Abstract
Background
Histone deacetylase 6 (HDAC6) exerts enzymatic deacetylation activity on histones and on non-histone substrates and plays a key role in microtubule dynamics and chaperone activities. In addition, previous studies have demonstrated its role in cancer progression. However, its clinical significance in esophageal squamous cell cancer (ESCC) has not been elucidated. We investigated the correlation of HDAC6 expression and clinical outcome in a group of T3N1–3M0 surgically resected ESCCs.
Methods
Tissue microarrays were conducted on 209 surgically resected T3N1–3M0 ESCC tumors, including 163 pairs of primary tumors (PTs) and their corresponding metastatic lymph nodes (MLNs). Immunohistochemistry was utilized to evaluate HDAC6 protein levels. The relationship between patient outcomes and HDAC6 expression was analyzed statistically.
Results
The level of HDAC6 expression in ESCC MLNs was found to be significantly lower than that in PTs (P<0.001). Patients with lower MLN HDAC6 expression demonstrated improved overall survival (P=0.011) and disease-free survival (P=0.012) than those with higher HDAC6 expression. HDAC6 expression levels in PTs revealed no prognostic significance. Multivariate analysis showed that the MLN HDAC6 expression level was an independent prognostic factor for both overall survival (HR 1.456, P=0.029) and disease-free survival (HR 1.432, P=0.033).
Conclusion
High expression of HDAC6 in MLNs but not in PTs suggests a poor prognosis for patients with resected T3N1–3M0 ESCC. We should take into account the protein expression of MLNs when assessing prognosis in patients with lymph-node involvement.
Introduction
Esophageal cancer constitutes one of the most severe forms of cancer, with a high annual death rate.Citation1 Esophageal cancer can be divided into adenocarcinoma and squamous cell carcinoma based upon histological origin, with adenocarcinoma being more predominant in western countries and squamous cell carcinoma more predominant in eastern countries.Citation1 The TNM staging system is the most useful way to assess prognosis.Citation2 Patients without distant metastasis are candidates for surgery. Among them, patients without nodal involvement exhibit a relatively improved survival outcome. While multiple treatment modalities have been introduced over the past few decades, patients who are positive for node involvement (N+) typically have poor prognosis.Citation3 To improve these patients’ treatment outcomes, it is important to select those eligible for surgery, perform surgery properly, and prescribe follow-up adjuvant therapy to patients with poor prognosis.
Several studies have focused on the issue of the number of metastatic lymph nodes (MLNs),Citation4 MLN stations,Citation5 MLN ratio (MLN number/examined lymph-node number),Citation6 and skip metastases,Citation7 which all proved to be essential in distinguishing patients with different outcomes, but disparities still persisted. Differences in the extent of lymphadenectomy (two-field vs three-field dissection) may also influence patient outcomes based on different numbers and stations of dissected lymph nodes.Citation8 Molecular profiling has been widely used in precision treatment guidance and outcome prediction in many kinds of cancers,Citation9–Citation11 including esophageal squamous cell cancer (ESCC).Citation12 Nonetheless, very few studies have concentrated on patients with MLNs who are still considered surgical candidates.
Histone deacetylases (HDACs) are enzymes involved in the regulation of multiple processes, including gene expression regulation, protein activity, and deacetylation of histone proteins.Citation13 HDAC6 is unique among the HDAC enzyme family, having two active catalytic domains and a unique physiological function.Citation14,Citation15 In addition to the deacetylation of histones, HDAC6 can exert deacetylase enzymatic activity on non-histone substrates, including Hsp90,Citation16 cortactin,Citation17 peroxiredoxin,Citation18 and prolyl isomerase Pin1,Citation19 hence playing a key role in microtubule dynamics, chaperone activities, and tumor progression.Citation20,Citation21 Its dysregulation relates to many kinds of cancers, with variable effects; high expression of HDAC6 has been shown to be associated with tumor development in hepatocellular cancer,Citation22 pancreatic cancer,Citation23 and glioblastoma,Citation24 while decreased expression has been found to be associated with the suppression of proliferation, migration, or invasion in breast cancer,Citation25 lung cancer,Citation26 and gastric cancer.Citation27 In ESCC, Li et alCitation28 found that downregulation of HDAC6 expression could inhibit cell proliferation and reduce cell migration in vitro. However, the role of HDAC6 in vivo and its prognostic value in ESCC patients have not yet been elucidated.
In the current study, the expression levels of HDAC6 protein in N+ ESCC primary tumors (PTs) and the corresponding levels in MLNs were evaluated. Our results revealed high levels of HDAC6 expression in PT tissues and a positive correlation between MLN HDAC6 expression and poor ESCC patient survival.
Patients and methods
Patient selection
The current study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center and all patients provided written informed consent.
Patients were selected retrospectively from those who had undergone esophagectomy with standard or extended dissection of thoracic and abdominal lymph nodes between July 1997 and December 2004 at the Department of Thoracic Surgery, Sun Yat-sen University Cancer Center. Additional selection criteria included 1) pathological proof of thoracic T3N1–3M0 ESCC according to the eighth edition American Joint Committee on Cancer TNM staging system,Citation2 2) the absence of neoadjuvant or adjuvant therapy, 3) complete surgical resection, 4) and sufficient formalin-fixed and paraffin-embedded PT and MLN samples for tissue micro-arrays (TMAs).
TMA construction
TMAs were constructed using a Beecher Instruments tissue microarrayer (Beecher Instruments, Sun Prairie, WI, USA). Three targeted core samples with a 1 mm diameter were punched from each specimen and arrayed on a recipient paraffin block, which was then cut into sections (4 µm) and placed on glass slides.
A total of 234 PTs and 639 regional MLN samples from the 234 selected T3N1–3M0 ESCC patients were utilized. A median of two (range 1–17) MLNs were resected from the patients. Each sample of H&E-stained sections was reviewed randomly from a single selected paraffin block to define representative tumor regions. In patients with only one MLN, the right MLN was chosen for TMA construction. However, in patients with multiple MLNs, the appropriate MLN that satisfied the aforementioned criteria was randomly selected. Altogether, 163 pairs of surgically resected ESCC PTs and their corresponding MLNs, as well as 71 PTs without eligible MLNs, were used.
Immunohistochemistry (IHC)
IHC was performed using an IHC kit (Maxim, Fuzhou, People’s Republic of China); the detailed procedure was described in our previous study.Citation29 In brief, after retrieving antigen and non-specific binding blocking, the tissue slides were incubated with rabbit polyclonal anti-HDAC6 (Santa Cruz Biotechnology Inc., Dallas, TX, USA; 1:50 dilution) at 4°C overnight. Then, a biotinylated goat anti-rabbit IgG was used as the secondary antibody, with incubation for 1 hour at 37°C, followed by a horseradish peroxidase conjugate streptavidin–peroxidase working solution for 20 minutes at 37°C. Finally, the slides were reacted with diaminobenzidine (Sigma-Aldrich, St Louis, MO, USA) and counterstained with hematoxylin. Negative controls were prepared using a normal rabbit IgG to replace the primary antibody. Protein expression of HDAC6 was recorded as negative if no staining was present in tumor cells; otherwise, it was recorded as positive.
Two experienced pathologists evaluated the HDAC6 expression in tumor cells independently, blinded to the patient’s clinicopathological information. A semiquantitative system consisting of staining intensity and proportion of positive cells on each slide was used to score the HDAC6 expression. Each score was calculated as “I × Prop”, in which I stands for staining intensity, stratified as: none (0), weak (1), moderate (2), and strong (3), and Prop represents the percentage of positive cells (0–100) in at least 200 cancer cells counted. Therefore, the score was calculated between 0 and 300. The expression level of each case was the average of the scores determined by the two pathologists.
Statistical analyses
Statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) or MedCalc 9.6.2.0 (MedCalc Software, Mariakerke, Belgium). A matched-pair Wilcoxon test was used to compare HDAC6 expression in paired PTs and MLNs. Receiver operating characteristics (ROC) curves were used to select the optimal cutoff value of HDAC6 expression in PTs and MLNs. The optional cutoff value maximizes both the sensitivity and specificity for survival outcome in the 18 months following the operation. The relationship between HDAC6 expression and clinicopathological characteristics was analyzed by the chi-squared test. Overall survival (OS) was calculated based on the time of surgery to the time of death from any cause, censoring patients who were still alive at the time of the last follow-up (June 4, 2016). Disease-free survival (DFS) was defined as the time from surgery to any regional relapse or distal metastasis, censoring patients who still had an absence of any malignancy at the last follow-up. Survival curves were analyzed by the Kaplan–Meier method and log-rank test. Multivariate analysis was performed using the Cox proportional hazard modes with potential factors whose P-values were less than 0.10 in the univariate analyses, constructed with the forward stepwise method. The result was considered significant when the two-tailed P-value was less than 0.05.
Results
Patients’ characteristics
Owing to the losses of cores during the IHC procedures, nine pairs of PTs and MLNs and 16 PTs without paired MLNs were excluded from the analyses. The remaining TMA included 155 PTs with paired MLNs and 54 PTs without paired MLNs. There were 172 male and 37 female patients, with a median age of 58 years. All of them were node positive, including N1 in 118 patients, N2 in 69 patients, and N3 in 22 patients.
The median length of follow-up for surviving patients was 140 (18–195) months. During the follow-up period, 186 patients (89.0%) died. The 5-year OS and DFS rates were 17.7% and 15.8%, respectively.
HDAC6 expression in ESCC PTs and MLNs
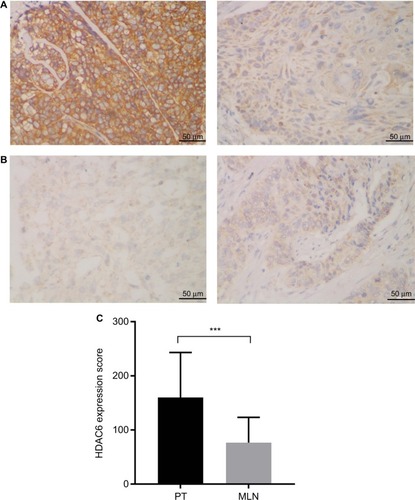
HDAC6 protein was found to be mainly localized to the cytoplasmic region (). Positive HDAC6 expression was detected in 96.7% (202/209) and 95.5% (148/155) of ESCC PTs and MLNs, respectively. However, based on their IHC score, HDAC6 expression in MLNs was significantly decreased compared to their paired PTs (Wil-coxon matched-pair signed-rank test, P<0.001) ().
Figure 1 Lower HDAC6 expression was exhibited in MLNs than in the corresponding esophageal squamous cell cancer PT.
Notes: (A) Representative immunohistochemical staining of a patient whose HDAC6 expression was higher in the PT (left) than in MLNs (right). (B) Representative immunohistochemical staining of a patient whose HDAC6 expression was lower in the PT (left) than in MLNs (right) (magnification ×400). (C) Paired comparison of HDAC6 staining scores of PTs and MLNs. ***P<0.001, Wilcoxon matched-pair signed-rank test.
Abbreviations: HDAC6, histone deacetylase 6; MLN, metastatic lymph node; PT, primary tumor.
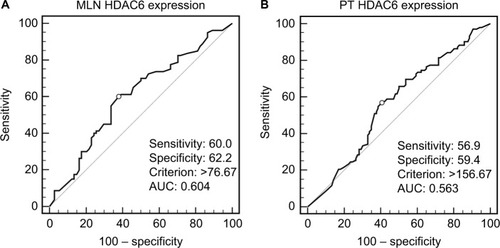
According to HDAC6 expression in ESCC PTs and MLNs, ROC curves were drawn to determine the optimal cutoff value with the best discriminatory power for prediction of survival outcome. As shown in , the cutoff score was 156.67 and 76.67 in PTs and MLNs, respectively, with values above these indicating high HDAC6 expression. Using this criterion, high HDAC6 expression was observed in 48.8% (102/209) of the ESCC PTs and 49.0% (76/155) of MLNs. When performing the comparison in 155 paired samples, we found that 50.3% (78/155) of PTs had high HDAC6 expression, but only 53.8% (42/78) of them retained high expression in their corresponding MLNs (P=0.228). HDAC6 expression (high vs low) in ESCC PTs and MLNs was analyzed with regard to their clinicopathological parameters (). No significant association was obtained between the high and low HDAC6 expression groups for gender, age, smoking, drinking, location, length, grade, and pN stage, in either PTs or MLNs.
Table 1 HDAC6 protein expression in PTs and MLNs and their correlations with clinicopathological features of patients with esophageal squamous cell cancer
Figure 2 ROC curves used HDAC6 expression scores of (A) MLNs and (B) PTs to select cutoff values. The optimal cutoff value was determined by maximizing an AUC to discriminate between survival and 18-month cancer-specific death.
Abbreviations: AUC, area under the ROC curve; HDAC6, histone deacetylase 6; MLN, metastatic lymph node; PT, primary tumor; ROC, receiver operating characteristics.
HDAC6 expression and ESCC patient survival
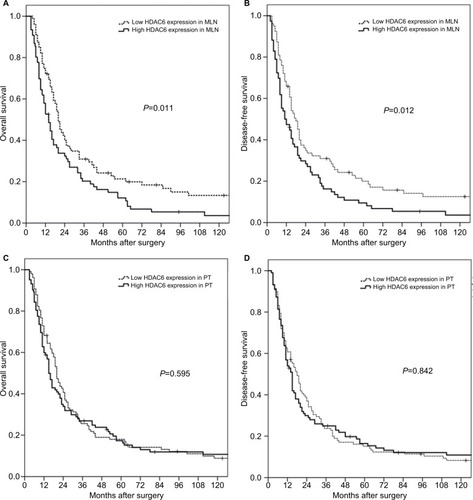
Based on Kaplan–Meier data analysis, higher HDAC6 expression in ESCC MLNs was significantly associated with poorer OS (P=0.011) and DFS (P=0.012). The 5-year OS rates for high and low HDAC6 expression in MLNs were 12.1% and 21.4%, respectively (). However, neither the OS (P=0.595) nor the DFS (P=0.842) was significantly influenced by the HDAC6 expression level of ESCC PTs. The 5-year OS rates for high and low HDAC6 expression in PTs were 17.4% and 18.0%, respectively ().
Figure 3 Kaplan–Meier survival analyses for surgically resected T3N1–3M0 esophageal squamous cell carcinomas revealed that HDAC6 expression levels in MLNs (A, B), but not in PTs (C, D), were prognostic factors.
Abbreviations: HDAC6, histone deacetylase 6; MLN, metastatic lymph node; PT, primary tumor.
As shown by the univariate analysis in , age and pN stage were also significant prognostic factors for OS (age, P=0.010; stage, P=0.006) and DFS (age, P=0.013; stage, P<0.001). Tumor grade was significantly correlated with DFS (P=0.041) but not with OS (P=0.068). Multivariate analysis using the Cox proportional hazards regression model with factors that might affect survival determined by univariate analysis showed that only pN stage and HDAC6 expression in MLNs were independent prognostic factors of OS (pN stage, P=0.004; HDAC6, P=0.029) and DFS (pN stage, P<0.001; HDAC6, P=0.033) in N+ ESCC patients ().
Table 2 Univariate and multivariate analyses of prognostic variables in patients with esophageal squamous cell cancer
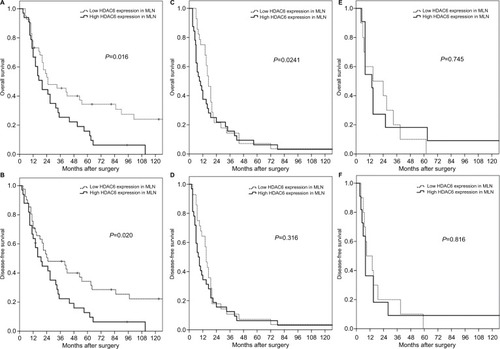
Subgroup analysis revealed that the significant prognostic value of HDAC6 expression in MLNs was only pronounced in pN1 patients (). In patients with pN2 and pN3, there was no significant difference in OS or DFS between high and low HDAC6 expression in MLNs ().
Figure 4 Subgroup analyses for surgically resected T3N1–3M0 esophageal squamous cell carcinomas according to different N status.
Note: The prognostic significance of HDAC6 expression in MLNs was found only for N1 patients (A, B), not N2 (C, D), or N3 patients (E, F).
Abbreviations: HDAC6, histone deacetylase 6; MLN, metastatic lymph node.
Discussion
Although adjuvant therapy following surgery has been validated to benefit N+ ESCC patients, their prognosis is still dismal.Citation30–Citation32 Multiple approaches have been made to improve the outcome for these patients; elucidating the molecular mechanisms for precision medicine is one of these options. However, most of the previous studies only focused on the biological characteristics of the PT. Although gene or protein expression in the PT is often related to lymphatic, distant metastases and prognosis, it has failed to show a similar predictive value in N+ ESCC patients.Citation33,Citation34 Tumor heterogeneity may be part of the reason for this phenomenon. It is now believed that intra-tumor heterogeneity reflects the ongoing linear and branching evolution, resulting in multiple simultaneous subclones that may individually be capable of giving rise to metastasis.Citation35 In this context, somatic genetic alterations are restricted or enriched in the metastatic lesions compared to their respective PTs.Citation36,Citation37 Our previous study also demonstrated the different epithelial–mesenchymal phenotypes between PTs and their corresponding MLNs. The transition of tumor cells from mesenchymal to epithelial phenotypes may be a key factor in the formation of metastasis.Citation29 In the present study, we again confirmed that there are differences in molecular expression in ESCC PTs and corresponding MLNs. The HDAC6 expression in MLNs was significantly decreased.
In addition, we found that HDAC6 expression in MLNs, but not in PTs, was associated with both the DFS and OS in survival analyses. This suggests that more attention should be paid to genomic expression in MLN in N+ patients, not only to elucidate the mechanism of cancer cell migration but also to help determine patient prognosis and guide treatment. Nonetheless, only a few studies have focused on this issue. In non-small-cell lung cancer with lymph-node metastases, Kil-vaer et alCitation38 found that a high level of intraepithelial CD45RO+ tumor infiltrative lymphocytes in MLNs was an independent positive prognostic factor for disease-specific patient survival. In stage II/III lymph-node-positive breast cancer patients, Bonin et alCitation39 determined that keratin 8 expression in MLNs, but not in PTs, indicated better survival. Our previous study also revealed that high expression of C-terminal Hsp-interacting protein (CHIP) in MLNs suggests a poor prognosis for patients with resected T3N1–3M0 ESCC.Citation40 Taking our findings together with these previous studies, we strongly recommend examining the genomic profiling of MLNs when assessing prognosis in lymph-node-positive cancer patients.
HDAC6 has been shown to be upregulated in a diverse number of tumors and cancer cell lines, suggesting an important role for this enzyme in cancer. It is essential in maintaining oncogenic phenotype and promoting anchorage-independent proliferation in transformed cellsCitation41 and leads to increased cell motility.Citation42 In an ESCC in vitro model, Li et alCitation28 confirmed its role in tumor progression by showing that cell proliferation and migration could both be significantly reduced after HDAC6 inhibition. Tao et alCitation43 found that HDAC6 facilitated ESCC development by regulating the acetylation of HSP90, and coadministration of HSP90 and HDAC6 inhibitors strongly inhibited tumor growth in mice. HDAC6 inhibitors, such as ricolinostat and ACY-241, stand apart from broad-spectrum HDAC inhibitors because of their druggability and unique function with the cells. Unlike other pan-HDAC inhibitors with adverse effects including hematological toxicity and QT prolongation, highly selective HDAC6 inhibitors are considered to have more potential for clinical use.Citation44 A number of clinical trials utilizing selective HDAC6 inhibitors are underway for treating multiple myeloma and lymphoid malignancies. Therefore, HDAC6 is not only a biomarker predicting patients’ outcome but also a potential therapeutic target.
Several limitations exist in this study. First, in patients with more than one MLN, samples were collected randomly. The heterogeneity between the different MLNs may impart some bias to our results. Second, most of the participants were from southern China, which may limit the generalization of our findings to other populations. Finally, the small sample size and retrospective nature of our study suggest the need to perform a large-scale prospective study to confirm our results.
Conclusion
Although HDAC6 was found to be highly expressed in most PTs and MLNs in ESCC, a substantial discordance between them was still present. HDAC6 expression was decreased in MLNs compared to their paired PTs and may serve as an independent predictor for prognosis of complete surgically resected T3N1–3M0 ESCC patients. More attention should be paid to HDAC6 expression in metastatic tumors for prognostic prediction and to the potential for HDAC6 inhibitor therapy in ESCC patients.
Abbreviations
| N+ | = | node involvement |
| MLN | = | metastatic lymph node |
| ESCC | = | esophageal squamous cell cancer |
| HDAC | = | histone deacetylase |
| PT | = | primary tumor |
| TMA | = | tissue microarray |
| IHC | = | immunohistochemistry |
| ROC | = | receiver operating characteristics |
| OS | = | overall survival |
| DFS | = | disease-free survival |
Acknowledgments
This work was supported by the National Science Foundation of China (grant nos 81672356 and 81602105), Guangzhou Science Technology and Innovation Commission (grant no. 201610010127), Guangdong Talents Special Support Program (grant no. 201629038), the Fundamental Research Funds for the Central Universities (grand nos 15ykpy34 and 16ykjc39), and Guangdong Esophageal Cancer Institute Science and Technology Program (grant no. M201701).
Author contributions
XX and KJL constructed the tissue microarrays, designed the study, analyzed the data, and drafted the manuscript. YL carried out data acquisition and performed the statistical analysis. YHL evaluated the protein expression and calculated the score of each sample. SSZ and XYX helped to construct the tissue microarrays and performed the immunohistochemistry. JW conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer Statistics, 2017CA Cancer J Clin201767173028055103
- RiceTWIshwaranHFergusonMKBlackstoneEHGoldstrawPCancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging PrimerJ Thorac Oncol2017121364227810391
- SjoquistKMBurmeisterBHSmithersBMAustralasian Gastro-Intestinal Trials GroupSurvival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysisLancet Oncol201112768169221684205
- SugawaraKYamashitaHUemuraYNumeric pathologic lymph node classification shows prognostic superiority to topographic pN classification in esophageal squamous cell carcinomaSurgery2017162484685628739092
- PengJWangWPDongTRefining the Nodal Staging for Esophageal Squamous Cell Carcinoma Based on Lymph Node StationsAnn Thorac Surg2016101128028626381757
- FuXLiuQLuoKLymph node station ratio: Revised nodal category for resected esophageal squamous cell carcinoma patientsJ Surg Oncol2017116793994628703872
- WangFZhengYWangZNodal Skip Metastasis in Esophageal Squamous Cell Carcinoma Patients Undergoing Three-Field LymphadenectomyAnn Thorac Surg201710441187119328669504
- RiceTWIshwaranHHofstetterWLEsophageal Cancer: Associations With (pN+) Lymph Node MetastasesAnn Surg2017265112212928009736
- WoodSLPernemalmMCrosbiePAWhettonADMolecular histology of lung cancer: from targets to treatmentsCancer Treat Rev201541436137525825324
- AdesFZardavasDBozovic-SpasojevicILuminal B breast cancer: molecular characterization, clinical management, and future perspectivesJ Clin Oncol201432252794280325049332
- CiomborKKWuCGoldbergRMRecent therapeutic advances in the treatment of colorectal cancerAnnu Rev Med2015661839525341011
- SuHHuNYangHHGlobal gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypesClin Cancer Res20111792955296621385931
- DelcuveGPKhanDHDavieJRRoles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitorsClin Epigenetics201241522414492
- ZouHWuYNavreMSangBCCharacterization of the two catalytic domains in histone deacetylase 6Biochem Biophys Res Commun20063411455016412385
- ZhangYGilquinBKhochbinSMatthiasPTwo catalytic domains are required for protein deacetylationJ Biol Chem200628152401240416272578
- BaliPPranpatMBradnerJInhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitorsJ Biol Chem200528029267292673415937340
- ZhangXYuanZZhangYHDAC6 modulates cell motility by altering the acetylation level of cortactinMol Cell200727219721317643370
- ParmigianiRBXuWSVenta-PerezGHDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulationProc Natl Acad Sci U S A2008105289633963818606987
- NoguésLRegleroCRivasVG Protein-coupled Receptor Kinase 2 (GRK2) Promotes Breast Tumorigenesis Through a HDAC6-Pin1 AxisEBioMedicine20161313214527720394
- AsthanaJKapoorSMohanRPandaDInhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cellsJ Biol Chem201328831225162252623798680
- WangXXWanRZLiuZPRecent advances in the discovery of potent and selective HDAC6 inhibitorsEur J Med Chem20181431406141829133060
- JungKHNohJHKimJKHistone deacetylase 6 functions as a tumor suppressor by activating c-Jun NH2-terminal kinase-mediated beclin 1-dependent autophagic cell death in liver cancerHepatology201256264465722392728
- LiDSunXZhangLHistone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cellsProtein Cell20145321422324474193
- LinTWChenMTLinLTTDP-43/HDAC6 axis promoted tumor progression and regulated nutrient deprivation-induced autophagy in glioblastomaOncotarget2017834566125662528915616
- SeoJMinSKParkHRExpression of Histone Deacetylases HDAC1, HDAC2, HDAC3, and HDAC6 in Invasive Ductal Carcinomas of the BreastJ Breast Cancer201417432333125548579
- YangCJLiuYPDaiHYNuclear HDAC6 inhibits invasion by suppressing NF-κB/MMP2 and is inversely correlated with metastasis of non-small cell lung cancerOncotarget2015630302633027626388610
- HeQLiGWangXA Decrease of Histone Deacetylase 6 Expression Caused by Helicobacter Pylori Infection is Associated with Oncogenic Transformation in Gastric CancerCell Physiol Biochem20174241326133528700998
- LiNTieXJLiuPJEffects of down-regulation of HDAC6 expression on proliferation, cell cycling and migration of esophageal squamous cell carcinoma cells and related molecular mechanismsAsian Pac J Cancer Prev201314268568923621219
- WenJLuoKJLiuQWThe epithelial-mesenchymal transition phenotype of metastatic lymph nodes impacts the prognosis of esophageal squamous cell carcinoma patientsOncotarget2016725375813758827147562
- ZhangSSYangHXieXAdjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studiesDis Esophagus201427657458423621119
- LyuXHuangJMaoYAdjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma?J Surg Oncol2014110786486824976079
- QinRQWenYSWangWPThe role of postoperative adjuvant chemotherapy for lymph node-positive esophageal squamous cell carcinoma: a propensity score matching analysisMed Oncol20163343126922662
- XieXZhangSSWenJPrognostic value of HOXB7 mRNA expression in human oesophageal squamous cell cancerBiomarkers201318429730323627614
- ChenYFXieJDJiangYCThe Prognostic Value of Peripheral Benzodiazepine Receptor in Patients with Esophageal Squamous Cell CarcinomaJ Cancer20178163343335529158807
- PodlahaORiesterMDeSMichorFEvolution of the cancer genomeTrends Genet201228415516322342180
- SchrijverWSelenicaPLeeJYMutation profiling of key cancer genes in primary breast cancers and their distant metastasesCancer Res201878123112312129615433
- SaberAHiltermannTJNKokKMutation patterns in small cell and non-small cell lung cancer patients suggest a different level of heterogeneity between primary and metastatic tumorsCarcinogenesis201738214415127993895
- KilvaerTKPaulsenEEKhanehkenariMRThe presence of intraepithelial CD45RO+ cells in resected lymph nodes with metastases from NSCLC patients is an independent predictor of disease-specific survivalBr J Cancer2016114101145115127167450
- BoninSPracellaDBarbazzaRSulfaroSStantaGIn stage II/III lymph node-positive breast cancer patients less than 55 years of age, keratin 8 expression in lymph node metastases but not in the primary tumour is an indicator of better survivalVirchows Arch2015466557158025724181
- WenJLuoKJHuYYangHFuJHMetastatic lymph node CHIP expression is a potential prognostic marker for resected esophageal squamous cell carcinoma patientsAnn Surg Oncol20132051668167523429937
- Aldana-MasangkayGISakamotoKMThe role of HDAC6 in cancerJ Biomed Biotechnol2011201187582421076528
- SajiSKawakamiMHayashiSSignificance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancerOncogene200524284531453915806142
- TaoHChenYYSunZWChenHLChenMSilence of HDAC6 suppressed esophageal squamous cell carcinoma proliferation and migration by disrupting chaperone function of HSP90J Cell Biochem201811986623663229665050
- BatchuSNBrijmohanASAdvaniAThe therapeutic hope for HDAC6 inhibitors in malignancy and chronic diseaseClin Sci (Lond)201613012987100327154743
