Abstract
Background
The patients with hepatocellular carcinoma (HCC) have poor prognosis due to being diagnosed at late stage or recurrence following surgery. It’s critical to identify effective biomarkers that can improve overall diagnosis and treatment of HCC.
Methods
We performed a meta-analysis of all relative studies reporting the clinicopathological significance of CDH1 hypermethylation in HCC by using Review Manager 5.2. A comprehensive literature search was performed in EMBASE, PubMed, Web of Science and Google Scholar databases. Kaplan Meier Plotter online database was used for the determination of correlation between CDH1 mRNA expression and overall survival in patients with HCC. Odds Ratios (OR) with 95% corresponding confidence intervals (CIs) were calculated. A total of 12 relevant studies were included in the meta-analysis with 981 patients.
Results
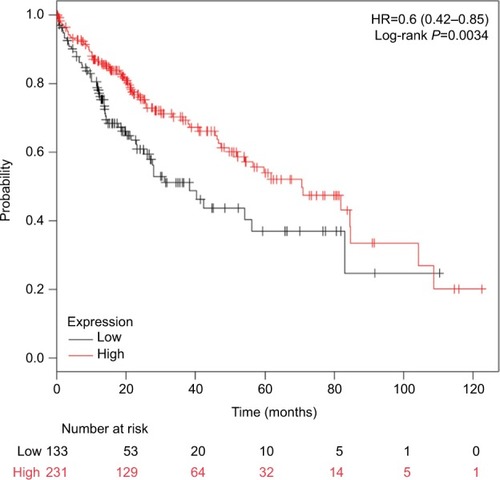
The positive rate of CDH1 hypermethylation was significantly higher in HCC than in normal liver tissue; and the pooled OR was 4.34 with 95% CI 2.50–7.56, P<0.00001. CDH1 promoter in HCC was more frequently hypermethylated compared to the group of chronic liver disease (CLD); OR was 4.83 with 95% CI 2.67–8.72, P<0.00001. However, the rate of CDH1 promoter hypermethylation was not correlated with different grades as well as stages. High CDH1 mRNA expression was significantly correlated to better overall survival in all 231 HCC patients compared to 133 HCC patients with low level CDH1 mRNA expression; HR was 0.6 with 95% CI 0.42–0.85, P=0.0034.
Conclusion
In summary, CDH1 promoter hypermethylation is a risk factor and promising biomarker for HCC carcinogenesis and diagnosis, as well as a predictor of poor prognosis.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the fourth leading cause of cancer mortality globally.Citation1 The patients with HCC have a poor prognosis due to being diagnosed at late stage or recurrence following surgery.Citation2 The only curative therapies are liver transplantation and resection. In order to qualify these two treatments, patients with HCC need to be diagnosed at early stage.Citation3 On the other hand, there is no good prognostic marker to predict survival outcome apart from tumor staging classification system. Therefore, it is very critical to find effective biomarkers for early diagnosis and biomarkers to predict prognosis.
Cadherins were defined as cell surface glycoproteins responsible for the Ca2+-dependent cell–cell adhesion mechanism,Citation4,Citation5 including E-cadherin, neural (N-) cadherin, and the placental (P-) cadherin.Citation5 E-cadherin functions as a mediator of cell–cell adhesion and the principal organizer of epithelial phenotype.Citation6,Citation7 Its adhesive function at cell surface holds cells together, facilitates other cell–cell interactions, and physically blocks the movement of cells. Additionally, E-cadherin homophilic binding leads to contact-mediated inhibition of growth via modulation of growth inhibitory signals including the Hippo, growth factor receptor tyrosine kinase (RTK), and Src family kinase signaling pathways. On the other hand, the cytoplasmic tail of E-cadherin is associated with intracellular molecules of alpha-, beta-, and gamacateninsCitation8,Citation9 that link to the cytoskeleton and mediate downstream growth factor signaling pathways such as Wnt, transforming growth factor β, and nuclear factor kappa-light-chain-enhancer of activated B cells.Citation10–Citation13 Loss of E-cadherin expression leads to induction of the epithelial to mesenchymal transition (EMT), causing cells to lose cell–cell contacts and acquire increased motility to spread into surrounding or distant tissues, also upregulates growth factor pathways and promoting proliferation. Therefore, loss of E-cadherin plays a crucial role in hepatocellular carcinogenesis and metastasis.Citation14 Cadherin-1 (CDH1) is the gene for E-cadherin, and its downregulation with hypermethylation of CpG islands of region promoter is found in various tumors originating from epithelial cells including HCC.Citation15,Citation16 Aberrant DNA methylation has been demonstrated in common HCC,Citation17,Citation18 and recent studies showed that CpG methylation around the promoter is very important in the transcriptional inactivation of CDH1 in HCC.Citation15 However, the positive rate of CDH1 promoter hypermethylation in HCC is inconsistent due to small size of samples in individual study.
In the present study, we conducted a meta-analysis study using the qualified studies and determined the association between CDH1 hypermethylation in HCC and its progression as well as prognosis.
Methods
Selection criteria and study search
We searched the three electronic databases EMBASE, PubMed, and Web of Science from the earliest date up to June 2018 for appropriated articles addressing the focused question. Electronic database searches were conducted by using the following terms: “liver,” “hepatocellular” and “cancer or tumor or neoplasm or carcinoma,” “methylation,” and “CDH1 or E-cadherin.” Additional studies were searched manually from the reference list of included articles. There were 37 articles from EMBASE, 73 articles found from PubMed, as well as 36 articles from Web of Science.
A total of 146 articles were reviewed and screened by article titles and abstracts. Studies were eligible for inclusion in the current analysis if they met the following criteria: 1) studies that evaluated the association between CDH1 hyper-methylation and clinicopathological parameters of HCC; 2) CDH1 hypermethylation detected in the primary HCC tissues; and 3) studies published in English or Chinese. The exclusion criteria were as follows: 1) expert opinion, case reports, reviews, conference abstracts, editorials, letters; 2) all publications regarding cell lines, in vitro/ex vivo studies, and human xenografts; 3) studies in which same population was involved; and 4) studies in which CDH1 protein expression was evaluated.
After exclusion of non-relevant and/or redundant publications from different databases, the 17 remaining papers were selected and reviewed in the full-text version for inclusion and exclusion criteria. Five articles were excluded due to lack of sufficient data for the present study.
Data extraction and study assessment
Two independent authors (XW and XY) extracted the data from the included studies. Disagreement regarding selection was resolved through discussion. If they could not reach an agreement, a third reviewer (LS) was consulted. The following information was collected from each study: year of publication, the first author name, sample source, number of cases, cancer TNM (tumor-node-metastasis) stage, methylation detection method, and CDH1 methylation status. Data for study characteristics and clinical responses were collected and organized into a standard table format. Heterogeneity of the included studies was assessed to determine whether or not the data of the various studies could be analyzed for a meta-analysis.
The assessment of methodological quality of included studies was done by two assessors based on a grading system developed by the Newcastle Ottawa Quality Assessment Scale (NOQAS).Citation19 The three reviewers graded the quality and compared them, and then they reached an agreement for each item. Those scales use a grading system to evaluate on comparability, the selection of quality, exposure, and outcomes for study participants. The NOQAS scores ranged between 0 and 9, and a study with a score of 7 or more indicated a good quality.
Statistical analysis
The meta-analysis was performed using Review Manager 5.2 (Cochrane Collaboration, Update Software, Oxford, UK). ORs with its 95% CIs were calculated. All the events represent the number of HCC with CDH1 hypermethylation. The evaluation of statistical heterogeneity was finished by using the Cochran’s Q statistic and I2 tests. When the I2 value was below 50%, fixed-effect model was generated, and when the I2 value was 50% or greater, a random-effect model was generated. Sensitivity analysis was used for exploring the reasons of statistical heterogeneity. The pooled frequency of CDH1 hypermethylation and 95% CIs were estimated. The rate of CDH1 hypermethylation was compared between different groups by tumor features. The pooled OR was estimated for the correlation between CDH1 hypermethylation and clinicopathological features. Overall survival was analyzed by using an online database Kaplan premier plotter that was established by using gene expression and the survival information of 364 liver cancer patients (http://kmplot.com/analysis/index.php?p=service&cancer=liver_rnaseq).Citation20 P-value less than 0.05 was regarded statistically significant. Publication bias was evaluated by using funnel plots reported by Egger et al.Citation21
Results
Identification of relevant studies and study quality
Twelve studies were included in the meta-analysis after screening 146 studies (). The following items were recorded from each study: first author, published year, country, tumor histology, CDH1 methylation status, differentiation and stages of tumors, as well as methylation detection methods ().
Table 1 Main characteristics of included studies
The correlation of CDH1 promoter hypermethylation with clinicopathological features
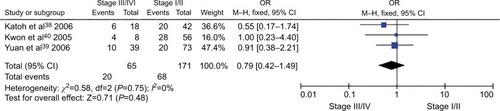
The frequency of CDH1 promoter hypermethylation was significantly higher in HCC than in normal liver tissue; the pooled OR was 4.34 with 95% CI 2.50–7.56, z=5.19, P<0.00001, I2=0% (). CDH1 promoter in HCC was more frequently hypermethylated compared to the group of chronic liver disease (CLD); OR was 4.83 with 95% CI 2.67–8.72, z=5.22, P<0.00001, I2=0% (). However, the rate of CDH1 promoter hypermethylation was not correlated with different grades; OR was 0.84 with 95% CI 0.40–1.78, z=0.46, P=0.65, I2=0% (). The rate of CDH1 promoter hypermethylation was not significantly different between early and advanced stages; OR was 0.79 with 95% CI 0.42–1.49, z=0.71, P=0.48, I2=0% (). High CDH1 mRNA expression was strongly associated with better overall survival in all 231 HCC patients compared to 133 HCC patients with low-level CDH1 mRNA expression; HR was 0.6 with 95% CI 0.42–0.85, P=0.0034 ().
Figure 2 Forest plot for CDH1 promoter hypermethylation in HCC and normal liver tissue.
Notes: The squares represent the weight of individual study in the meta-analysis and the line width indicates the corresponding 95% CI. The diamond represents the pooled OR and the width of diamond indicates 95% CI.
Abbreviations: HCC, hepatocellular carcinoma; NLT, normal liver tissue; M–H, Mantel–Haenszel.
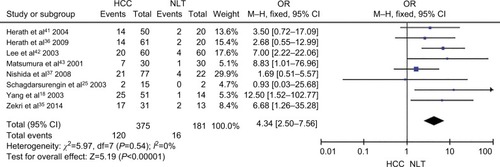
Figure 3 Forest plot for CDH1 promoter hypermethylation in HCC and CLD.
Notes: The squares represent the weight of individual study in the meta-analysis and the line width indicates the corresponding 95% CI. The diamond represents the pooled OR and the width of diamond indicates 95% CI.
Abbreviations: CLD, chronic liver disease; HCC, hepatocellular carcinoma; M–H, Mantel–Haenszel.
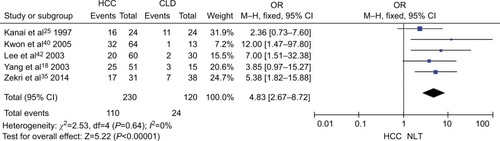
Figure 4 Forest plot for CDH1 promoter hypermethylation in high and low grade of histological differentiation.
Notes: The squares represent the weight of individual study in the meta-analysis and the line width indicates the corresponding 95% CI. The diamond represents the pooled OR and the width of diamond indicates 95% CI.
Abbreviation: M–H, Mantel–Haenszel.
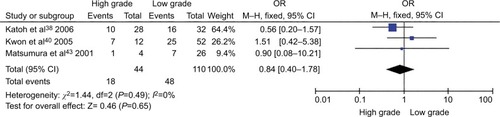
Figure 5 Forest plot for CDH1 promoter hypermethylation in different stage of HCC.
Notes: The squares represent the weight of individual study in the meta-analysis and the line width indicates the corresponding 95% CI. The diamond represents the pooled OR and the width of diamond indicates 95% CI.
Abbreviations: HCC, hepatocellular carcinoma; M–H, Mantel–Haenszel.
Sensitivity analyses and publication bias
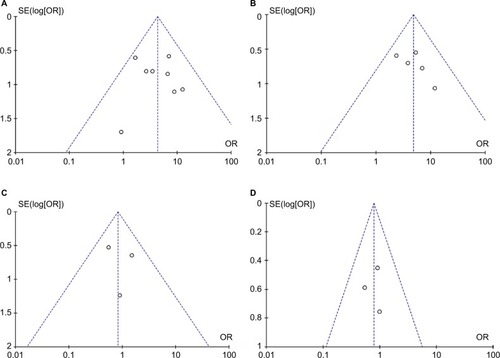
The quality of each study was evaluated using the NOQAS. This scale for non-randomized case-controlled studies and cohort studies was used to evaluate the quality of selection, comparability, exposure, and outcomes for study participants, with the maximum score being 9 points. Among the 12 studies, three of them scored 8 points, seven scored 7 points, and two scored 6 points (data not shown). The score for each study showed a relatively high quality. A sensitivity analysis was performed by removing one study at a time; the result remained stable, indicating the stability of the present analysis. The funnel plots were largely symmetric (), indicating there were no publication biases in the meta-analysis of CDH1 methylation and clinicopathological features.
Figure 7 Funnel plot for publication bias.
Notes: (A) CDH1 promoter hypermethylation in HCC and normal liver tissue; (B) CDH1 promoter hypermethylation in HCC and CLD; (C) CDH1 promoter hypermethylation in high and low grade of histological differentiation; (D) CDH1 promoter hypermethylation in different stage of HCC. Area of the circle represents the weight of individual study.
Abbreviations: CLD, chronic liver disease; HCC, hepatocellular carcinoma; SE, standard error.
Discussion
E-cadherin, a CaCitation2+-dependent cell adhesion molecule, encoded by CDH1, regulates cell polarity and tissue morphology.Citation8 Disturbance of cell polarity and destruction of normal tissue morphology initiate carcinogenesis. Knocking-down of CDH1 results in the activation of oncogenic signaling pathways and leads to the transition of epithelial to mesenchymal in the development of epithelial tumor.Citation22–Citation24 Cancer invasion is initiated by the dissociation of cells from primary cancer nests due to loosened cell adhesion. Recent studies reported that CDH1 promoter hypermethylation was observed in HCC. However, the frequency varied from 13.3% to 66.7%.Citation25,Citation26 We pooled nine studies including 580 patients, and evaluated the frequency of CDH1 promoter hypermethylation. The frequency of CDH1 promoter hypermethylation in HCC was 32% – 4.34 times higher than the one in normal liver tissue. Additionally, the rate of CDH1 promoter hypermethylation in HCC was 4.83 times higher than the one in CLD. Our findings suggest that CDH1 promoter hypermethylation could be a biomarker for HCC diagnosis. DNA methyltransferases (DNMTs) are pivotal regulators of the methylation of CpG islands. Previous evidence indicated that increased expression of DNMT1 was correlated with the stages, portal venous invasion, and prognosis of HCC.Citation27,Citation28 It has been reported that inhibitors of DNA methyltransferase inhibitors (DNMTis) such as 5-Aza-2′-deoxycytidine (5-Aza-CdR) and 5-fluoro-2-deoxycytidine (FdCyd) are applied to human breast and lung cancer cells, and currently FdCyd is in clinical trials for the treatment of breast cancer and other solid tumors.Citation29–Citation31 Therefore, inhibitors for DNA methylation could be a new therapy strategy for HCC patients with CDH1 promoter hypermethylation.
Overall survival was evaluated by using an online database Kaplan–Meier Plotter that was generated by using mRNA expression and the survival data of 364 liver cancer patients (http://kmplot.com/analysis/index.php?p=service&cancer=liver_rnaseq). Our findings showed that low expression of E-cadherin mRNA was significantly correlated with poor overall survival in HCC patients. Gene methylation is a major mechanism to inactivate gene in HCC.Citation18 Thus, CDH1 promoter hypermethylation could be a predictor of poor overall survival. Previous evidence revealed that EMT is an important process correlated with the propensity for invasion and metastases of many types of tumors including HCC.Citation32–Citation34 The process of EMT is initiated by suppression of the epithelial markers such as E-cadherin. Loss of E-cadherin function initiates the dissociation of cells from primary cancer nests because of loosened intercellular adhesion, thus resulting in invasion and metastasis. Therefore, CDH1 promoter hypermethylation in HCC leads to a poor overall survival in patients with HCC. However, our find ings showed that CDH1 promoter hypermethylation was not significantly associated with HCC differentiation and stages. The evaluation needs to be performed when more relative studies are available in future.
Limitations
There were a few limitations of the present study. First, the selection bias was unavoidable due to the selection restricted to the articles published in English and Chinese. Second, the heterogeneity existed in the frequency of CDH1 promoter hypermethylation between HCC and normal liver tissue. This phenomenon may be caused by the moderate number of samples in the involved studies and inconsistent criteria in selection of controls. Further evaluation with a larger size of samples will be carried out in future for a more reliable conclusion.
Conclusion
In summary, CDH1 promoter hypermethylation is a promising biomarker for HCC diagnosis and a predictor of poor prognosis.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. The corresponding author had full access to all data and the final responsibility for the decision to submit the article for publication.
Acknowledgments
This work was supported by the National Science Foundation of China (81373990, 81402523, 81672990 to XYW), Jiangsu Province “Six talents” high peak plan (2015-WSN-052 to XYW), Project of Jiangsu Provincial Bureau of Traditional Chinese Medicine (JD201510 to XYW and XQY), and the six “1” Project of Jiangsu Province (LGY2016012 to XYW). The funding institutions did not have any roles in the study design, data collection, or analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- AlacaciogluASomaliISimsekIEpidemiology and survival of hepatocellular carcinoma in Turkey: outcome of multicenter studyJpn J Clin Oncol2008381068368818753360
- VillanuevaAMinguezBFornerAReigMLlovetJMHepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapyAnnu Rev Med20106131732820059340
- YangJDRobertsLRHepatocellular carcinoma: A global viewNat Rev Gastroenterol Hepatol20107844845820628345
- TakeichiMCadherins: a molecular family essential for selective cell-cell adhesion and animal morphogenesisTrends Genet198738213217
- TakeichiMHattaKNoseANagafuchiAIdentification of a gene family of cadherin cell adhesion moleculesCell Differ Dev198825Suppl91943061598
- GumbinerBStevensonBGrimaldiAThe role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complexJ Cell Biol19881074157515873049625
- GumbinerBMRegulation of cadherin-mediated adhesion in morphogenesisNat Rev Mol Cell Biol20056862263416025097
- TakeichiMCadherin cell adhesion receptors as a morphogenetic regulatorScience19912515000145114552006419
- MareelMBoterbergTNoëVE-cadherin/catenin/cyto-skeleton complex: a regulator of cancer invasionJ Cell Physiol199717322712749365535
- PaduaDMassaguéJRoles of TGFbeta in metastasisCell Res20091918910219050696
- QianXKarpovaTSheppardAMMcnallyJLowyDRE-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinasesEmbo J20042381739178415057284
- OzawaMBaribaultHKemlerRThe cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different speciesEmbo J198986171117172788574
- MendonsaAMNaTYGumbinerBME-cadherin in contact inhibition and cancerOncogene201837354769478029780167
- GiannelliGKoudelkovaPDituriFMikulitsWRole of epithelial to mesenchymal transition in hepatocellular carcinomaJ Hepatol201665479880827212245
- YoshiuraKKanaiYOchiaiAShimoyamaYSugimuraTHirohashiSSilencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomasProc Natl Acad Sci U S A19959216741674197543680
- GraffJRHermanJGLapidusRGE-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomasCancer Res19955522519551997585573
- HuangJCurrent progress in epigenetic research for hepatocarcinomagenesisSci China C Life Sci2009521314219152082
- YangBGuoMHermanJGClarkDPAberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinomaAm J Pathol200316331101110712937151
- WellsGASheaBO’ConnellDThe Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-AnalysesOttawa, ONOttawa Hospital Research Institute2013
- GyorffyBSurowiakPBudcziesJLanczkyAOnline survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancerPLoS One8e82241
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- van RoyFBerxGThe cell-cell adhesion molecule E-cadherinCell Mol Life Sci200865233756378818726070
- BerxGvan RoyFInvolvement of members of the cadherin superfamily in cancerCold Spring Harb Perspect Biol200916a00312920457567
- RodriguezFJLewis-TuffinLJAnastasiadisPZE-cadherin’s dark side: possible role in tumor progressionBiochim Biophys Acta201218261233122440943
- KanaiYUshijimaSHuiAMThe E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomasInt J Cancer19977133553599139867
- SchagdarsurenginUWilkensLSteinemannDFrequent epigenetic inactivation of the RASSF1A gene in hepatocellular carcinomaOncogene200322121866187112660822
- OhBKKimHParkHJDNA methyltransferase expression and DNA methylation in human hepatocellular carcinoma and their clinicopathological correlationInt J Mol Med2007201657317549390
- SaitoYKanaiYNakagawaTIncreased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomasInt J Cancer2003105452753212712445
- BeumerJHPariseRANewmanEMConcentrations of the DNA methyltransferase inhibitor 5-fluoro-2′-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU)Cancer Chemother Pharmacol200862236336817899082
- GowherHJeltschAMechanism of inhibition of DNA methyl-transferases by cytidine analogs in cancer therapyCancer Biol Ther20043111062106815539938
- NewmanEMMorganRJKummarSA phase I, pharmacokinetic, and pharmacodynamic evaluation of the DNA methyltransferase inhibitor 5-fluoro-2′-deoxycytidine, administered with tetrahydrouridineCancer Chemother Pharmacol201575353754625567350
- GiannelliGBergaminiCFransveaESgarraCAntonaciSLaminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinomaGastroenterology200512951375138316285938
- GaoDVahdatLTWongSChangJCMittalVMicroenvironmental regulation of epithelial-mesenchymal transitions in cancerCancer Res201272194883488923002209
- LeeTKPoonRTYuenAPTwist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transitionClin Cancer Res200612185369537617000670
- ZekriARBahnasyAAShoeabFEMethylation of multiple genes in hepatitis C virus associated hepatocellular carcinomaJ Adv Res201451274025685469
- HerathNIPurdieDMKewMCVarying etiologies lead to different molecular changes in Australian and South African hepatocellular carcinomasInt J Oncol20093551081108919787262
- NishidaNNagasakaTNishimuraTIkaiIBolandCRGoelAAberrant methylation of multiple tumor suppressor genes in aging liver, chronic hepatitis, and hepatocellular carcinomaHepatology200847390891818161048
- KatohHShibataTKokubuAEpigenetic instability and chromosomal instability in hepatocellular carcinomaAm J Pathol200616841375138416565510
- YuanYWangJLiJFrequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinomaClin Cancer Res200612226687669517121887
- KwonGYYooBCKohKCChoJWParkWSParkCKPromoter methylation of E-cadherin in hepatocellular carcinomas and dysplastic nodulesJ Korean Med Sci200520224224715831995
- HerathNIWalshMDKewMCYoungJLeggettBAMacdonaldGACadherin/catenin complex appears to be intact in hepatocellular carcinomas from Australia and South AfricaJ Gastroenterol Hepatol200419667668215151624
- LeeSLeeHJKimJHLeeHSJangJJKangGHAberrant CpG island hypermethylation along multistep hepatocarcinogenesisAm J Pathol200316341371137814507645
- MatsumuraTMakinoRMitamuraKFrequent down-regulation of E-cadherin by genetic and epigenetic changes in the malignant progression of hepatocellular carcinomasClin Cancer Res20017359459911297254