Abstract
Background
The purpose of this study was to investigate the value of the postsurgical pathological T and N (ypTN) category combined with the American Joint Committee on Cancer-tumor regression grade (AJCC-TRG) in evaluating the prognosis of neoadjuvant chemoradiation therapy (NeoCRT) for locally advanced rectal cancer (LARC) to screen for a subgroup of patients with the worst prognosis.
Patients and methods
In total, 265 patients with LARC were enrolled in the trial. All patients received NeoCRT. Total mesorectal excision was performed 6–8 weeks after the completion of radiotherapy. The surgical specimens were re-evaluated based on the AJCC-TRG (seventh edition) and the AJCC-tumor-node-metastasis (TNM; seventh edition) systems. We followed up these patients and calculated their overall survival (OS), disease-free survival (DFS), local recurrence-free survival (RFS), and distant metastasis (DM)-free survival (MFS) rates through the Kaplan–Meier analysis. The logrank test was further applied to evaluate the predictive value of the ypTN stage combined with AJCC-TRG for several survival indexes.
Results
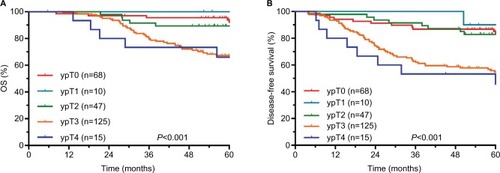
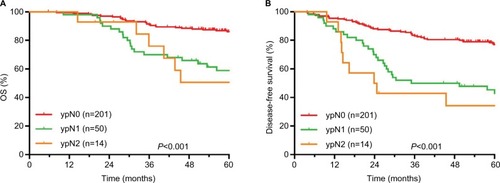
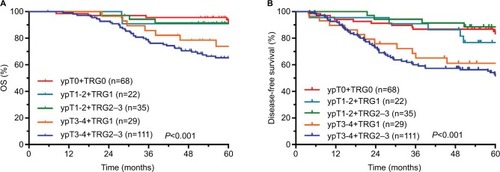
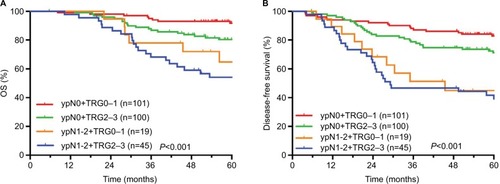
The median follow-up period was 65.1 months. The 5-year OS, DFS, RFS, and MFS rates were 79.4%, 68.8%, 94.4%, and 76.5%, respectively. There were significant differences in OS, DFS, and MFS rates among different ypT+AJCC-TRG and ypN+AJCC-TRG subgroups. The 5-year OS, DFS, and MFS rates for ypT3–4+TRG 1 and ypT3–4+TRG2–3 subgroups were 73.9% vs 65.3%, 61.2% vs 52.9%, and 65.0% vs 61.5%, respectively. The 5-year OS, DFS, and MFS rates for ypN1–2+TRG 0–1 and ypN1–2+TRG2–3 subgroups were 64.8% vs 54.1%, 44.9% vs 41.7%, and 61.4% vs 46.3%, respectively.
Conclusion
The ypTNM category combined with the AJCC-TRG can more accurately evaluate the prognosis of patients with LARC and identify the subgroup of patients with the worst prognosis and high risk of developing DM, thereby demonstrating clinical significance in guiding individualized postoperative adjuvant therapy and follow-up for LARC.
Introduction
The postsurgical pathological T and N (ypTN) category is an important factor influencing the prognosis of neoadjuvant chemoradiation therapy (NeoCRT) before surgery for locally advanced rectal cancer (LARC).Citation1–Citation3 However, the prognostic value of postsurgical pathological T category (ypT) has often been challenged. For example, patients are often thought to have ypT3 or ypT4 category disease when postoperative pathological examination shows a small number of deformed atypical cells in the outer membrane layer of the rectal wall or outside the serosa of the rectal wall, but in fact, these patients have good prognosis. In addition, the accuracy of ypN category depends on the number of lymph nodes dissected during surgery, which is further influenced by the age of patients, tumor grade and stage, location of lesions, and quality of surgery.Citation4,Citation5 If the number of dissected lymph nodes is not enough, the postsurgical pathological N category (ypN) is not accurate. Therefore, it is still not very reliable to evaluate the prognosis of LARC based on the ypTN category alone.
Several studies have shown that the tumor regression grade (TRG) had significant correlation with the prognosis of LARCCitation6–Citation10 and is gradually being used to assess the progression of rectal cancer. Thus far, mainly six types of TRG systems have been used, and the American Joint Committee on Cancer (AJCC)-TRG has been found to be better than any other system as it can be more accurate in predicting the occurrence of distant metastasis (DM) due to rectal cancer.Citation11
The purpose of this study is to investigate the value of ypTNM category combined with the AJCC-TRG in evaluating the prognosis of NeoCRT for LARC to screen for the subgroup of patients with the worst prognosis and to provide the foundation for individualized postoperative adjuvant therapy and follow-up.
Patients and methods
Patients
Patients with pathologically diagnosed, nonmetastatic, and resectable LARC were enrolled in the trial between October 1, 2004, and December 30, 2012. All the patients received NeoCRT before surgery; clinical stage was stage II (T3–4N0M0) or stage III (T1–4N1–2M0) according to the seventh edition of the Union for International Cancer Control (UICC)/AJCC tumor-node-metastasis (TNM) classification,Citation12 and the Eastern Cooperative Oncology Group (ECOG) performance status was ≤2. The baseline clinical characteristics of the patients are shown in .
Table 1 Baseline clinical characteristics and treatment results of the study population
Ethics approval and consent to participate
Before treatment, all patients received detailed oral and written information on the treatment protocol and possible adverse effects and signed an informed consent. The trial was approved by the institutional review board of our hospital (Sun Yat-sen University Cancer Center) and conducted in accordance with the Declaration of Helsinki.
Grading standard and evaluation method of TRG
According to the tumor regression grading standard of the seventh edition of AJCC,Citation11 TRG0 refers to no residual tumor cells, TRG1 refers to single cells or small groups of cells, TRG2 refers to residual cancer with desmoplastic response, while TRG3 refers to minimal evidence of tumor response. The TRG was independently evaluated by two experienced pathology specialists by reviewing the tissues sections. When the evaluation results were inconsistent, two experienced pathology specialists discussed and reviewed the tissues sections together and then gave the final evaluation.
Treatment
Radiotherapy
All patients were immobilized at a prone position using an AIO Bellyboard and Pelvic Solution System (AIO Solution; Orfit Industries, Wijnegem, Belgium). Volumetric-modulated arc therapy was the irradiation treatment modality used in this study. After a computed tomography (CT)-based simulation, target volumes were delineated according to the guidelines of the International Commission on Radiation Units and Measurements (ICRU) reports 50 and 62. Gross tumor volume (GTV) included the macroscopic tumor and enlarged lymph nodes as visualized on CT or magnetic resonance (MR) images. Clinical target volume (CTV) covered the GTV with a radial margin of 2 cm and included high-risk regions of lymphatic drainage. Conventional fractionation radiotherapy was conducted (2 Gy per fraction, 1 fraction per day, 5 days per week), in which the total doses of GTV and CTV were 50 and 46 Gy, respectively.
Neoadjuvant chemotherapy
During radiotherapy, all patients received neoadjuvant chemotherapy. In total, 242 cases received the oxaliplatin and capecitabine (XELOX) chemotherapy regimen repeated every 21 days, in which 100 mg/m2 oxaliplatin (OXA) was administered on day 1 and 1,000 mg/m40 capecitabine (CAP) was administered twice daily from day 1–14. The other 23 cases received the fluorouracil, leucovorin, oxaliplatin (FOLFOX6) chemotherapy regimen repeated every 14 days, in which 85 mg/m2 OXA, 400 mg/m2 calcium folinate (CF), and intravenous injection of 5-fluorouracil (400 mg/m2) were administered on day 1, with continuous intravenous pump infusion of 5-fluorouracil (2,400 mg/m2) for 46–48 hours.
Radical resection
All patients planned to receive radical rectal resection 6–8 weeks after NeoCRT. The surgery was performed according to the principles of total mesorectal excision (TME).
Adjuvant chemotherapy
Adjuvant chemotherapy with XELOX or FOLFOX6 was started 3–4 weeks after surgery, with a median of four cycles.
Follow-up and endpoints
The frequency of follow-up was once in every 3–4 months within 2 years after treatment and then once in every 6 months thereafter. Follow-up evaluations mainly involved a complete physical examination, digital rectal examination, thoracoabdominal CT scan, endoscopic ultrasonography, pelvic MR imaging scan, and tests of the levels of carcinoembryonic antigen (CEA), and carbohydrate antigen-199 (CA-199).
The primary endpoint was overall survival (OS) rate, which referred to the percentage of patients who were alive after a certain time period from diagnosis. Secondary endpoints were disease-free survival (DFS), local recurrence-free survival (RFS), and DM-free survival (MFS) rates. The DFS rate was defined as the percentage of living patients without local recurrence or DM after a certain time period from diagnosis. In addition, the RFS/MFS ratio was defined as the percentage of patients without local recurrence or DM after a certain time period.
Statistical analyses
OS, DFS, RFS, and MFS rates were determined using the Kaplan–Meier analysis. The logrank test was performed to evaluate whether ypT category, ypN category, AJCC-TRG, ypT+AJCC-TRG, and ypN+AJCC-TRG were candidate risk factors for OS, DFS, RFS, and MFS.
Statistical analyses were conducted using SPSS Statistics Version 19.0 software (IBM Corporation, Armonk, New York, USA). A two-sided P-value <0.05 was considered statistically significant.
Results
Survival
The deadline for follow-up was November 2017, with a follow-up rate of 94.1%. The median follow-up time was 65.1 months (range, 5.7–152.6 months). There were 67 cases of death in the whole group – 1 patient died of pulmonary infection, 1 patient died of diabetes complication, and the other 65 patients died of tumor recurrence or metastasis. The 5-year OS, DFS, RFS, and MFS rates were 79.4%, 68.8%, 94.4%, and 76.5%, respectively. The results are shown in .
Table 2 Univariate survival analysis of patients according to different factors
Associations between ypTN or TRG and prognosis
The 5-year survival rates of different ypT, ypN, and TRG groups are shown in . The comparisons of survival curves among different ypT subgroups are shown in . The comparisons of survival curves among different ypN subgroups are shown in .
ypTN stage combined with AJCC-TRG to evaluate prognosis
The comparisons of OS and DFS among different ypT+AJCC-TRG subgroups are shown in . The comparisons of OS and DFS among different ypN+AJCC-TRG subgroups are shown in . There were significant differences in OS, DFS, and MFS among different ypT+AJCC-TRG and ypN+AJCC-TRG subgroups. Patients in the ypT3−4+TRG2–3 and ypN1−2+ TRG2–3 subgroups had the worst prognosis. The 5-year OS, DFS, and MFS rates for ypT3−4+TRG1 and ypT3−4+TRG2–3 subgroups were 73.9% vs 65.3% (P<0.001), 61.2% vs 52.9% (P<0.001), and 65.0% vs 61.5% (P<0.001), respectively. The 5-year OS, DFS, and MFS rates for ypN1−2+TRG0–1 and ypN1−2+TRG2–3 subgroups were 64.8% vs 54.1% (P<0.001), 44.9% vs 41.7% (P<0.001), and 61.4% vs 46.3% (P<0.001), respectively.
Discussion
In our study, the prognosis of patients with ypT3–4 and ypN1–2 diseases was significantly worse than that of other patients, with 5-year OS rates of 67.1% and 57.5%, 5-year DFS rates of 54.6% and 42.8%, and 5-year MFS rates of 62.3% and 50.6%, respectively. However, subgroup analysis showed that the prognosis of patients with TRG2–3 disease was worst; the 5-year OS rates of the ypT3–4+TRG2–3 and ypN1–2+TRG2–3 subgroups were 65.3% and 54.1%, the 5-year DFS rates were 52.9% and 41.7%, and the 5-year MFS rates were 61.5% and 46.3%, respectively.
Several studiesCitation1–Citation3 have reported that ypT and ypN categories are prognostic factors for OS, DFS, and MFS and that ypN category is an independent prognostic factor. The results of our study further confirmed these conclusions. However, the prognostic value of ypT category is often challenged. For example, patients are often thought to have ypT3 or ypT4 category disease when postoperative pathological examination shows a small number of deformed atypical cells in the outer membrane layer of the rectal wall or outside the serosa of the rectal wall, but in fact, these patients have good prognosis. Our results also suggest that some patients with ypT3-4 disease had a better prognosis than patients with ypT1-2 disease. The accuracy of ypN category depends on the number of lymph nodes dissected during surgery, which is further influenced by the age of patients, tumor grade and stage, location of lesions, and quality of surgery.Citation4,Citation5 If the number of dissected lymph nodes is not enough, the ypN category is not accurate. Therefore, it is not very reliable to evaluate the prognosis of LARC based on the ypTN category alone.
TRG is a type of grading metric used in the histological stratification of tumor response after chemoradiotherapy, and it was first used in the evaluation of the efficacy of concurrent chemoradiotherapy in esophageal, gastric, bladder, and head and neck cancers.Citation13–Citation17 Later, studies reported that TRG had a significant correlation with the prognosis of LARC,Citation6–Citation10 and it was gradually used to evaluate the progression of rectal cancer. There are mainly 6 types of TRG systems at present, which divide the TRG into 3–5 grades, but no unified standards have yet been established. Through the comparison of evaluation results of each system, the AJCC-TRG system was found to be superior to other systems, as it could more accurately predict the occurrence of DM due to rectal cancer.Citation11 The results of this study also showed that the OS, DFS, RFS, and MFS rates were different in LARC patients with different AJCC-TRGs, and the TRG was a factor influencing OS, DFS, and MFS rates (P-values were 0.003, 0.011, and 0.004, respectively). Moreover, we noticed that the TRG was determined by the proportion of residual tumor cells,Citation11,Citation18 which is highly dependent on the technology and experience of pathologist and might be difficult to accurately determine in some cases. In addition, the TRG mainly focuses on the response of primary tumor to treatment, without considering lymph node metastasis. In this study, TRG2–3 subgroups included patients with ypN0 and ypN1–2 stage diseases, and subgroup analysis showed that the 5-year OS, DFS, and MFS rates in the ypN1–2+TRG2–3 subgroups were significantly worse than those in the ypN0+TRG2–3 subgroup (54.1% vs 80.4%, 41.7% vs 71.1%, and 46.3% vs 79.7%, respectively; the P-values were all <0.001). Therefore, it is not accurate to evaluate the prognosis of LARC with AJCC-TRG alone.
The results of this study showed that the main mode of failure for LARC treatment was DM, consistent with the results reported in other studies.Citation19,Citation20 The 5-year MFS rates of the ypT0+TRG0, ypT1–2+TRG1, ypT1–2+TRG2–3, ypT3–4+TRG1, and ypT3–4+TRG2–3 subgroups in this study were 92.6%, 85.6%, 94.0%, 65.0%, and 61.5%, respectively (P<0.001). In addition, the 5-year MFS rates of the ypN0+TRG0–1, ypN0+TRG2–3, ypN1–2+TRG0–1, and ypN1–2+TRG2–3 subgroups in this study were 89.0%, 79.7%, 61.4% and 46.3%, respectively (P<0.001). Obviously, the prognosis of patients in the ypT3–4+TRG2–3 and ypN1–2+TRG2–3 subgroups was significantly worse than that in other subgroups. Hence, minimizing the rates of DM in these subgroups may achieve better long-term results. Therefore, reinforcing postoperative adjuvant therapy is recommended for patients with ypT3–4 or ypN1–2 disease combined with AJCC-TRG2–3, and stronger postoperative adjuvant therapy may improve the long-term survival of these patients. At the same time, these patients also need close follow-up and observation.
Conclusion
The ypTNM category combined with the AJCC-TRG can more accurately evaluate the prognosis of patients with LARC and screen the subgroup of patients with the worst prognosis and high risk of developing DM, thereby demonstrating clinical significance in guiding individualized postoperative adjuvant therapy and follow-up for LARC.
Disclosure
The authors report no conflicts of interest in this work.
References
- QuahHMChouJFGonenMPathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiationCancer20081131576418442099
- ParkIJYouYNAgarwalANeoadjuvant treatment response as an early response indicator for patients with rectal cancerJ Clin Oncol201230151770177622493423
- RödelCMartusPPapadoupolosTPrognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancerJ Clin Oncol200523348688869616246976
- SarliLBaderGIuscoDNumber of lymph nodes examined and prognosis of TNM stage II colorectal cancerEur J Cancer200541227227915661553
- Luna-PérezPRodríguez-RamírezSAlvaradoIGutiérrez de La BarreraMLabastidaSPrognostic significance of retrieved lymph nodes per specimen in resected rectal adenocarcinoma after preoperative chemoradiation therapyArch Med Res200334428128612957524
- FokasELierschTFietkauRTumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trialJ Clin Oncol201432151554156224752056
- VecchioFMValentiniVMinskyBDThe relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancerInt J Radiat Oncol Biol Phys200562375276015936556
- CapirciCValentiniVCioniniLPrognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patientsInt J Radiat Oncol Biol Phys20087219910718407433
- DhaddaASDickinsonPZaitounAMGandhiNBessellEMPrognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancerEur J Cancer20114781138114521220198
- ZhangLNXiaoWWXiSYPathological assessment of the ajcc tumor regression grading system after preoperative chemoradiotherapy for chinese locally advanced rectal cancerMedicine2016953e227226817863
- TrakarnsangaAGönenMShiaJComparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatmentJ Natl Cancer Inst201410610
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- MandardAMDalibardFMandardJCPathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlationsCancer19947311268026868194005
- BeckerKMuellerJDSchulmacherCHistomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapyCancer20039871521153014508841
- LowyAMMansfieldPFLeachSDPazdurRDumasPAjaniJAResponse to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancerAnn Surg1999229330330810077040
- RödelCGrabenbauerGGKühnRCombined-modality treatment and selective organ preservation in invasive bladder cancer: long-term resultsJ Clin Oncol200220143061307112118019
- BraunOMNeumeisterBPoppWHistologic tumor regression grades in squamous cell carcinoma of the head and neck after preoperative radiochemotherapyCancer1989636109711002492898
- ShiaJGuillemJGMooreHGPatterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcomeAm J Surg Pathol200428221522315043311
- SineshawHMJemalAThomasCRMitinTChanges in treatment patterns for patients with locally advanced rectal cancer in the United States over the past decade: An analysis from the National Cancer Data BaseCancer2016122131996200327074300
- SauerRLierschTMerkelSPreoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 yearsJ Clin Oncol201230161926193322529255