Abstract
Breast cancer has a high incidence worldwide. The results of substantial studis reveal that inflammation plays an important role in the initiation, development, and aggressiveness of many malignancies. The use of celecoxib, a novel NSAID, is repetitively associated with the reduced risk of the occurrence and progression of a number of types of cancer, particularly breast cancer. This observation is also substantiated by various meta-analyses. Clinical trials have been implemented on integration treatment of celecoxib and shown encouraging results. Celecoxib could be treated as a potential candidate for antitumor agent. There are, nonetheless, some unaddressed questions concerning the precise mechanism underlying the anticancer effect of celecoxib as well as its activity against different types of cancer. In this review, we discuss different mechanisms of anticancer effect of celecoxib as well as preclinical/clinical results signifying this beneficial effect.
Keywords:
Introduction
Breast cancer (BC) is the most frequent cancer in women, the second most common cancer worldwide, and the second primary cause of cancer-related deaths.Citation1 One in eight women who live to age 85 years will develop BC over the course of their lifetime.Citation2 Previous studies suggest that inflammation is associated with cancer, and a robust correlation exists between the manifestation of inflammation and the progress of precancerous lesions at a number of anatomic sites.Citation3 On the other hand, cancer cells might exploit components of the inflammatory process to induce angiogenesis, inhibit apoptosis, and enhance proliferation, migration, and metastasis,Citation4 such as NF-κB, cytokines or cytokine receptors, chemokines or chemokine receptors, fibroblast growth factor or receptor (FGF or FGFR), and vascular endothelial growth factor (VEGF). Increasing evidence demonstrates the key role of chronic inflammation markers in increased BC riskCitation5; for example, a meta-analysis suggested a significant dose–response correlation for C-reactive protein (CRP) with BC risk.Citation6 The pro-inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, induce BC cells to penetrate the blood vessels, contributing to metastasis.Citation7
Owing to its significant pro-tumor effects, inflammation has become a promising target for cancer prevention and treatment. Among various inflammatory factors, cyclooxygenase 2 (COX-2) is the most commonly studied anti-inflammatory/anticancer target.Citation8,Citation9 Unlike COX-1, COX-2 is undetectable in normal breast tissue, but in tumor tissue, it overexpresses by 40%,Citation10 and in ductal carcinoma in situ (DCIS) it overexpresses by approximately 80%.Citation11 The overexpression of COX-2 has been reported in different tumor cells and neovascular endothelial cells.Citation12 The overexpressed COX-2 converts arachidonic acid (AA) into prostaglandin E2 (PGE2),Citation13 which promotes BC progression through different mechanisms, for instance, suppression of antitumor immunity,Citation14 promotion of invasiveness,Citation15 migration,Citation15 stem-like cell (SLC) formation,Citation16 angiogenesis,Citation17 and lymphangiogenesis.Citation18
Over 20 years ago, NSAIDs were reported to have anti-colon cancer effects.Citation19 Abundant epidemiological and preclinical/clinical studies demonstrated that celecoxib, a specific COX-2 inhibitor, was related to suppression of cancer cell proliferation and decrease in cancer incidents. In this article, different mechanisms underlying anticancer effect of celecoxib as well as preclinical/clinical results signifying this beneficial effect are discussed.
Celecoxib and BC
Celecoxib is the international nonproprietary name of 4-[5-(4-methylphenyl)–3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide, a COX-2-selective NSAID. Its oral capsule form was initially approved by US Food and Drug Administration (FDA) and marketed by Pfizer, Inc. (New York, NY, USA) in 1999. As a selective COX-2 inhibitor, celecoxib is used as an analgesic, anti-inflammatory, and antipyretic drug. Numerous preclinical evidence suggests that celecoxib may provide a strong chemopreventive activity against BC. Celecoxib treatment (500–1,500 mg/kg diet) can significantly decrease incidence, multiplicity, and tumor volume in several animal models of BC.Citation20,Citation21 In addition, metastasis to the lung and brain could also be prevented.Citation22,Citation23 Besides preclinical studies, clinical trials also showed positive results. Two case–control studies, including 323Citation24 and 18,368Citation25 BC cases, respectively, illustrated that a standard dose intake of celecoxib (200 mg/day) for more than 12 months was associated with significant reduced risk of BC. However, in these studies, OR was different,Citation25 and this might be caused by a longer duration of drug intake in the former study (more than 2 years vs more than 1 year).
Mechanisms of celecoxib’s antitumor action
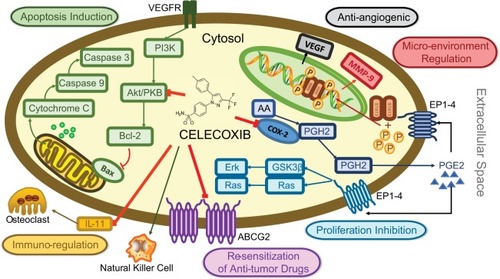
In spite of the encouraging efficacy mentioned earlier, the mechanisms of celecoxib’s antitumor action still need to be explored. COX-2 plays an important role in tumorigenesis and development. Thus, celecoxib, as a selective COX-2 inhibitor, is believed to have several potential antitumor mechanisms, including inhibition of proliferation, induction of apoptosis, immunoregulation, regulation of tumor microenvironment, antiangiogenic effect, and resensitization of other antitumor drugs. Meanwhile, COX-2-independent pathways also contribute to the antitumor effect of celecoxib.
Inhibition of proliferation
Celecoxib represses the proliferation of BC cells in vitro and also prevents the incidence of BC chemically induced by 7,12-dimethylben anthracene (DMBA) in rats. Thus, celecoxib shows an anticancer activity and seems to be effective in anticancer treatment.Citation26 Bocca et alCitation27 assessed the antiproliferative activity of celecoxib on human BC cells with different COX-2 expression levels. Celecoxib treatment induces a robust inhibition of cell growth in estrogen receptor (ER)α (+) MCF-7 cells, which is accompanied by a decrease in expression and activation of aromatase and ERα. The related mechanism may involve ERK and Akt inhibition as well as induction of PP2A and PTEN. In this cell line, celecoxib shows only weak effect on COX-2 level. In contrast, in ERα (–) MDA-MB-231 cells, celecoxib induces a striking suppression of COX-2, which is associated with a decrease in aromatase expression and cell proliferation. These results imply that celecoxib may exert antiproliferative activity in BC cells through COX-2-dependent or COX-2-independent pathways. A study identifying transcriptional changes in BC tissues of patients treated with celecoxib suggested that short-term COX-2 inhibition by the drug stimulates transcriptional programs that facilitate antitumor activity in primary BC tissue. The influence on proliferation-related genes is reflected by a decrease in Ki-67 (+) cells.Citation28 Basu et alCitation29 explored the mechanisms by which celecoxib affects tumor growth of two human BC cell lines such as MDA-MB-231 (highly invasive) and MDA-MB-468 (moderately invasive). They demonstrated that the distinct molecular mechanisms of celecoxib-induced growth suppression depend on the expression level of COX-2 and invasiveness in different human BC cell lines. The studies suggest that COX-2 plays an important role not only in the cancer cell growth but also in activating the angiogenic pathway via modulating levels of VEGF. Taken together, these results provide a theoretical and experimental basis for the clinical anticancer effect of celecoxib.
Induction of apoptosis
Apoptosis is an evolutionary conserved programmed cellular suicide mechanism that is vital for tissue homeostasis in multicellular organisms. It also causes the cytotoxic effects in response to standard genotoxic chemotherapy/radiotherapy. Intriguingly, some researchers found that the tumor growth inhibition effect of celecoxib was mainly caused by inducing apoptosisCitation30 rather than disturbing cell proliferation.Citation31 Celecoxib-resistant cell lines with COX-2 overexpressionCitation32 exhibit a reduced level of Bax, a pro-apoptosis protein, and increased levels of anti-apoptosis proteins such as Bcl-2 or Bcl-xL. COX-2 knockdown by its specific siRNA significantly decreases clonogenicity and levels of Bcl-xL and Bcl-2 in these cells. In MDA-MB-231Citation29,Citation30 and MCF-7 cell lines,Citation33 celecoxib induces apoptosis by decreasing phosphorylation of Akt, then increasing the expression of Bax and activation of caspase 3 and caspase 7. Wang et alCitation34 showed that celecoxib induces apoptosis of the BC cell line, MDA-MB-231, by inhibiting the NF-κB pathway. Another mechanism of celecoxib-induced apoptosis in most cellular systems involves p53-independent mitochondrial apoptosis pathway, which is COX-2 independent and could not be inhibited by the overexpression of Bcl-2.Citation35,Citation36
Some studies suggest that the antineoplastic effects of celecoxib are attributed to its unspecific inhibitory actions on β-catenin signaling,Citation37,Citation38 which is a fundamental component of the canonical Wnt pathway. Overactivation of Wnt/β-catenin signaling is associated with the progression of different types of cancer and promotion of cancer cell growth, survival, and malignant phenotype. Furthermore, recent studies suggest that Wnt/β-catenin signaling plays an important role in the modulation of cancer stem cells.Citation39,Citation40 GSK-3β phosphorylates and marks β-catenin for ubiquitination and succeeding proteasomal degradation. Binding of Wnt ligands to their receptors initiates a signaling cascade that averts GSK-3β from tagging β-catenin for degradation, leading to its accumulation, translocation to the nucleus, binding to the T cell factor (TCF) family of transcription factors, and activation of Wnt target gene expression. Celecoxib treatment induces GSK-3β dephosphorylation, contributing to β-catenin phosphorylation induced by GSK-3β and suppression of the Wnt/β-catenin-dependent gene transcription, for example, c-Myc or cyclin D1, COX-2, and VEGF.Citation38,Citation41 It is noteworthy that GSK-3β is a direct downstream target of Akt/PKB and activated Akt/PKB phosphorylates and consequently inactivates GSK-3β. Hence, celecoxib inhibition of Akt/PKB might at least partly be responsible for the reduction in the β-catenin levels.
Survivin is an “inhibitor of apoptosis” (IAP) protein that also functions as a mitotic regulator essential for cell division.Citation42 It represses apoptosis through hindering the activation of caspases. Intriguingly, celecoxib treatment downregulates survivin levels of cancer cells in vitro and in vivo.Citation43–Citation47 The degree of survivin inhibition of celecoxib correlates with its efficacy to impede cancer cell growth and to promote apoptosis among different types of cancer cells. Since prostaglandins elevate survivin expression, the downregulation of survivin may be partly attributable to celecoxib-induced suppression of COX-2.Citation48 In view of the role played by survivin in apoptosis resistance of cancer cells, the inhibitory effects of celecoxib on this protein might be particularly relevant to its use in anticancer treatment.Citation49,Citation50 Whether the induction of apoptosis leads to clinical benefits is still debatable. A study showed that celecoxib increased apoptosis and reduced the levels of PG and VEGF expression.Citation51 However, this effect could not postpone tumor appearance and reduce tumor progression and development.
Immunoregulation
A thorny problem in the process of treating tumors is a repressed cell-mediated immunity, characterized by the failure of immune effector cells to induce effective antitumor responses. CD4+ or CD8+ T cells were mostly involved in local tumor suppression, while natural killer (NK) cells were involved in tumor metastasis.Citation52 Immunosuppressive factors, produced by the tumor, cause this problem according to tolerance. COX-2 plays an important role in BC immune escape. PGE2 has a series of adverse effects on the immune response to tumors in the body, for example, negatively influencing the activity of T/B lymphocytes, NK cells, and dendritic cells, reducing TNF-α synthesis and increasing the activity of immunosuppressive IL-10.Citation53 These unfavorable effects ablate the effectiveness of host defenses in monitoring and eliminating malignant cells, thus resulting in their unrestrained proliferation.Citation54 Previous studies demonstrate that the reduced expression of COX-2 in BC cells promotes tissue infiltration of cytotoxic T lymphocytes (CD8+), implying a role of COX-2 in immunosuppression. COX-2 inhibitors such as celecoxib regulate antitumor immunity in local and metastasis BC individually.Citation55 In addition, the application of celecoxib in human BCs prompts an increased number of immune cells in the tumor microenvironment.Citation54 A study by Gallouet et alCitation56 demonstrated that follicular lymphoma stromal cells release great amounts of PGE2. This production can be abolished by celecoxib treatment that targets the COX-2 isoenzyme associated with PGE2 synthesis. Interestingly, they also found that celecoxib promotes apoptosis in primary follicular lymphoma B cells cocultured with stromal cells, nonetheless, independently of the PGE2/COX-2 axis.
Substantial studies suggested that celecoxib might switch the function of immune cells to a more tumor-killing phenotype via impeding tumors from releasing prostaglandins and via hindering COX activity in immune effector cells. Lang et alCitation57 suggested that cancer cells suppress the physiological function of immune cells and that celecoxib, to a certain extent, recovers this function. These results deliver an in-depth understanding of the anticancer effect of celecoxib and support its prophylactic use in high-risk patients. In a study evaluating the influence of celecoxib administration on tumor-infiltrating lymphocyte (TIL) subsets (CD3[+]CD4[+]CD8[+]CD25[+] and T cell receptor [TCR]-zeta-expressing cells) and tryptase(+) mast cells in human cervical cancers, Ferrandina et alCitation58 provided the first evidence that this drug can restore zeta expression by TIL in primary cervical cancers. Overall, these results suggest that a positive regulation of immune function might serve as a crucial mechanism underlying the antitumor effect of celecoxib.
Regulation of tumor microenvironment
During chronic inflammation, pro-inflammatory molecules including cytokines, ROS, NF-κB, and inducible nitric oxide synthase (iNOS) are increased and provide an advantageous microenvironment for cancer cell growth. Therefore, inflammation might result in the initiation of cancer and provide the suitable environment to support tumor growth.Citation59 The tumor microenvironment consists of a variety of cell and molecular components, such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMP)-1. Tumor microenvironment has profound impacts on tumor cell proliferation, migration, and apoptosis.Citation60,Citation61 Celecoxib inhibits 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced MMP-9 expression in a dose-dependent manner by increasing the activity of TIMP-1.Citation62,Citation63
The stimulation of constitutive expression of COX-2 is a crucial factor in the tumorigenic process. Various key risk factors associated with cancer causativeness are capable of stimulating COX-2. These factors comprise certain essential dietary fatty acids, nicotine and its metabolites, growth factors, infectious agents, hypoxia, hormones, ultraviolet B, and free radicals; oncogenic proteins; and endotoxins; etc.Citation64–Citation67 Some microenvironmental stimuli, for example, bacterial lipopolysaccharides, TNF, and by-products of protein synthesis and degradation, also induce constitutive COX-2 expression. In addition, because the COX-2 gene contains various promoter binding sites, nuclear transcription factors including NF-κβ or NF-IL6 might also mediate its upregulation.Citation68 Genetic induction of COX-2 in BC cells triggers local constitutive estrogen synthesis through activating the promoter II region of the aromatase gene (CYP-19) in adjacent fat and muscle cells.Citation69 Terry et alCitation70 revealed a vital relationship between COX-2 overexpression and mammary tumorigenesis induced by estrogen. Hence, COX-2 tumorigenesis seems to involve synergistic interactions between many microenvironmental and genetic cofactors. Accordingly, recent studies suggested that regular use of aspirin and other coxibs has noteworthy therapeutic impact in cancer patients.Citation71,Citation72 Expression of COX-2 can also be increased by large amount of collagen, which contributes to high breast densityCitation73 and growing incidence of BC.Citation74,Citation75 This effect can be inhibited by celecoxib through reducing overall collagen deposition and the levels of COX-2, PGE2, and Ki-67 expression.Citation76
Tumor-associated macrophages are associated with cancer cell survival. In a microenvironment study of BC cells, Li et alCitation77 demonstrated that COX-2 is plentifully expressed in breast tumor-associated macrophages, which is associated with poor prognosis in BC patients. Their studies suggested that COX-2 serves as an important cancer-promoting factor through prompting a positive feedback loop between macrophages and BC cells. Apparently, COX-2 inhibitor, celecoxib, is favorable in disturbing this feedback loop in the cancer microenvironment. Accordingly, COX-2 can be exploited as a target for BC prevention and therapy. These findings provide solid molecular evidence to support the anti-BC effect of celecoxib, which has potential to raise positive expectations for clinical use.
Antiangiogenic effect
Angiogenesis refers to the generation of new blood vessels through the extension of preexisting vasculature. It is modulated by an equilibrium between the pro- and antiangiogenic factors. During tumorigenesis, the role of pro-angiogenic factors exceeds that of their counterpart and triggers the growth of new capillaries to supply more blood flow and overcome hypoxia inside the cancer microenvironment, leading to tumor growth and metastasis. At the molecular level, one of the mechanisms underlying COX-2-dependent neoplastic initiation and development in BC involves its proangiogenic activity.Citation78,Citation79 Actually, COX-2 activates MMPs in an intricate mechanism involving NF-κB. This protein also promotes endothelial migration by thromboxane A2 (TXA2).Citation78,Citation80 Moreover, the augmented activity of COX-2 contributes to the release of proangiogenic factors by epithelial and endothelial neoplastic cells, fibroblasts, and macrophages.Citation80,Citation81 In detail, COX-2-dependent angiogenesis begins with the formation of proangiogenic prostaglandins (primarily PGE2) by tumor cells, which enhances the levels of VEGF and bFGF. VEGF directly induces COX-2 in ECs, while bFGF induces COX-2 in fibroblasts to synthesize PGs, which can stimulate the PKA pathway via the EP2 receptor. In addition to their direct pro-angiogenic action, PGs may also induce angiogenesis indirectly, via activating monocytes that infiltrate tumor tissues. Subsequently, the activated vascular COX-2 leads to the elevated permeability, proliferation, and morphogenesis of vasculatures.Citation81 The high microvessel density results in the greater metastatic potential of tumor cells and poor patient prognosis.Citation78,Citation81 Furthermore, PGE2 can also promote angiogenesis through activating EP4 and its second messenger PKA in ECs.Citation82
Celecoxib could inhibit PGE2-induced angiogenesis and lymphangiogenesis and sequentially inhibit tumor growth and metastasis, especially in COX-2-overexpressed cell lines,Citation17,Citation83 together with the reduction in microvessel density, microtubule formation, and serum VEGF level.Citation84,Citation85 This inhibition effect was associated with PGE2 receptor 4 (EP4) and could be reversed by exogenous PGE2.Citation86 Microvascular permeability could also be reduced by celecoxib.Citation87,Citation88 Tamoxifen (TAM) is an ER modulator and widely used in the treatment of BC as an adjuvant therapy against recurrence after surgery. Nevertheless, prolonged TAM administration increases VEGF levels in BC patients, stimulating new blood vessel formation and thus limiting its effectiveness. Kumar et alCitation89 demonstrated that celecoxib can relieve TAM-induced angiogenesis via ROS-dependent VEGF/VEGFR2 autocrine signaling. In addition, Vaish and SanyalCitation90 reported that celecoxib can inhibit angiogenesis during the early neoplasm of colon through regulating PI3-K/PTEN/Akt and the canonical Wnt/β-catenin signaling pathway. In short, these findings shed light on molecular mechanisms underlying celecoxib’s anticancer effect from another perspective, which enhances the positive anticipation of its clinical application.
Integrating celecoxib into BC treatment
Celecoxib has been examined for improvement in chemotherapy effectiveness in cancer clinical trials. In fact, it has been reported that celecoxib could stimulate sensitivity to chemotherapy of BC cellsCitation91–Citation93 via affecting the activation of multidrug resistance protein 1 (MDR1) which induces drug resistance and could be upregulated by COX-2.Citation94,Citation95 Instead of affecting the pump function of MDR1, celecoxib downregulates its expression by inducing hypermethylation of MDR1 gene promoterCitation96 and inhibiting the DNA-binding activity and expression of nuclear transcription factors such as AP-1 and NF-κB, which can combine with putative binding sites of human MDR1 gene promoter.Citation97 In addition to the activation of MDR1, of pertinence to this review, celecoxib was recently demonstrated to significantly sensitize other antitumor drugs with multiple mechanisms.
Combination with chemotherapy
Considering its own antitumor competence and resensitization of other antitumor drugs, celecoxib could be a potential candidate for combination therapy. Preclinical research suggested that the antitumor effect of several agents can be enhanced by combining with celecoxib,Citation98 including doxorubicinCitation99 and 5-fluorouracil (5-FU).Citation100 In several Phase II studies, the combination of celecoxib and capecitabine, an orally administered pro-drug of 5-FU, could provide a clinical benefit rate at 42.1–47.5% and an unexpected lower toxicity than capecitabine alone in metastatic BC (MBC) patients.Citation101,Citation102 In a single-arm, mono-institutional, nonrandomized, Phase II, two-step clinical trial, celecoxib was combined with cyclophosphamide, and the clinical benefit of this combination came to 55% in 20 advanced BC (ABC) patients.Citation103
Celecoxib can also be integrated into multidrug chemotherapy regimens, in which FEC (5-FU, epirubicin, and cyclophosphamide) is the most common one. A study containing 50 patients showed that preoperative FEC with celecoxib (FECC) could provide lower intensity staining for COX-2, Ki-67, and p53 in 90% patients, while no difference was observed on tumor size, grade, or axillary lymph node status.Citation104 In a Phase II, multicenter, open-label, single-arm study (N001),Citation105 64 invasive BC patients received four cycles of FEC (500, 100, 500 mg/m2) followed by four cycles of docetaxel (100 mg/m2) with celecoxib (200 mg twice daily) as neoadjuvant therapy (NAT). After NAT, 43 patients achieved clinical complete response (cCR) and 13 achieved clinical partial response (cPR). In addition, despite potential side effects on cardiac system, the cardiac safety of celecoxib has been declared to be acceptable.Citation106,Citation107
It has also been reported that celecoxib increases the sensitivity of drug-resistant KBV20C cancer cells to anti-mitotic drugs.Citation108 This sensitization mechanism is independent of the suppression of p-glycoprotein, indicating that the KBV20C cells are sensitized via targeting of signaling pathways by celecoxib. Moreover, it has also been observed that celecoxib intensely sensitizes KBV20C cells to vin-blastine and paclitaxel, as indicated by microscopic observation, determination of Annexin V staining, and cleaved poly(ADP-ribose) polymerase (cleaved PARP). These results suggest that COX-2 inhibitors such as celecoxib can be used for cancer patients with potential resistance, without the toxic effects of p-glycoprotein suppression. Of interest, as detailed previously,Citation109 celecoxib promotes (sorafenib + sildenafil) lethality in multiple ovarian cancer cell lines, concomitant with a decrease in the expression of several chaperone proteins in parallel with decreased levels of the drug efflux pumps such as ABCB1/ABCG2. The cytotoxicity by the triple combination was induced by caspase 9-dependent apoptotic pathway and RIP-1/caspases 2, 4/AIF-dependent necroptotic pathway. In addition, the triple combination significantly reverted platinum chemotherapy resistance. Combined with the previous studies substantiating in vivo the combinations of “celecoxib + sildenafil” and “sorafenib + sildenafil” as cytotoxic to various cancer cell types, it has been suggested that the celecoxib/sorafenib/sildenafil combination ought to be investigated in a Phase I trial in ovarian cancer.Citation109 summarizes the drugs suggested for use in combination with celecoxib for BC treatment based on preclinical data.
Table 1 Preclinical studies on combination of celecoxib and other therapeutic drugs in BC
Not all the studies showed positive results, especially in patients with HER2-negative tumor. A multicenter randomized controlled Phase II clinical trial showed that celecoxib did not improve pathological complete response (pCR) rates in addition to epirubicin–cyclophosphamide–docetaxel (EC-D) regimen.Citation110 Moreover, the REMAGUS-02 multicenter randomized Phase II trialCitation111 demonstrated that the addition of celecoxib could not provide an increased pCR rate in HER2-negative patients. The long-term follow-up indicated that, in the HER2-negative subgroup, the addition of celecoxib led to smaller tumor size and lower expression of progesterone receptor (PgR) status, but no association of disease-free survival (DFS) benefit. In BC patients, COX-2 overexpression can be induced by HER2 oncogene activationCitation112 and provide a positive feedback through its product PGE2 which induces HER2 expression.Citation113 However, the combination of celecoxib (400 mg twice daily) and trastuzumab (2 mg/kg intravenous injection weekly or 6 mg/kg intravenous injection every 3 weeks) provided no significant enhancement in a Phase II study.Citation114 Of note, El-Awady et alCitation115 explored the ability of celecoxib to sensitize different types of cancer cells (HeLa, HCT116, HepG2, MCF-7, and U251) to a number of anticancer drugs (5-FU, cisplatin, doxorubicin, and etoposide). Interaction of celecoxib with these chemotherapeutic drugs is antagonistic in the BC cells, MCF-7, but not in other cells, suggesting that celecoxib exerts distinct molecular actions in different cancer cells. Mechanistic investigations demonstrated that celecoxib increases drug-triggered G2/M arrest in MCF-7 cells allowing more time to repair drug-elicited DNA damage before access into mitosis, leading to decrease in cell death and thus contributing to antagonism. These findings, if substantiated in vivo, suggest that celecoxib is not an appropriate chemo-sensitizer for BC. Therefore, the combination of celecoxib with other chemotherapeutic drugs must be customized to the cancer type. To obtain a more accurate conclusion, more in-depth and extensive clinical trials are needed. summarizes the clinical trials of celecoxib or celecoxib combined with chemotherapy on BC patients.
Table 2 Clinical trials of celecoxib on BC (ClinicalTrial.gov)
Combination with endocrinal therapy
It was demonstrated that the expression of aromatase CYP19 might be potentially regulated by PGE2 through cAMP-mediated pathways, and it makes further influence on aromatase activityCitation116,Citation117 and estrogen biosynthesis.Citation69 Linear positive correlation was shown between CYP19 and COX-2 by semi-quantitative reverse transcriptase PCR (RT-PCR), suggesting that the combination of COX-2 and aromatase inhibitors (AIs) could have synergistic effect on hormone-dependent BC.Citation69 The inhibition of aromatase by celecoxib was observed at transcriptional level by real-time PCR and appeared to be dose dependent.Citation118 Anastrozole, an AI, was combined with celecoxib to treat BC in rats.Citation119 The results showed that this combination might be workable for clinical therapy.
Besides laboratory investigations, clinical trials also conducted to combine celecoxib with selective ER modulator (TAM) and AIs (exemestane). In the Celecoxib Anti-Aromatase Neoadjuvant (CAAN) trial,Citation120 a combination of exemestane (25 mg daily) and celecoxib (400 mg twice daily) gained significantly lowered cholesterol and low-density lipoprotein (LDL) levels and higher bone mineral density (BMD) and BC subscale scores compared with single-agent groups of exemestane (25 mg daily) and letrozole (2.5 mg daily), in postmenopausal women with histologically proven local ABC (LABC). So, although the final outcomes showed no statistical difference on clinical response and tumor volume,Citation121 which meant that different neoadjuvant anti-aromatase therapies have similar efficacy, the combination with celecoxib may provide some additional benefits. Some other studies, including a Phase II studyCitation122 and a Phase III study,Citation123 positively supported the combination of celecoxib and exemestane in postmenopausal MBC patients. A Phase II trial of neoadjuvant exemestane (25 mg daily) plus celecoxib (400 mg twice daily) demonstrated that the combination was tolerated and anticancer response was observed in the majority of postmenopausal women with BC. Statistically, noteworthy reduction could also be found in the expression of ER, PgR, Ki-67, and COX-2.Citation124 Nevertheless, some other research provided a different opinion. A study established by Dirix et alCitation125 showed that the demographic characteristics, prognostic factors, and time to progression (TTP) were all similar no matter whether celecoxib was added to endocrine therapy or not, and the lack of COX-2 expression may attribute to this result.Citation126 Moreover, it was suggested that the anticancer effect of combination therapy might have mainly resulted from exemestane instead of celecoxib.Citation127
TAM is extensively used in BC therapy as a preventive drug against recurrence after surgical treatment, but the long-term TAM treatment enhances patients’ VEGF levels, stimulates neovascularization, and thus restrains its effectiveness. Kumar et alCitation89 demonstrated that the combination of TAM and celecoxib at nontoxic concentrations exerted antiangiogenic effects via explicitly targeting VEGF–VEGFR2 pathway through ROS formation. In addition, their preclinical studies suggested that the TAM/celecoxib combination is a feasible strategy for the treatment of BCs with VEGF/VEGFR2 over-expression. This inventive combination exhibits encouraging effect in anti-metastasis and stimulation of apoptosis and may be a superior personalized clinical regimen vs TAM alone for BC treatment. The clinical trials on the combination of endocrine therapy and celecoxib are summarized in .
Table 3 Summary of clinical trials on the combination of endocrine therapy and celecoxib
Combination with other antitumor treatments
Dendritic cell-based cancer vaccine, from tumor lysate-pulsed dendritic cell, is a popular candidate for cancer immunotherapy. Hahn et alCitation85 tested the antitumor immune response using the combination of celecoxib, vaccine, and GM-CSF in 4T1 cells, a cell line with COX-2 expression, poorly immunogenic, and highly metastatic ability. The triple combination therapy successfully suppressed primary tumor growth and significantly reduced the incidence of lung metastases. This effect was achieved by a tumor-specific immune response which could be observed as increased interferon (IFN)-γ and IL-4 secretion by CD4+ T cells and infiltration of CD4+ and CD8+ T cells to the tumor site. Basu et alCitation128 also combined celecoxib with dendritic cell-based cancer vaccine and reconfirmed that the combination gained its antitumor effect by downregulating the expression of indoleamine 2,3-dioxygenase (IDO), a negative regulator of T cell activity. A recent study by Li et alCitation129 using alginate hydrogel system to locally deliver celecoxib and programmed death 1 (PD-1) monoclonal antibody (mAb) to treat 4T1 MBC mouse model demonstrated a significant improvement in the anticancer activities of celecoxib, PD-1 mAb, or both combined. The persistent high levels of the drugs in peripheral circulation and within local tumor areas were observed. Importantly, the concurrent dual local delivery of celecoxib and PD-1 synergistically elevated the levels of CD4+ IFN-γ+/CD8+ IFN-γ+ T cells in the tumor and the immune system, implying that the combinatorial therapy synergistically enhances antitumor immunity. In addition, this combination treatment induces the production of two antiangiogenic chemokines such as C–X–C motif ligand (CXCL)9 and CXCL10 as well as inhibits the intratumoral formation of IL-1, IL-6, and COX2, indicating a diminished pro-cancer angiogenic and inflammatory microenvironment. This celecoxib/PD-1 mAb combination treatment provides a promising regimen for treating human BC.
By combining celecoxib with the HIV protease inhibitor, nelfinavir (Viracept), Cho et alCitation130 explored the aggravation of endoplasmic reticulum stress caused by this combination, which led to apoptosis in chemoresistant BC cells. Furthermore, unmethylated celecoxib (UMC), with superior COX-2 inhibitory efficacy, showed substantially weaker antitumor effect. Therefore, they speculated that the antitumor effect of celecoxib was COX-2 independent in chemoresistant BC. Chloroquine is another material that can play a role in antitumor effect with celecoxib through endoplasmic reticulum stress response.Citation131 In addition, treatment with the combination of anti-IL-17 antibody and celecoxib can significantly decrease bone and lung metastasis in SKG mice with mammary gland tumors and autoimmune arthritis.Citation132 Preclinical studies also suggested that celecoxib could be combined with peroxisome proliferator-activated receptor gamma agonist for the treatment of spontaneous BCCitation133 and minocycline hydrochloride for osseous metastasis in BC.Citation134 These combination treatments were strikingly more effective than celecoxib alone. Moreover, many plant-derived materials were combined with celecoxib and emerged synergistic effect on antitumor effect via VEGF/Akt/NF-κB signaling, including matrine,Citation135 berbamine,Citation136 and luteolin.Citation137,Citation138 ResveratrolCitation139 can also enhance the tumor prevention effect of celecoxib, but the exact mechanism is still under investigated.
Several epidemiological studies have shown that vitamin D has beneficial effects against the carcinogenesis and development of BC.Citation140,Citation141 Recent studies revealed an association between vitamin D and PGE2 metabolism. Thill et alCitation142 demonstrated that a synergistic growth-inhibiting effect in BC cell lines can be elicited by the combination of celecoxib and calcitriol (1,25-dihydroxycholecalciferol or 1,25-[OH]2D3), which is a biologically active form of vitamin D.Citation143 Calcitriol could also inhibit COX-2 expression at both protein and mRNA levels. New perspectives emerge from the growing knowledge of innovative combination of celecoxib, and other anticancer agents, which act in a complementary way, increase the efficacy and minimize toxicity.
Side effects and clinical complications
Celecoxib is the only FDA-approved COX-2 inhibitor for use in the USA. Although celecoxib is usually a well-tolerated drug, it is not harmless. Its typical doses range from 200 to 400 mg/day; nonetheless, the dose for acute gout can reach 800 mg once, followed by 400 mg on the first day, then 400 mg twice daily for 7 days. A higher dose of celecoxib (800 mg per day) might be related to augmented cardiovascular risk according to the Adenoma Prevention with Celecoxib (APC) study.Citation144 The cardiotoxicity side effects may be attributed to its off-target effect, namely modulating calcium levels within the cell, according to the immediate time-dependent cell response profiles (TCRPs) for celecoxib.Citation145 In fact, previous studies have shown that celecoxib therapy induces an immediate increase in intracellular calcium levels.Citation146 In addition, clinical data indicate that chronic use of celecoxib may damage normal skeletal function resulting in reduced BMD in older male patients.Citation147 Serious allergic reactions to celecoxib have also been reported.Citation148
When celecoxib is recommended in advanced cancer patients, the pros and cons need to be considered prudently. A meta-analysis by Chen et alCitation149 suggested that celecoxib has certain benefits in the treatment of cancer, but increases the risk of cardiovascular events. Specifically, they demonstrated an increase in grade 3 and 4 toxicities of cardiovascular events with the incorporation of celecoxib to the treatment of advanced cancers. Other toxicities include rash, hepatotoxicity, and gastrointestinal events, and there is no statistically significant difference among them. Of note, the risk of anemia in the celecoxib group is also significant. Although the risk of grade 3 and 4 cardiovascular events increases by 1.78 times after celecoxib use for a long time, the risk is acceptable, considering that this is a prescription for life-threatening diseases. Nevertheless, clinical monitoring of side effects, for example, cardiovascular events should be strengthened. According to these findings, it is necessary to carefully consider the benefit vs harm when recommending celecoxib in the treatment of patients with advanced cancer, especially those with a history of heart disease. Further studies are needed to confirm these results with large samples.
Conclusion
In addition to being widely used for treating inflammatory diseases such as rheumatoid arthritis and osteoarthritis,Citation150 celecoxib may also play an important role in the cancer prevention and treatment. Preclinical evidence demonstrates that celecoxib seems to suppress the proliferation and growth of different types of cancer through various mechanisms (). Results of abundant clinical studies, although unconvincing, suggest that celecoxib administration is related not only to diminished incidence of cancer but also to the better prognosis in cancer patients. In view of the potential variations in response to celecoxib in cancer patients, it seems critical to ascertain target populations for its use. Nevertheless, factors that contribute to better outcome in celecoxib consumers are still to be explicated. The data on the effectiveness of celecoxib as neoadjuvant treatment in cancer patients are deficient. There are substantial clinical studies evaluating the role of celecoxib in the cancer treatment. The results will permit evaluation of the position of celecoxib in cancer prevention and therapy and identify the target populations in the near future. Of note, comparative studies should be designed to ascertain the optimal dosage, duration, side effects (especially the gastrointestinal and cardiovascular systems), and its cost-effectiveness. As was originally pointed out more than 10 years ago, “there exists an urgent need for clinical trials of this compound so as to accelerate its effective application in the chemoprevention and treatment of cancer.” NSAIDs, and especially celecoxib, represent an inspiring proposition for repurposing as anticancer drugs with low toxicity, hence demonstrating how understanding cancer-relevant molecular signaling pathways in combination with clinical data will contribute to further development of oncology.
Acknowledgments
This study was supported in part by NIH-NIMHD U54MD007598, NIH/NCI1 U54CA14393, U56CA101599-01; Department-of-Defense Breast Cancer Research Program (grant BC043180), NIH/NCATS CTSI UL1TR000124 to JV Vadgama, and Accelerating Excellence in Translational Science Pilot (grants G0812D05), NIH/NCI (SC1CA200517) to Y Wu; the National Natural Science Foundation of China 81630049; National Key R&D Program of China 2017YFC0113302; China Scholarship Council 201706165022.
Disclosure
The authors report no conflicts of interest in this work.
References
- EvansDGHowellABreast cancer risk-assessment modelsBr Cancer Res200795 213
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin 20182018681 73029313949
- RayburnEEzellSJZhangRAnti-Inflammatory Agents for Cancer TherapyMol Cell Pharmacol200911 294320333321
- CoussensLMWerbZInflammation and cancerNature20024206917 86086712490959
- AllenMDJonesLJThe role of inflammation in progression of breast cancer: Friend or foe? (Review)Int J Oncol2015473 79780526165857
- ChanDSBanderaEVGreenwoodDCNoratTCirculating C-Reactive Protein and Breast Cancer Risk-Systematic Literature Review and Meta-analysis of Prospective Cohort StudiesCancer Epidemiol Biomarkers Prev20152410 1439144926224798
- LandskronGde La FuenteMThuwajitPThuwajitCHermosoMAChronic inflammation and cytokines in the tumor microenvironmentJ Immunol Res201420142 1491851924901008
- GallieraECorsiMBonecchiRLocatiMMantovaniAChemokines as pharmacological targetsMini Rev Med Chem200887 63864618537719
- KnowlesMANovel therapeutic targets in bladder cancer: mutation and expression of FGF receptorsFuture Oncol200841 718318241002
- BoscoJLPalmerJRBoggsDAHatchEERosenbergLRegular aspirin use and breast cancer risk in US Black womenCancer Causes Control20112211 1553156121877122
- Díaz-CruzESShapiroCLBrueggemeierRWCyclooxygenase Inhibitors Suppress Aromatase Expression and Activity in Breast Cancer CellsJ Clin Endocrinol Metab2005905 2563257015687328
- MasferrerJLKokiASeibertKCOX-2 inhibitors. A new class of antiangiogenic agentsAnn N Y Acad Sci19998891 CANCER PREVEN 848610668485
- BennettABerstockDACarrollMAStamfordIFWilsonAJBreast cancer, its recurrence, and patient survival in relation to tumor prostaglandinsAdv Prostaglandin Thromboxane Leukot Res1983122993026221606
- LalaPKAl-MutterNOrucevicAEffects of chronic indomethacin therapy on the development and progression of spontaneous mammary tumors in C3H/HEJ miceInt J Cancer1997733 3713809359485
- TimoshenkoAVXuGChakrabartiSLalaPKChakrabortyCRole of prostaglandin E2 receptors in migration of murine and human breast cancer cellsExp Cell Res20032892 26527414499627
- MajumderMXinXLiuLGirishGVLalaPKProstaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functionsCancer Sci20141059 1142115124981602
- ChangS-HLiuCHConwayRRole of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progressionProc Natl Acad Sci U S A20041012 59159614688410
- BhattacharjeeRNTimoshenkoAVCaiJLalaPKRelationship between cyclooxygenase-2 and human epidermal growth factor receptor 2 in vascular endothelial growth factor C up-regulation and lymphangiogenesis in human breast cancerCancer Sci20101019 2026203220608938
- KuneGAKuneSWatsonLFColorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer StudyCancer Res19884815 439944043390835
- KangHFWangXJLiuXXDaiZJXueFJXueXHChemopreventive effect of celecoxib against DMBA-induced breast cancer and its mechanismNan Fang Yi Ke Da Xue Xue Bao20062611 1599160217121709
- WoditschkaSHaagJDMauBLubetRAGouldMNChemopreventive effects of celecoxib are limited to hormonally responsive mammary carcinomas in the neuinduced retroviral rat modelBreast Cancer Res2008101 R1818279516
- Lanza-JacobySMillerSFlynnJThe cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu miceCancer Epidemiol Biomarkers Prev20031212 1486149114693742
- LiuYKosakaAIkeuraMPremetastatic soil and prevention of breast cancer brain metastasisNeuro Oncol2013157 89190323595625
- HarrisREBeebe-DonkJAlshafieGAReduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitorsBMC Cancer200661 2716445867
- AshokVDashCRohanTESprafkaJMTerryPDSelective cyclooxygenase-2 (COX-2) inhibitors and breast cancer riskBreast2011201 667020724158
- DaiZ-JMaX-BKangH-FAntitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in Vitro and in VivoCancer Cell Int2012121 5323249419
- BoccaCBozzoFBassignanaAMigliettaAAntiproliferative effects of COX-2 inhibitor celecoxib on human breast cancer cell linesMol Cell Biochem20113501–2 597021140284
- BrandãoRDVeeckJvan de VijverKKA randomised controlled phase II trial of pre-operative celecoxib treatment reveals anti-tumour transcriptional response in primary breast cancerBreast Cancer Res2013152 R2923566419
- BasuGDPathangeyLBTinderTLGendlerSJMukherjeePMechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cellsBreast Cancer Res200574 R42243515987447
- BasuGDPathangeyLBTinderTLLagioiaMGendlerSJMukherjeePCyclooxygenase-2 inhibitor induces apoptosis in breast cancer cells in an in vivo model of spontaneous metastatic breast cancerMol Cancer Res2004211 63264215561779
- BarnesNLPWarnbergFFarnieGCyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancerBr J Cancer2007964 57558217285134
- SinghBIrvingLRTaiKLucciAOverexpression of COX-2 in celecoxib-resistant breast cancer cell linesJ Surg Res20101632 23524320691996
- BaumannKHKlusmeierEEggemannIEffects of celecoxib and ly117018 combination on human breast cancer cells in vitroBreast Cancer20093233421556247
- WangLLiuLHShanBEZhangCSangMXLiJCelecoxib promotes apoptosis of breast cancer cell line MDA-MB-231 through down-regulation of the NF-kappaB pathwayAi Zheng2009286 569574 Chinese19635192
- JendrossekVTargeting apoptosis pathways by Celecoxib in cancerCancer Lett20133322 31332421345578
- LiZHaoQLuoJUSP4 inhibits p53 and NF-κB through deubiquitinating and stabilizing HDAC2Oncogene20163522 2902291226411366
- TuynmanJBVermeulenLBoonEMCyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancerCancer Res2008684 1213122018281498
- XiaJ-JPeiL-BZhuangJ-PCelecoxib Inhibits β-Catenin-Dependent Survival of the Human Osteosarcoma MG-63 Cell LineJ Int Med Res2010384 1294130420926002
- Takahashi-YanagaFKahnMTargeting Wnt signaling: can we safely eradicate cancer stem cells?Clin Cancer Res20101612 3153316220530697
- FoddeRBrabletzTWnt/β-catenin signaling in cancer stemness and malignant behaviorCurr Opin Cell Biol2007192 15015817306971
- MaierTJJanssenASchmidtRGeisslingerGGröschSTargeting the beta-catenin/APC pathway: a novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cellsFASEB J20051910 1353135515946992
- AltieriDCNew wirings in the survivin networksOncogene20082748 6276628418931693
- KimY-YLeeE-JKimY-KAnti-cancer effects of celecoxib in head and neck carcinomaMol Cells2010292 18519420082220
- KardoshASorianoNPyrkoPReduced survivin expression and tumor cell survival during chronic hypoxia and further cytotoxic enhancement by the cyclooxygenase-2 inhibitor celecoxibJ Biomed Sci2007145 64766217440835
- HsiaoP-WChangC-CLiuH-FTsaiC-MChiuTHChaoJ-IActivation of p38 mitogen-activated protein kinase by celecoxib oppositely regulates survivin and gamma-H2AX in human colorectal cancer cellsToxicol Appl Pharmacol20072221 9710417540426
- FukadaKTakahashi-YanagaFSakoguchi-OkadaNCelecoxib induces apoptosis by inhibiting the expression of survivin in HeLa cellsBiochem Biophys Res Commun20073574 1166117117466271
- Sakoguchi-OkadaNTakahashi-YanagaFFukadaKCelecoxib inhibits the expression of survivin via the suppression of promoter activity in human colon cancer cellsBiochem Pharmacol2007739 1318132917270149
- BaiX-MJiangHDingJ-XProstaglandin E2 upregulates survivin expression via the EP1 receptor in hepatocellular carcinoma cellsLife Sci2010865–6 21422320035770
- ZhaoSCaiJBianHGuiLZhaoFSynergistic inhibition effect of tumor growth by using celecoxib in combination with oxaliplatinCancer Invest2009276 63664019387877
- GaiserTBeckerMRHabelATRAIL-mediated apoptosis in malignant glioma cells is augmented by celecoxib through proteasomal degradation of survivinNeurosci Lett20084422 10911318634847
- Tran-ThanhDButtarsSWenYWilsonCDoneSJCyclooxygenase-2 inhibition for the prophylaxis and treatment of preinvasive breast cancer in a her-2/neu mouse modelCancer Prev Res201032 202211
- LangersIRenouxVMThiryMDelvennePJacobsNNatural killer cells: role in local tumor growth and metastasisBiologics20126738222532775
- HoweLSubbaramaiahKBrownAMDannenbergAJCyclooxygenase-2: a target for the prevention and treatment of breast cancerEndocr Relat Cancer200182 9711411397667
- MarkosyanNChenEPEvansRANdongVVonderheideRHSmythEMMammary carcinoma cell derived cyclooxygenase 2 suppresses tumor immune surveillance by enhancing intratumoral immune checkpoint activityBreast Cancer Res2013155 R7524004819
- KunduNWalserTCMaXFultonAMCyclooxygenase inhibitors modulate NK activities that control metastatic diseaseCancer Immunol Immunother20055410 98198715891886
- GallouetA-STravertMBresson-BepoldinLCOX-2-independent effects of celecoxib sensitize lymphoma B cells to TRAIL-mediated apoptosisClin Cancer Res20142010 2663267324637636
- LangSPicuAHofmannTCOX-inhibitors relieve the immunosuppressive effect of tumor cells and improve functions of immune effectorsInt J Immunopathol Pharmacol2006192 40941916831307
- FerrandinaGCelecoxib Up-Regulates the Expression of the Chain of T Cell Receptor Complex in Tumor-Infiltrating Lymphocytes in Human Cervical CancerClin Cancer Res2006127 2055206016609015
- SarkarDFisherPBMolecular mechanisms of aging-associated inflammationCancer Lett20062361 132315978720
- JoyceJAPollardJWMicroenvironmental regulation of metastasisNat Rev Cancer200994 23925219279573
- BissellMJHinesWCWhy don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progressionNat Med2011173 32032921383745
- KimSKimSHHurSMSilibinin prevents TPA-induced MMP-9 expression by down-regulation of COX-2 in human breast cancer cellsJ Ethnopharmacol20091262 25225719715751
- AfsharimaniBCabotPJParatM-OEffect of lysine antifibrinolytics and cyclooxygenase inhibitors on the proteolytic profile of breast cancer cells interacting with macrophages or endothelial cellsBr J Anaesth2014113Suppl 1 i22i3124418973
- JaimesEATianR-XPearseDRaijLUp-regulation of glomerular COX-2 by angiotensin II: Role of reactive oxygen speciesKidney Int2005685 2143215316221213
- ChangY-WEPutzerKRenLDifferential regulation of cyclo-oxygenase 2 expression by small GTPases Ras, Rac1, and RhoAJ Cell Biochem2005962 31432916088958
- KaulRVermaSCMurakamiMLanKChoudhuriTRobertsonESEpstein-Barr virus protein can upregulate cyclo-oxygenase-2 expression through association with the suppressor of metastasis Nm23-H1J Virol2006803 1321133116415009
- Diaz-CruzEBrueggemeierRInterrelationships between cyclo-oxygenases and aromatase: unraveling the relevance of cyclo-oxygenase inhibitors in breast cancerAnticancer Agents Med Chem200663 22123216712450
- HarrisRBeebeJAlshafieGAReduction in cancer risk by selective and nonselective cyclooxygenase-2 (COX-2) inhibitorsJ Exp Pharmacol20124919627186121
- BrueggemeierRWQuinnALParrettMLJoarderFSHarrisRERobertsonFMCorrelation of aromatase and cyclooxygenase gene expression in human breast cancer specimensCancer Lett19991401–2 273510403538
- TerryMBGammonMDZhangFAssociation of frequency and duration of aspirin use and hormone receptor status with breast cancer riskJAMA200429120 2433244015161893
- HolmesMDChenWYLiLHertzmarkESpiegelmanDHankinsonSEAspirin intake and survival after breast cancerJ Clin Oncol2010289 1467147220159825
- ChanATOginoSFuchsCSAspirin use and survival after diagnosis of colorectal cancerJAMA20093026 64965819671906
- UrsinGHovanessian-LarsenLPariskyYRPikeMCWuAHAhWGreatly increased occurrence of breast cancers in areas of mammographically dense tissueBreast Cancer Res200575 R60560816168104
- BoydNFMammographic density and risk of breast cancerAm Soc Clin Oncol Educ Book2013
- PetterssonAGraffREUrsinGMammographic density phenotypes and risk of breast cancer: a meta-analysisJ Natl Cancer Inst20141065
- EsbonaKInmanDSahaSCOX-2 modulates mammary tumor progression in response to collagen densityBreast Cancer Res2016181 3527000374
- LiHYangBHuangJCyclooxygenase-2 in tumor-associated macrophages promotes breast cancer cell survival by triggering a positive-feedback loop between macrophages and cancer cellsOnco-target2015630 2963729650
- GuptaGPNguyenDXChiangACMediators of vascular remodelling co-opted for sequential steps in lung metastasisNature20074467137 76577017429393
- LeahyKKokiAMasferrerJRole of cyclooxygenases in angiogenesisCurr Med Chem2000711 1163117011032965
- ZhaSYegnasubramanianVNelsonWGIsaacsWBde MarzoAMCyclooxygenases in cancer: progress and perspectiveCancer Lett20042151 12015374627
- FosslienEReview: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesisAnn Clin Lab Sci2001314 32534811688844
- ZhangYDaakaYPGE2 promotes angiogenesis through EP4 and PKA C pathwayBlood201111819 5355536421926356
- BasuGDLiangWSStephanDAA novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cellsBreast Cancer Res200686 R6917156488
- FifeRSStottBCarrREEffects of a selective cyclooxygenase-2 inhibitor on cancer cells in vitroCancer Biol Ther200432 22823214726667
- HahnTAlvarezIKobieJJShort-term dietary administration of celecoxib enhances the efficacy of tumor lysate-pulsed dendritic cell vaccines in treating murine breast cancerInt J Cancer20061189 2220223116331615
- SunTWZhWWengXSCelecoxib can suppress expression of genes associated with PGE2 pathway in chondrocytes under inflammatory conditionsInt J Clin Exp Med201587 109021091026379884
- FournierLSNovikovVLucidiVMR monitoring of cyclooxygenase-2 inhibition of angiogenesis in a human breast cancer model in ratsRadiology20072431 10511117329684
- HanFZhangSZhangLHaoQThe overexpression and predictive significance of MMP-12 in esophageal squamous cell carcinomaPathol Res Pract201721312 1519152229033183
- KumarBNPRajputSDeyKKCelecoxib alleviates tamoxifen-instigated angiogenic effects by ROS-dependent VEGF/VEGFR2 autocrine signalingBMC Cancer2013131 27323731702
- VaishVSanyalSNRole of Sulindac and Celecoxib in the regulation of angiogenesis during the early neoplasm of colon: Exploring PI3-K/PTEN/Akt pathway to the canonical Wnt/β-catenin signalingBiomed Pharmacother2012665 35436722397759
- KalaliniaFElahianFBehravanJPotential role of cyclooxygenase-2 on the regulation of the drug efflux transporter ABCG2 in breast cancer cell linesJ Cancer Res Clin Oncol20111372 32133020422426
- KalaliniaFElahianFMosaffaFBehravanJCelecoxib Up Regulates the Expression of Drug Efflux Transporter ABCG2 in Breast Cancer Cell LinesIran J Pharm Res2014134 1393140125587329
- RatnasingheDDaschnerPJAnverMRCyclooxygenase-2, P-glycoprotein-170 and drug resistance; is chemoprevention against multidrug resistance possible?Anticancer Res2001213C 2141214711501838
- MillerBPatelVASorokinACyclooxygenase-2 rescues rat mesangial cells from apoptosis induced by adriamycin via upregulation of multidrug resistance protein 1 (P-glycoprotein)J Am Soc Nephrol2006174 97798516540558
- SorokinACyclooxygenase-2: potential role in regulation of drug efflux and multidrug resistance phenotypeCurr Pharm Des2004106 64765714965327
- XiaWZhaoTLvJCelecoxib enhanced the sensitivity of cancer cells to anticancer drugs by inhibition of the expression of P-glycoprotein through a COX-2-Independent MannerJ Cell Biochem20091081 18119419562670
- ChenCShenHLYangJChenQYXuWLWlXPreventing chemoresistance of human breast cancer cell line, MCF-7 with celecoxibJ Cancer Res Clin Oncol20111371 91720229271
- HidaTKozakiKItoHSignificant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase 2 inhibitor, JTE-522, and conventional anticancer agentsClin Cancer Res200287 2443244712114451
- van WijngaardenJvan BeekEvan RossumGCelecoxib enhances doxorubicin-induced cytotoxicity in MDA-MB231 cells by NF-κB-mediated increase of intracellular doxorubicin accumulationEur J Cancer2007432 43344217097285
- IrieTTsujiiMTsujiSSynergistic antitumor effects of celecoxib with 5-fluorouracil depend on IFN-γInt J Cancer20071214 87888317450522
- YoungSDLafrenieRMClemonsMJPhase ii trial of a metronomic schedule of docetaxel and capecitabine with concurrent celecoxib in patients with prior anthracycline exposure for metastatic breast cancerCurr Oncol2012192 e758322514500
- FabiAMetroGPapaldoPImpact of celecoxib on capecitabine tolerability and activity in pretreated metastatic breast cancer: results of a phase II study with biomarker evaluationCancer Chemother Pharmacol2008624 71772518071704
- PerroudHAAlasinoCMRicoMJMetastatic breast cancer patients treated with low-dose metronomic chemotherapy with cyclophosphamide and celecoxib: clinical outcomes and biomarkers of responseCancer Chemother Pharmacol2016772 36537426721701
- ChowLWLooWTWaiCCLuiELZhuLToiMStudy of COX-2, Ki67, and p53 expression to predict effectiveness of 5-flurouracil, epirubicin and cyclophosphamide with celecoxib treatment in breast cancer patientsBiomed Pharmacother200559Suppl 2 S298S30116507397
- ChowLWTungSYNgTYTyNConcurrent celecoxib with 5-fluorouracil/epirubicin/cyclophosphamide followed by docetaxel for stages II - III invasive breast cancer: the OOTR-N001 studyExpert Opin Investig Drugs2013223 299307
- ChowLWLooWTYipAYNgELAcceptable cardiac safety profile of neoadjuvant 5-fluorouracil, epirubicin, cyclophosphamide and celecoxib (FEC-C) for breast cancer: a subanalysis of biomarkers for cardiac injuryInt J Biol Markers2013281 3237
- UenoTChowLWToiMIncreases in circulating VEGF levels during COX-2 inhibitor treatment in breast cancer patientsBiomed Pharmacother2006606 27727916806797
- LimJSParkYLeeBMKimHSYoonSCo-treatment with Celecoxib or NS398 Strongly Sensitizes Resistant Cancer Cells to Antimitotic Drugs Independent of P-gp InhibitionAnticancer Res20163610 5063507027798865
- WebbTCarterJRobertsJLCelecoxib enhances [sorafenib + sildenafil] lethality in cancer cells and reverts platinum chemotherapy resistanceCancer Biol Ther20151611 1660167026417912
- PiergaJYDelalogeSEspiéMA multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patientsBreast Cancer Res Treat20101222 42943720480225
- GiacchettiSHamyASDelalogeSLong-term outcome of the REMAGUS 02 trial, a multicenter randomised phase II trial in locally advanced breast cancer patients treated with neoadjuvant chemotherapy with or without celecoxib or trastuzumab according to HER2 statusEur J Cancer20177532333228279941
- SubbaramaiahKHoweLRPortERHER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanismCancer Res20066610 5504551116707480
- BenoitVRelicBLeval XdXChariotAMervilleMPBoursVRegulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2Oncogene2004238 1631163514985703
- DangCTDannenbergAJSubbaramaiahKPhase II study of celecoxib and trastuzumab in metastatic breast cancer patients who have progressed after prior trastuzumab-based treatmentsClin Cancer Res20041012 Pt 1 4062406715217939
- El-AwadyRASalehEMEzzMElsayedAMInteraction of celecoxib with different anti-cancer drugs is antagonistic in breast but not in other cancer cellsToxicol Appl Pharmacol20112553 27128621763710
- GatesMATworogerSSEliassenAHMissmerSAHankinsonSEAnalgesic use and sex steroid hormone concentrations in postmenopausal womenCancer Epidemiol Biomarkers Prev2010194 1033104120332258
- MarshallSFBernsteinLAnton-CulverHNonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor statusJ Natl Cancer Inst20059711 80581215928301
- BrueggemeierRWDíaz-CruzESLiPKSugimotoYLinYCShapiroCLTranslational studies on aromatase, cyclooxygenases, and enzyme inhibitors in breast cancerJ Steroid Biochem Mol Biol2005951–5 12913615964185
- SunXLvMWangBComparative pharmacokinetics study of anastrozole after single administration and combination with celecoxibXenobiotica2018483 1628010169
- ChowLWChengCWWongJLToiMSerum lipid profiles in patients receiving endocrine treatment for breast cancer--the results from the Celecoxib Anti-Aromatase Neoadjuvant (CAAN) TrialBiomed Pharmacother200559Suppl 2 S302S30516507398
- ChowLWYipAYLooWTLamCKToiMCelecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancerJ Steroid Biochem Mol Biol20081111–2 131718514508
- CanneyPAMachinMACurtoJA feasibility study of the efficacy and tolerability of the combination of Exemestane with the COX-2 inhibitor Celecoxib in post-menopausal patients with advanced breast cancerEur J Cancer20064216 2751275617027257
- FalandryCDebledMBachelotTCelecoxib and exemestane versus placebo and exemestane in postmenopausal metastatic breast cancer patients: a double-blind phase III GINECO studyBreast Cancer Res Treat20091163 50150819020973
- LustbergMBPovoskiSPZhaoWPhase II trial of neoadjuvant exemestane in combination with celecoxib in postmenopausal women who have breast cancerClin Breast Cancer2011114 22122721729671
- DirixLYIgnacioJNagSTreatment of advanced hormone-sensitive breast cancer in postmenopausal women with exemestane alone or in combination with celecoxibJ Clin Oncol2008268 1253125918323548
- BonebergEMLeglerDFSennHJFürstenbergerGReduced expression of cyclooxygenase-2 in primary breast cancerJ Natl Cancer Inst200810014 1042104318612134
- AristarcoVSerranoDGandiniSA Randomized, Placebo-Controlled, Phase II, Presurgical Biomarker Trial of Celecoxib Versus Exemestane in Postmenopausal Breast Cancer PatientsCancer Prev Res201695 349356
- BasuGDTinderTLBradleyJMCyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDOJ Immunol20061774 2391240216888001
- LiYFangMZhangJHydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunityOncoimmunology201652 e107437427057439
- ChoHYThomasSGoldenEBEnhanced killing of chemo-resistant breast cancer cells via controlled aggravation of ER stressCancer Lett20092821 879719345476
- ThomasSSharmaNGoldenEBPreferential killing of triple-negative breast cancer cells in vitro and in vivo when pharmacological aggravators of endoplasmic reticulum stress are combined with autophagy inhibitorsCancer Lett20123251 637122664238
- das RoyLPathangeyLBTinderTLSchettiniJLGruberHEMukherjeePBreast-cancer-associated metastasis is significantly increased in a model of autoimmune arthritisBreast Cancer Res2009114 R5619643025
- MustafaAKrugerWDSuppression of tumor formation by a cyclo-oxygenase-2 inhibitor and a peroxisome proliferator-activated receptor gamma agonist in an in vivo mouse model of spontaneous breast cancerClin Cancer Res20081415 4935494218676768
- NiuGLiaoZCaiLWeiRSunLThe combined effects of celecoxib and minocycline hydrochloride on inhibiting the osseous metastasis of breast cancer in nude miceCancer Biother Radiopharm2008234 46947618771351
- YuPLiuQLiuKYagasakiKWuEZhangGMatrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-kappaB signalingCytotechnology2009593 21922919760125
- WangSLiuQZhangYSuppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of actionMol Cancer200988119796390
- JeonYWAhnYEChungWSChoiHJSuhYJSynergistic effect between celecoxib and luteolin is dependent on estrogen receptor in human breast cancer cellsTumour Biol2015368 6349635925851346
- JeonYWSuhYJSynergistic apoptotic effect of celecoxib and luteolin on breast cancer cellsOncol Rep2013292 81982523229294
- KiskováTJendželovskýRRentsenEResveratrol enhances the chemopreventive effect of celecoxib in chemically induced breast cancer in ratsEur J Cancer Prev2014236 50651325254309
- KimYJeYVitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysisBr J Cancer201411011 2772278424714744
- RoseAAElserCEnnisMGoodwinPJBlood levels of vitamin D and early stage breast cancer prognosis: a systematic review and meta-analysisBreast Cancer Res Treat20131413 33133924104883
- ThillMReichertKWoesteACombined treatment of breast cancer cell lines with vitamin D and COX-2 inhibitorsAnticancer Res2015352 1189119525667510
- ThillMTerjungAFriedrichMBreast cancer--new aspects of tumor biology: are calcitriol and cyclooxygenase-2 possible targets for breast cancer?Eur J Gynaecol Oncol2014354 34135825118473
- HowesLGSelective COX-2 inhibitors, NSAIDs and cardiovascular events - is celecoxib the safest choice?Ther Clin Risk Manag200735 83184518473007
- AbassiYAXiBZhangWKinetic cell-based morphological screening: prediction of mechanism of compound action and off-target effectsChem Biol2009167 71272319635408
- PyrkoPKardoshALiuYTCalcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxibMol Cancer Ther200764 1262127517431104
- O’ConnorJPLyszTNSAIDs and the skeletonDrugs Today2008449 69370919137124
- LeeJHParkHKHeoJDrug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugsJ Korean Med Sci2008233 52152518583892
- ChenJShenPZhangXCZhaoMDZhangXGYangLEfficacy and safety profile of celecoxib for treating advanced cancers: a meta-analysis of 11 randomized clinical trialsClin Ther2014368 1253126325016505
- MegeffCEStrayerSMCelecoxib for rheumatoid arthritisJ Fam Pract2000492 10810910718683