with liver metastases. Specifically, there were significant differences in patients with bone and liver metastases vs those with bone metastases only (log-rank test, P<0.001) and patients with lung and liver metastases vs those with lung metastases only (log-rank test, P<0.001). However, there was no significant difference in OS between patients with brain and liver metastases vs those with brain metastases only (log-rank test, P=0.261). Furthermore, the abovementioned findings were observed in different breast cancer subtypes except for the HR−/HER2+ subtype.
Table 4 Median OS of breast cancer patients stratified by extent of extrahepatic metastatic disease
Univariate and multivariate Cox proportional hazards models were conducted to assess the prognostic factors of patients with BCLM (). In the univariate Cox models, age at diagnosis, type of breast cancer surgery treatment, number of extrahepatic metastatic sites, breast cancer subtype, marital status, and insurance status were significantly associated with OS and BCSS (P<0.05). The multivariate Cox analysis identified that age at diagnosis, pathological grade, type of breast cancer surgery treatment, number of extrahepatic metastatic sites, breast cancer subtype, marital status, and insurance status were independent prognostic factors for OS and BCSS (P<0.05).
Table 5 Cox regression of OS and BCSS among patients with liver metastasis
Discussion
Distant metastasis of breast cancer is a main public health concern affecting women worldwide.Citation1 Liver metastases is the third most common distant metastatic site for breast cancer.Citation25 In addition, BCLM remains an essential clinical problem due to its poor prognosis and limited response to systemic treatment. Golubnitschaja and Sridhar summarized the information about risk assessment and prognostic factors of liver metastases in colorectal, breast, and prostate cancers and recommended the analysis of molecular and pathology specific patterns in blood samples of individual patient, which could provide help for the diagnosis and treatment of liver metastases.Citation26 Besides, the multi-omics approach like proteomics, metabolomics, and bioinformatic analysis in the blood samples could be of great clinical utility for disease prevention, patients stratification, and disease treatment.Citation27,Citation28 There are several studies evaluating the distribution and prognosis of patients with BCLM; however, most mainly evaluate the occurrence of liver metastases after the diagnosis of early-stage breast cancer, while few focus on the patients who present with liver metastases upon initial diagnosis of breast cancer.Citation29–Citation36 Since the previous systemic therapies and disease-free intervals may modify the natural course of liver metastases in recurrent breast cancer,Citation3,Citation37 it is meaningful to evaluate the prognostic factors and outcomes of patients with BCLM at initial diagnosis in a large population-based analysis.
In this retrospective study, we identified 3,276 cases of liver metastases upon initial diagnosis of breast cancer between 2010 and 2014, accounting for 1.5% of all patients with breast cancer and 27.3% of the metastatic subgroup. This result is slightly different from the previously published literature. Diamond et al reported that nearly half of metastatic breast cancer patients have metastasis to the liver during the disease course.Citation20 In addition, Hoe et al reported an incidence of 5.2% among 912 breast cancer patients treated between 1982 and 1987.Citation19 However, these two papers studied the liver metastases of patients diagnosed with early-stage breast cancer and not with synchronous liver metastases upon initial diagnosis of breast cancer. In addition, these two studies were based on small sample size analyses. Furthermore, the incidence of liver metastases may have changed dramatically over time due to the practice of early screening for breast cancer and the improvement of disease awareness.
There were significant differences in the clinicopathological characteristics of patients with BCLM when stratified by molecular subtype (). Compared with other patients, the HER2+ subtype and triple-negative subtype breast cancer patients with liver metastases more presented with high pathological grade, young age at diagnosis, and extrahepatic metastatic disease (P<0.001). Additionally, we found an obvious discrepancy in the incidence of liver metastases when stratified by breast cancer subtype. Specifically, the HR+/HER2+ subtype and HR−/HER2+ subtype had the highest incidence of liver metastases, while the HR+/HER2− subtype had the lowest. Additionally, patients with the HR+/HER2+ subtype, HR−/HER2+ subtype, and triple-negative breast cancer had significantly greater odds of presenting liver metastases at initial diagnosis than patients with the HR+/HER2− subtype among the entire cohort and within the metastatic subgroup. These observations are in accordance with the findings of other publications studying the metastatic behaviors of breast cancer subtypes.Citation9,Citation11,Citation12,Citation38–Citation42 The HER2+ enriched subtype and triple-negative subtype are more aggressive and have a tendency for visceral metastases. Upregulation of the chemokine receptor CXCR4 and enrichment of the PIK3– AKT–mTOR pathway, both relative to HER2 activation, are involved in promoting the metastasis of tumor cells to liver.Citation43,Citation44 Besides, Kimbung et al found that downregulation of extracellular matrix genes was associated with BCLM by whole-genome transcriptional profiling and significant analysis of microarray analyses.Citation45 The activities of matrix metalloproteinases MMP-2 and MMP-9 had increased specifically after radiotherapy treatment in patients with BCLM, which was associated with poorer disease outcome.Citation46 The Notch1 signal pathway had been found to act as metastatic suppressor in the liver microenvironment.Citation47
In addition to the breast cancer subtypes mentioned earlier, we found that age at diagnosis, sex, pathological grade, and number of extrahepatic metastatic sites were associated with the presence of liver metastases in the multivariate logistic regression model among both the entire population and the metastatic subgroup. Similarly, Polivka et al studied the risk factors of brain metastases in patients diagnosed with breast cancer which included young age, premenopausal status, high tumor grade, breast cancer subtype, and specific protein and genetic markers.Citation48 Interestingly, our current result indicated that patients with older age at diagnosis had a decreased risk of liver metastases, which was consistent with the findings of previous studies.Citation15,Citation17,Citation49 The multistep process from primary breast cancer tissue to liver metastasis is complicated. Semenza has summarized that the process of blood vessel metastases of breast cancer includes intravasation, circulation, margination, extravasation, and colonization.Citation50 Both breast cancer cell itself and the liver microenvironment are involved in this process.Citation51 In addition, it may be possible that the capacity of these factors to facilitate metastases is compromised by the pathophysiological changes associated with aging. Purushotham et al discussed that deterioration of the immune system and alteration of the extracellular matrix accompanied by aging may explain this striking phenomenon.Citation49 In addition, we found that female patients had an increased risk of liver metastases compared with that of male patients in both the entire population and metastatic subgroup. Previous studies have reported that there exists a discordance in clinicopathological features between male breast cancer and female breast cancer.Citation52,Citation53 The association between gender and biological behavior of liver metastases needs further research. Here, we evaluated the predictive factors associated with liver metastases upon initial diagnosis of breast cancer, which may provide some reference for clinicians to distinguish those patients with a relatively high risk of liver metastases during the clinical course.
The median OS of patients with BCLM was 20 months, while patients with metastases confined to the liver had a median OS of 29 months. Recent studies have reported a median survival ranging from 24 to 33 months among patients with BCLM,Citation54,Citation55 which is in accordance with our results. Remarkably, we observed that the median survival of patients with BCLM varied significantly when stratified by tumor subtype. Specifically, HR+/HER2+ patients had the longest survival (median survival, 36.0 months), while triple-negative breast cancer patients had the shortest survival (median survival, 9.0 months). This result was slightly different from former publications. In a retrospective study, Duan et al had found that the median OS among patients with BCLM was 21–30, 32, and 41 months for the triple-negative, HR−/HER2+, HR+/HER2−, and HR+/HER2+ subtypes, respectively.Citation34 In the multivariate Cox regression model, compared with HR+/HER2− breast cancer, the HR+/HER2+ subtype had a 33.4% reduction in hazards of overall mortality, while triple-negative patients experienced a 128% increase in overall mortality. The HR+/HER2+ subtype had a more favorable outcome than triple-negative subtype, which was consistent with some previous retrospective publications.Citation35,Citation42,Citation56 We speculated that it may be mainly due to the improvement of HER2-targeted therapy, endocrine therapy, and the incorporation of chemotherapeutics, such as paclitaxel and anthracyclines.
The impact of the presence of extrahepatic metastases on the survival of patients with BCLM has been studied in previous studies.Citation30–Citation32,Citation57 Wyld et al found that the presence of extrahepatic metastases was not significantly associated with the OS in patients with BCLM,Citation31 while some papers reported that patients with liver and extrahepatic metastases had poorer survival than patients with metastases confined to the liver only.Citation30,Citation57 Atalay et al found that patients with liver metastases alone had longer survival than patients with liver plus other sites of metastases by a retrospective analysis of two prospective, randomized metastatic breast cancer trials.Citation32 In the present study, we found that patients with only liver metastases had better survival than those simultaneously with extrahepatic metastases. Additionally, in the multivariate Cox regression model, we observed that patients with more extra-hepatic metastatic sites had a much higher hazard in overall mortality. Specifically, we identified that patients presenting with extrahepatic metastatic sites such as the lung, bone, and brain independently had worse survival than those without such metastatic sites by the survival comparison. Moreover, the influence of liver metastases on the OS of patients with extrahepatic metastases was evaluated. We found that among those patients with lung or bone metastases, the presence of liver metastases led to worse survival. However, the comparison did not reach significance in patients with brain metastases. We speculated that this result was due to the poor prognosis of breast cancer brain metastases itself with a median survival of 10 months.Citation58
Some limitations in our study should be acknowledged. First, the SEER database provides information about only four metastatic sites, namely, the lung, bone, brain, and liver. The presence of metastases in other sites, such as pleura and adrenal glands, is unknown, which may cause some bias in the prognostic assessment of the extrahepatic metastases subgroup. Furthermore, information on the extension and lesion of liver metastases is not available, which has been identified as an important prognostic factor among patients with BCLM.Citation34 Moreover, the database does not offer information about recurrence and later metastatic sites of disease. Therefore, patients who developed liver metastases would not be captured in the analysis. In addition, we do not have treatment information, such as information about whether patients received endocrine therapy, HER2-targeted therapy, or chemotherapy. Additionally, information about the local treatment of liver metastases was not available, which may contribute to some disparities in the survival analyses. Local treatment such as surgical liver resection or radiofrequency ablation has been studied, though the current guidelines for patients with BCLM include mainly systemic palliative therapy.Citation20 In addition, the classification of breast cancer subtypes in the SEER database is mainly based on receptor status acquired by medical records from primary breast cancer. Recent studies have reported that some discordance exists in receptor status between primary and metastatic lesions, which may influence the statistical analysis of incidence and disease outcome of patients when stratified by breast cancer subtype.Citation59
Conclusion and expert recommendations
Studies have indicated that liver metastases from primary tumor is a complex process.Citation26,Citation50,Citation51 Not only factors associated with breast cancer cells (inflammatory factors, chemokine and chemokine receptors, cell adhesion molecules, claudins, and breast cancer subtypes) but also factors associated with liver microenvironment (hypoxia-inducible factor-regulated genes, vasculature, and sinusoidal capillaries) are involved in this process.Citation51 The advancement of multi-omics approaches of clinical samples from patients with BCLM individually would further unveil the mystery of liver metastases.Citation27,Citation28 Nowadays, distant metastases of breast cancer are related to poor disease outcome,Citation2 and it is meaningful to study the risk factors of breast cancer patients predisposed to liver metastases for early intervention and prognostic factors of patients with BCLM for personalized therapy, which was our current study’s aim. To our knowledge, this is the first large population-based study of patients with liver metastases at initial diagnosis of breast cancer. Our study has strong external validity and provides essential information about the clinicopathological characteristics, incidence, and prognosis of patients with BCLM. The results can inform early screening, risk evaluation, and prognosis guidance for patients with BCLM. However, whether these changes in clinical course could have an impact on disease outcome warrants further prospective research.
Acknowledgments
We would like to thank SEER for providing open access to the database. This study was supported by a grant from the Ministry of Science and Technology of China (MOST2016YFC0900300, National Key R&D Program of China), the National Natural Science Foundation of China (81672601–81602311), and the Shanghai Committee of Science and Technology Funds (15410724000). The funders had no role in the study design, collection and analysis of the data, decision to publish, or manuscript preparation.
Author contributions
Conception and design: HYZ, YG, FGY, HL, and XH. Development of methodology: HYZ, YG, and FGY. Acquisition of data: HYZ and FGY. Analysis and interpretation of data: HYZ, YG, FGY, HL, and HX. Writing, review, and/or revision of manuscript: HYZ, HL, and HX. Study supervision: HL and XH. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- DesantisCMaJBryanLJemalABreast cancer statistics, 2013CA Cancer J Clin2014641526224114568
- DawoodSBroglioKEnsorJHortobagyiGNGiordanoSHSurvival differences among women with de novo stage IV and relapsed breast cancerAnn Oncol201021112169217420427349
- RedigAJMcallisterSSBreast cancer as a systemic disease: a view of metastasisJ Intern Med2013274211312623844915
- PerouCMSørlieTEisenMBMolecular portraits of human breast tumoursNature2000406679774775210963602
- PratAPerouCMDeconstructing the molecular portraits of breast cancerMol Oncol20115152321147047
- SørlieTTibshiraniRParkerJRepeated observation of breast tumor subtypes in independent gene expression data setsProc Natl Acad Sci U S A2003100148418842312829800
- RouzierRPerouCMSymmansWFBreast cancer molecular subtypes respond differently to preoperative chemotherapyClin Cancer Res200511165678568516115903
- SmidMWangYZhangYSubtypes of breast cancer show preferential site of relapseCancer Res20086893108311418451135
- SoniARenZHameedOBreast cancer subtypes predispose the site of distant metastasesAm J Clin Pathol2015143447147825779997
- GerratanaLFanottoVBonottoMPattern of metastasis and outcome in patients with breast cancerClin Exp Metastasis201532212513325630269
- HarrellJCPratAParkerJSGenomic analysis identifies unique signatures predictive of brain, lung, and liver relapseBreast Cancer Res Treat2012132252353521671017
- KastKLinkTFriedrichKImpact of breast cancer subtypes and patterns of metastasis on outcomeBreast Cancer Res Treat2015150362162925783184
- ChanSFriedrichsKNoelDProspective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancerJ Clin Oncol19991782341235410561296
- LeeYTBreast carcinoma: pattern of metastasis at autopsyJ Surg Oncol19832331751806345937
- MartyMCognettiFMaraninchiDRandomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study groupJ Clin Oncol200523194265427415911866
- CummingsMCSimpsonPTReidLEMetastatic progression of breast cancer: insights from 50 years of autopsiesJ Pathol20142321233124122263
- O’ReillySMRichardsMARubensRDLiver metastases from breast cancer: the relationship between clinical, biochemical and pathological features and survivalEur J Cancer19902655745772144744
- HoeALRoyleGTTaylorIBreast liver metastases – incidence, diagnosis and outcomeJ R Soc Med199184127147161774744
- DiamondJRFinlaysonCABorgesVFHepatic complications of breast cancerLancet Oncol200910661562119482250
- PerezJEMachiavelliMLeoneBABone-only versus visceral-only metastatic pattern in breast cancer: analysis of 150 patients. A GOCS study. Grupo Oncológico Cooperativo del SurAm J Clin Oncol19901342942982198793
- MussHBEndocrine therapy for advanced breast cancer: a reviewBreast Cancer Res Treat199221115261382723
- AdamRAloiaTKrissatJIs liver resection justified for patients with hepatic metastases from breast cancer?Ann Surg2006244689790817122615
- BubnovRPolivkaJZuborPKonieczkaKGolubnitschajaO“Pre-metastatic niches” in breast cancer: are they created by or prior to the tumour onset? “Flammer syndrome” relevance to address the questionEpma J20178214115728725292
- HessKRVaradhacharyGRTaylorSHMetastatic patterns in adenocarcinomaCancer200610671624163316518827
- GolubnitschajaOSridharKCLiver metastatic disease: new concepts and biomarker panels to improve individual outcomesClin Exp Metastasis201633874375527541751
- GolubnitschajaOFilepNYeghiazaryanKBlomHJHofmann-ApitiusMKuhnWMulti-omic approach decodes paradoxes of the triple-negative breast cancer: lessons for predictive, preventive and personalised medicineAmino Acids2018503–438339529249020
- FröhlichHPatjoshiSYeghiazaryanKKehrerCKuhnWGolubnitschajaOPremenopausal breast cancer: potential clinical utility of a multi-omics based machine learning approach for patient stratificationEpma J20189217518629896316
- ErOFryeDKKauSWClinical course of breast cancer patients with metastases limited to the liver treated with chemotherapyCancer J2008141626818303485
- PentheroudakisGFountzilasGBafaloukosDMetastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 womenBreast Cancer Res Treat200697323724416322882
- WyldLGutteridgeEPinderSEPrognostic factors for patients with hepatic metastases from breast cancerBr J Cancer200389228429012865918
- AtalayGBiganzoliLRenardFClinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trialsEur J Cancer200339172439244914602130
- GeQDLvNKongYNClinical characteristics and survival analysis of breast cancer molecular subtypes with hepatic metastasesAsian Pac J Cancer Prev201213105081508623244114
- DuanXFDongNNZhangTLiQThe prognostic analysis of clinical breast cancer subtypes among patients with liver metastases from breast cancerInt J Clin Oncol2013181263222041927
- LobbezooDJvan KampenRJVoogdACPrognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcomeBreast Cancer Res Treat2013141350751424104881
- BartmannCDiessnerJBlettnerMFactors influencing the development of visceral metastasis of breast cancer: a retrospective multi-center studyBreast201731667527816834
- YardleyDAKaufmanPABrufskyATreatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancerBreast Cancer Res Treat2014145372573424706168
- DawoodSBroglioKBuzdarAUHortobagyiGNGiordanoSHPrognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based reviewJ Clin Oncol2010281929819933921
- Leyland-JonesBHuman epidermal growth factor receptor 2-positive breast cancer and central nervous system metastasesJ Clin Oncol200927315278528619770385
- ChenXSunLCongYBaseline staging tests based on molecular subtype is necessary for newly diagnosed breast cancerJ Exp Clin Cancer Res20143312824628817
- LeoneBAVallejoCTRomeroAOPrognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosisBreast Cancer Res Treat2017161353754827975154
- PressDJMillerMELiederbachEYaoKHuoDDe novo metastasis in breast cancer: occurrence and overall survival stratified by molecular subtypeClin Exp Metastasis201734845746529288366
- LiYMPanYWeiYUpregulation of CXCR4 is essential for HER2-mediated tumor metastasisCancer Cell20046545946915542430
- PierobonMRamosCWongSEnrichment of PI3K-AKT-mTOR pathway activation in hepatic metastases from breast cancerClin Cancer Res201723164919492828446508
- KimbungSJohanssonIDanielssonATranscriptional profiling of breast cancer metastases identifies liver metastasis-selective genes associated with adverse outcome in luminal a primary breast cancerClin Cancer Res201622114615726276891
- GolubnitschajaOYeghiazaryanKStrickerHTrogDSchildHHBerlinerLPatients with hepatic breast cancer metastases demonstrate highly specific profiles of matrix metalloproteinases MMP-2 and MMP-9 after SIRT treatment as compared to other primary and secondary liver tumoursBMC Cancer201616135727277077
- BanerjeeDHernandezSLGarciaANotch suppresses angiogenesis and progression of hepatic metastasesCancer Res20157581592160225744722
- PolivkaJKralickovaMPolivkaJKaiserCKuhnWGolubnitschajaOMystery of the brain metastatic disease in breast cancer patients: improved patient stratification, disease prediction and targeted prevention on the horizon?Epma J20178211912728824737
- PurushothamAShamilECariatiMAge at diagnosis and distant metastasis in breast cancer – a surprising inverse relationshipEur J Cancer201450101697170524768572
- SemenzaGLCancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasisOncogene201332354057406323222717
- MaRFengYLinSMechanisms involved in breast cancer liver metastasisJ Transl Med20151316425885919
- MiaoHVerkooijenHMChiaKSIncidence and outcome of male breast cancer: an international population-based studyJ Clin Oncol201129334381438621969512
- AndersonWFJatoiITseJRosenbergPSMale breast cancer: a population-based comparison with female breast cancerJ Clin Oncol201028223223919996029
- PockajBAWasifNDueckACMetastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer: time for a second look?Ann Surg Oncol20101792419242620232163
- CrumpMGluckSTuDRandomized trial of high-dose chemotherapy with autologous peripheral-blood stem-cell support compared with standard-dose chemotherapy in women with metastatic breast cancer: NCIC MA.16J Clin Oncol2008261374318025439
- TaoLChuLWangLIOccurrence and outcome of de novo metastatic breast cancer by subtype in a large, diverse populationCancer Causes Control20162791127113827496200
- AbbasHErridgeSSodergrenMHBreast cancer liver metastases in a UK tertiary centre: outcomes following referral to tumour board meetingInt J Surg20174415215928645556
- MartinAMCagneyDNCatalanoPJBrain metastases in newly diagnosed breast cancer: a population-based studyJAMA Oncol2017381069107728301662
- SchrijverWAMESuijkerbuijkKPMvan GilsCHvan der WallEMoelansCBvan DiestPJReceptor conversion in distant breast cancer metastases: a systematic review and meta-analysisJ Natl Cancer Inst2018110656858029315431
