Abstract
Purpose
Following surgery for early stage non-small-cell lung cancer (NSCLC), de novo pulmonary tumors are common. This study aimed to assess the efficacy, patterns of failure, and toxicity of stereotactic body radiotherapy (SBRT) in the treatment of de novo pulmonary tumors following curative resection of early stage NSCLC.
Patients and methods
We reviewed the medical data of patients who had received definitive intent SBRT for small lung cancer at Zhongshan Hospital, Fudan University, between June 2011 and December 2017. Patients who had experienced complete resection for prior early stage NSCLC before SBRT were identified for further analysis. Incidences of locoregional recurrence (LR) and distant metastasis (DM) were evaluated using the alternative cumulative incidence competing risk method. The probability of survival was estimated using the Kaplan–Meier method.
Results
A total of 33 patients with 36 lesions were eligible and included in this study. The median follow-up time was 32 months. Estimated incidences of LR and DM were 37.62% and 15.92%, respectively, at 1 year and 48.02% and 21.23%, respectively, at 2 years. The progression-free survival and overall survival of all patients were 62.40% and 90.30%, respectively, at 1 year and 52.00% and 69.90%, respectively, at 2 years. In all, 26 patients experienced grade 1 SBRT-related toxicity, 11 patients experienced grade 2 SBRT-related toxicity, and three patients experienced grade 3 toxicity. There were no grade 4/5 toxicities or SBRT-related deaths during the follow-up period.
Conclusion
SBRT appears to be a safe and potentially effective alternative therapeutic option for de novo pulmonary tumors following early stage NSCLC radical resection, despite impaired pulmonary reserve.
Introduction
Lung cancer is one of the leading causes of cancer worldwide. Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, representing ~85% of all cases.Citation1 Surgery is the preferred treatment modality for patients with resectable NSCLC and results in 5-year overall survival (OS) rates ranging from 24% to 73%.Citation2 Extended survival outcomes after curative resection of early stage NSCLC come with an increased risk for development of novel pulmonary tumors, which are associated with a particularly poor prognosis.Citation3–Citation7 Surgical resection remains the preferred modality for those patients who developed pulmonary tumors following curative resection and has yielded considerable survival achievement;Citation7–Citation11 however, a large proportion of cases are not suited for repeated pulmonary resection due to inadequate postoperative pulmonary reserve, and there are few other options for further treatment.Citation12–Citation14
Stereotactic body radiotherapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), has been recommended as a standard alternative treatment to surgery for early stage NSCLC patients who are not surgical candidates. This type of radiotherapy (RT) has been shown to achieve local control rates (LCRs) comparable to those seen with radical resection in multiple prospective trials.Citation15–Citation20 Moreover, SBRT yields better clinical outcomes for early stage NSCLC than conventional fractionated RT.Citation6,Citation7,Citation20,Citation21 In addition, several reports support the efficacy of SBRT for multiple primary lung cancers (MPLCs).Citation16,Citation17,Citation22 However, the efficacy and safety of SBRT in patients with de novo pulmonary tumors after lung resection remain unclear. The pulmonary reserve of this patient population is impaired, which limits the ability of these patients to tolerate further antitumor therapy. Therefore, we conducted this retrospective study to evaluate our experience regarding the therapeutic effectiveness, feasibility, and safety of SBRT for de novo pulmonary tumors in patients with completely resected early stage NSCLC.
Patients and methods
Patient population
A review was conducted of the medical data of patients who had received definitive intent SBRT for small lung cancer between June 2011 and December 2017 at Zhongshan Hospital, Fudan University. Patients who had experienced complete resection for the prior early stage NSCLC before SBRT were identified for further analysis. A multidisciplinary tumor board team, including at least a thoracic radiologist, thoracic surgeon, pulmonologist, and pathologist, determined the diagnosis and treatment strategy of the de novo pulmonary tumors. Inclusion criteria were as follows: 1) margin-negative radical resection (R0) for the prior lung cancer with postoperative pathological confirmation; 2) a clinical presentation consistent with malignant tumor based on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan before SBRT in patients without pathological confirmation of de novo pulmonary nodules; 3) three or less lesions confined to the lung; 4) no evidence of regional lymph node metastases, mediastinal spread, or systemic metastases at the time of SBRT; 5) treatment with curative intent; 6) no antitumor therapy other than SBRT until the disease progression; and 7) at least 6 months of follow-up. Patients who had other malignancies in other sites or had an Eastern Cooperative Oncology Group (ECOG) performance status of >2 were excluded.
A total of 33 patients with 36 lesions met the eligibility criteria for inclusion in this study. Medical records were reviewed to obtain each patient’s baseline characteristics and clinical and therapeutic data. During the data screening process, patients with >5% missing data were excluded. We staged every lesion for each patient independently according to the tumor, node, and metastasis system based on the American Joint Committee on Cancer (AJCC) seventh edition and retrospectively coded the lesions according to the AJCC eighth edition.Citation14 The interval between the two lesions was defined as the time between the date of surgery for the first tumor and the date of radiological diagnosis of the de novo pulmonary lesion.Citation17,Citation23
This retrospective study was approved by the Zhongshan Hospital Ethics Committee. Written informed consent was obtained from each patient for the use of his or her clinical data in clinical studies.
SBRT techniques
Our institution’s SBRT treatment planning and delivery method has been previously described.Citation24 Briefly, each patient was immobilized in the supine position with arms overhead using a customized vacuum cushion. All patients underwent respiration-correlated helical four-dimensional (4D)-computed tomography (CT) scans with a 3 mm slice thickness under free quiet respiration using a 16-slice CT scanner (Siemens Somatom CT, Sensation Open; Siemens Healthcare, Munich, Germany). A breath-hold or respiration-gated technique was considered for cases with tumor movement of >1 cm in any direction. The gross tumor volume (GTV) was defined as a lesion visible in the lung window on CT and/or PET/CT. There was no clinical target volume (CTV) construction. The internal target volume (ITV) was created based on the maximum-intensity projection image obtained in the 4D-CT. The planning target volume (PTV) was generated by adding a uniform 5 mm margin expansion to the ITV for setup uncertainty. Dose constraints for the organs at risk (OAR) were based on the Radiation Therapy Oncology Group (RTOG) 0236 guidelines.Citation19 All SBRT treatments were administered using a Helical Tomotherapy (HT) Hi-Art Treatment System (Accuray, Madison, WI, USA). Daily image-guided RT was performed with a megavoltage computed tomography (MVCT) scan before each treatment, and automatic adjustments were made to confirm the position of the tumor throughout the course of treatment. Dose/fractionation schedules were applied depending on the tumor size, tumor location, and lung function parameters. A total dose of 50–65 Gy in five to 10 fractions was delivered. Treatments were performed over 5–14 days (median 10 days).
Follow-up evaluations
Follow-up evaluations after SBRT were conducted based on regular CT scans of the chest and clinical examinations that were obtained from the medical records. These evaluations were acquired every 3 months during the first 2 years, then every 6 months for another 3 years, and annually thereafter. An 18F-FDG PET/CT scan was performed if clinical recurrence or metastasis was suspected. The follow-up started on the first date of SBRT and ended on May 31, 2018. Clinical response to therapy was assessed 6 months after SBRT based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Locoregional recurrence (LR) after SBRT was defined as a new lesion reappearance in the radiation field, in the same lobe, or in the ipsilateral hilar/mediastinal lymph nodes. All other sites of failure were considered as distant metastasis (DM). The follow-up time was calculated from the initial date of SBRT to the date of the last followup visit. OS was calculated from the initial date of SBRT to the date of death from any cause. Progression-free survival (PFS) was calculated from the initial date of SBRT to the date of disease progression or the date of death. Patients were censored at the date of the last available follow-up if alive. A radiation oncologist or pulmonologist diagnosed radiation pneumonitis (RP) based on clinical symptoms and radiographic changes that occurred during the first 12 months after completion of SBRT and if there was no evidence indicating other competing diagnoses. The National Cancer Institute’s Common Toxicity Criteria (CTC) Version 3.0 was used for grading adverse events.Citation25
Statistical analyses
Continuous variable data were summarized as medians and ranges. Categorical variable data were expressed as percentages. The median follow-up time was calculated using the reverse Kaplan–Meier method. OS and PFS were calculated using the Kaplan–Meier method. LR and DM rates were evaluated using the alternative cumulative competing risk method, with death as a competing risk. Statistical analyses were performed using SPSS statistical software (version 23.0; IBM Corporation, Armonk, NY, USA) and R statistical software (version 3.4.4) using the cmprsk and survival packages (R Foundation, Vienna, Austria).
Results
Patients’ characteristics
A total of 33 patients with 36 lesions were eligible and included in this study. There were three patients with two intrapulmonary lesions, and all received SBRT for both lesions. Only one tumor had marginal recurrences to atypical resections, whereas other tumors were de novo pulmonary tumors apart from the surgical sites. All patients completed the course of SBRT without unscheduled interruption. The median follow-up was 32 months (range, 8–84 months) for all patients and 36 months for living patients (range, 8–84 months). In 17 patients, the time between the first tumor and the second novel lesion was less than 2 years, and in the remaining 16 patients, it was longer than 2 years. At the end of the follow-up period, 15 patients developed recurrence or metastasis. Baseline demographics, clinical characteristics, and treatment characteristics are summarized in .
Table 1 Baseline characteristics (N=33)Table Footnotea
Local control and patterns of failure
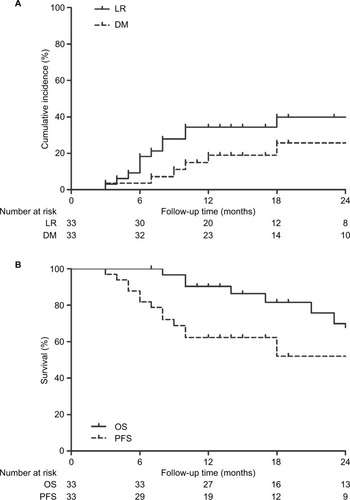
Six months after the completion of SBRT, complete response (CR) was reported for five (13.89%) lesions, partial response (PR) for 14 (38.89%) lesions, stable disease (SD) for 10 (27.78%) lesions, and progression disease (PD) for 7 (19.44%) lesions. The tumor response rate was 52.78%. By the end of the follow-up periods, five (15.15%) patients developed LR combined with DM, eight (24.24%) patients developed LR alone, and two (6.06%) patients developed DM alone. The 1- and 2-year cumulative incidences of LR were 37.62% (95% CI, 36.09%–39.15%) and 48.02% (95% CI, 46.00%–50.04%), respectively. The corresponding incidences of DM were 15.92% (95% CI, 15.06%–16.78%) and 21.23% (95% CI, 19.92%–22.54%), respectively (). Among patients with LR, six (18.18%) developed LR within the radiation field or the involved lobe and seven (21.21%) developed regional lymph node recurrence. Bone (two cases, 6.06%) and liver (two cases, 6.06%) were the most common sites of DM, followed by the contralateral lung (one case, 3.03%), brain (one case, 3.03%), adrenal gland (one case, 3.03%), and celiac lymph nodes (one case, 3.03%). All patients with PD received further treatment for the recurrence or metastasis.
Figure 1 Cumulative incidences of LR and DM for all patients following SBRT (A) and Kaplan–Meier curves showing OS and PFS for all patients following SBRT (B).
Abbreviations: DM, distant metastasis; LR, locoregional recurrence; OS, overall survival; PFS, progression-free survival; SBRT, stereotactic body radiotherapy.
Survival
In all, 25 patients were alive at the time of the last followup. Of these patients, 18 were alive with no evidence of disease. Among the eight deaths, four patients died as a result of systemic spread, two patients died of local control failure, and two patients died of other cancer-related pathology. The median PFS was 53 months (95% CI, 5.60–100.40 months) for all patients, whereas the median estimated OS was not reached. The 1-year and 2-year PFS rates for all patients were 62.40% (95% CI, 45.54%–79.26%) and 52.00% (95% CI, 32.79%–71.21%), respectively, and the 1-year and 2-year OS rates were 90.30% (95% CI, 79.90%–100.69%) and 69.90% (95% CI, 50.30%–89.50%), respectively ().
Toxicity evaluation
Adverse events were carefully recorded during the treatment and follow-up period (). In all, 26 patients experienced grade 1 SBRT-related toxicity, 11 patients experienced grade 2 toxicity, and three patients experienced grade 3 toxicity (). There were no grade 4/5 toxicities or SBRT-related deaths during the follow-up period.
Table 2 Adverse effects after SBRT
Discussion
With the development of diagnostic modalities and treatment innovations, survival outcomes in patients with resected early stage NSCLC have improved. However, the risk of developing a new lesion/disease recurrence in this population is also steadily increasing.Citation5,Citation26,Citation27 Although a surgical approach remains the frontline treatment for de novo tumors after lung resection, a significant proportion of cases do not qualify for additional surgery due to the impaired pulmonary reserve.Citation8,Citation9 The results presented here demonstrate that SBRT is a safe and potentially effective alternative therapeutic option for de novo pulmonary tumors following lung cancer surgical resection. Over the course of the study, with a median follow-up of 32 months, there were no grade 4/5 toxicities or SBRT-related deaths. The 2-year OS and PFS rates of all patients were 66.90% and 52.00%, respectively, with a tumor response rate of 52.78% at 6 months.
There are currently no systematic or authoritative guidelines for the treatment of de novo pulmonary tumors in patients with completely resected early stage first lung cancer. Zuin et al assessed clinical outcomes for different operation methods in the treatment of second primary lung cancer (SPLC) in patients who had received pulmonary resection.Citation23 They reported that the overall 5-year survival rate following the second surgery was 42%. Additionally, Stella et alCitation28 described the surgical management for pulmonary metastasis and SPLC after curative resection for the index lung cancer. The 2-year OS rate after the second resection of pulmonary metastasis was 29%, whereas the corresponding survival rate of SPLC was 81%, with a 2-year OS rate of ~60%. The overall 2-year survival rate of 33 patients after SBRT in our cohort was 69.90%, which appeared to be slightly higher than that of surgical resection.Citation28 Although the subjects in our study were medically inoperable, these data suggest that clinical outcomes of SBRT for de novo pulmonary tumors after curative resection were not inferior to those of additional surgery. Previous retrospective studies of the role of SBRT in patients with early stage MPLC found 2-year OS rates of 73.2%, 56.0%, and 58.5%, which were slightly higher or comparable to that of our patients.Citation17,Citation22,Citation29 However, some of the de novo tumors in our patients were pulmonary metastases from the prior lung cancer, which tend to have poorer prognosis than MPLC.Citation30,Citation31 Therefore, the present data and prior reports lead us to believe that SBRT can be a safe and effective alternative to further pulmonary resection for de novo pulmonary tumors after NSCLC radical resection when repeated surgery is not possible due to poor pulmonary reserve.
Of note, LR and DM rates in this current analysis were inferior to those reported previously in patients undergoing SBRT for early stage NSCLC.Citation27,Citation32 In the series of Horne et al,Citation33 the 2-year LCR of SBRT for residual/recurrent and new primary NSCLC was 78.4%. The LR and DM rates that we observed here may be attributable to several factors, including poor pulmonary function capacity, stage III or IV disease de novo tumors due to pulmonary recurrences or metastases, and because almost 40% of our patients received a biologically effective dose (BED) of <100 Gy. As the radiation dose is crucial for local tumor control and a BED of >100 Gy to the target volume is needed to achieve optimal local control,Citation19,Citation34 we compared the survival of patients who did and did not receive ≥100 Gy. We did not detect any difference in OS (P=0.667) or PFS (P=0.603) between these two groups. The reason for this phenomenon may be attributed to the small sample size of our patients. In addition, there were six patients in the current study who developed disease progression within 6 months following SBRT. This short interval suggests that microscopic metastatic disease may have been present at the time of SBRT. All patients in our study received SBRT delivered via HT, which is associated with excellent clinical outcome and normal tissue sparing because of its sharp dose gradient. HT-based SBRT for primary lung cancer showed improved LCR for primary lung cancer. The 2-year LCR was 97% for primary lung cancer using HT, whereas that for linac-based SBRT waŝ87%.Citation35 In our previous report, 3-year LCR was >90% for stage I lung cancer via HT.Citation25 It is essential to accurately differentiate SPLC from pulmonary metastases or recurrence before performing SBRT because the prognosis and treatment are markedly different.Citation35 MPLC was first described by BeyreutherCitation36 in 1924. The most recent widely used and recognized criteria for diagnosing MPLC are still those outlined by Martini and Melamed in 1975.Citation37 These criteria have recently been revised and updated in the American College of Chest Physicians Lung Cancer Guideline. The update extended the time interval, further differentiated pathological subtypes, and added clinical assessment of molecular genetic characteristics to improve the diagnostic accuracy.Citation38 In clinical practice, there is still ambiguity in distinguishing a new primary lesion from intrapulmonary metastasis or a satellite lesion derived from the prior index cancer for tumors with the same pathological diagnosis. In addition, with the development of newer scanning techniques, such as thin-section CT, and treatment for early stage NSCLC, the detection of small-sized lung cancer in patients who have undergone surgical resection for early stage lung cancer has increased. However, a substantial proportion of these lesions are not suitable for biopsy confirmation due to associated medical issues, perceived risk of potential complications, or the relatively too small lesion to obtain pathologic specimen. Therefore, establishing a diagnosis of MPLC based on a comprehensive clinical and radiological assessment would be particularly important. Matsunaga et alCitation39 proposed a set of new simple radiological criteria for MPLC based on prognosis. They suggested that a tumor with a ground-glass opacity and clinical N0 should not be diagnosed as MPLC because its prognosis is satisfactory. Ono et al distinguished MPLC from intrapulmonary by analyzing differential protein expression profiles.Citation31 Further research on accurate selection of radiological criteria for MPLC is necessary to provide clinical guidelines in early detection of multiple pulmonary lesions, especially metachronous lesions.
Despite limited pulmonary reserve after previous pulmonary resection of the patients in this study, SBRT was well tolerated with an acceptable toxicity profile (). Results from the RTOG 0236 trail analyzing patients treated with SBRT indicate that poor baseline pulmonary function test does not correlate with pulmonary toxicity and OS following SBRT in medically inoperable, early stage NSCLC.Citation19 No significant changes in pulmonary function test were observed in the RTOG 0236 trial; therefore, a poor baseline pulmonary function test alone should not be used to exclude patients with early stage lung cancer from treatment with SBRT, a conclusion supported by other reports.Citation40,Citation41
RP was the most common SBRT-related toxicity in our study. Three of the 33 patients in our study (9.67%) developed grade 3 SBRT-related toxicity, which was at a lower rate than the 12.7% reported in RTOG 0236.Citation19 During the follow-up period of our study, there were no grade 4/5 toxicities or SBRT-related deaths. The rate and severity of other toxicities were considered acceptable and comparable to those previously published for SABR for early stage NSCLC.Citation12,Citation18,Citation23,Citation27
Our study did have several limitations. First, because of the retrospective nature and relatively small sample size, we were unable to draw conclusions regarding risk factors for any form of treatment failure. Second, several patients did not have pathological confirmation of de novo pulmonary nodules due to the difficulty and perceived risk in obtaining a pathologic specimen from a small lesion. Third, we could not distinguish between an SPLC and metastasis because of the limited patient data. Fourth, there were 13 patients who received a BED of <100 Gy, 10 patients who received 96 Gy (~100 Gy), and three patients who received 75 Gy in our study. The patients in our study were old (median age: 68 years) and had undergone pulmonary resection, which diminished their pulmonary reserve and physical conditions and limited their ability to tolerate high doses (>100 Gy) of BED. However, all patients in our clinical study received SBRT via HT, which is associated with excellent clinical outcomes. Each tumor was sliced based on HT and received a high dose within few minutes, which is in contrast to the CyberKnife or intensity-modulated radiotherapy with linear accelerator, which takes dozens of minutes. The radiobiology may be different among the RT equipment, but the results warrant further study. In our prior clinical experience, the 3-year LCR could reach 90% for stage I lung cancer with BED=96 Gy via HT.Citation24 Additional studies are needed to validate our conclusions.
Conclusion
We reported on a series of de novo tumors after curative resection treated with SBRT in our institution. Our data demonstrate that SBRT is a reasonable and potentially effective alternative therapeutic option for de novo tumors in patients with previous lung resection and has clinically acceptable treatment toxicity. Additional large, randomized prospective trials are needed to confirm our results and help refine treatment recommendations for de novo tumors in patients with completely resected early stage initial NSCLC.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- YangHXLong-term survival of early-stage non-small cell lung cancer patients who underwent robotic procedure: a propensity score-matched studyChin J Cancer20163516627389329
- ReaFZuinACallegaroDBortolottiLGuanellaGSartoriFSurgical results for multiple primary lung cancersEur J Cardiothorac Surg200120348949511509268
- RahnRD3rdThakurSMakaniSSandhuAStereotactic body radiation therapy (SBRT) for multiple primary lung cancers (MPLC): a review and case seriesJ Radiosurg SBRT20132213514029296352
- YuYCHsuPKYehYCSurgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers?Ann Thorac Surg20139661966197424021769
- FedorDJohnsonWRSinghalSLocal recurrence following lung cancer surgery: incidence, risk factors, and outcomesSurg Oncol201322315616123702313
- SuboticDVan SchilPGrigoriuBOptimising treatment for postoperative lung cancer recurrenceEur Respir J201647237437826828046
- JungEJLeeJHJeonKTreatment outcomes for patients with synchronous multiple primary non-small cell lung cancerLung Cancer201173223724221145616
- UsudaJIchinoseSIshizumiTManagement of multiple primary lung cancer in patients with centrally located early cancer lesionsJ Thorac Oncol201051626819952800
- van BodegomPCWagenaarSSCorrinBBaakJPBerkelJVander-schuerenRGSecond primary lung cancer: importance of long term follow upThorax198944107887932595619
- RosengartTKMartiniNGhosnPBurtMMultiple primary lung carcinomas: prognosis and treatmentAnn Thorac Surg19915247737781929628
- TanvetyanonTRobinsonLSommersKERelationship between tumor size and survival among patients with resection of multiple synchronous lung cancersJ Thorac Oncol2010571018102420453687
- OkadaMTsubotaNYoshimuraMMiyamotoYOperative approach for multiple primary lung carcinomasJ Thorac Cardiovasc Surg199811548368409576219
- CaiXWXuLYWangLComparative survival in patients with postresection recurrent versus newly diagnosed non-small-cell lung cancer treated with radiotherapyInt J Radiat Oncol Biol Phys20107641100110519540063
- YanoTOkamotoTFukuyamaSMaeharaYTherapeutic strategy for postoperative recurrence in patients with non-small cell lung cancerWorld J Clin Oncol2014551048105425493240
- RahnDA3rdThakurSMakaniSSandhuAStereotactic body radiation therapy (SBRT) for multiple primary lung cancers (MPLC): a review and case seriesJ Radiosurg SBRT20132213514029296352
- ChangJYLiuYHZhuZStereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancerCancer2013119183402341023798353
- ChangJYSenanSPaulMAStereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trialsLancet Oncol201516663063725981812
- TimmermanRPaulusRGalvinJStereotactic body radiation therapy for inoperable early stage lung cancerJAMA2010303111070107620233825
- PalmaDVisserOLagerwaardFJBelderbosJSlotmanBJSenanSImpact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysisJ Clin Oncol201028355153515921041709
- JeppesenSSSchytteTJensenHRBrinkCHansenOStereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival ratesActa Oncol20135271552155823902274
- GriffioenGHLagerwaardFJHaasbeekCJSmitEFSlotmanBJSenanSTreatment of multiple primary lung cancers using stereotactic radiotherapy, either with or without surgeryRadiother Oncol2013107340340823746675
- ZuinAAndrioloLGMarulliGIs lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer?Eur J Cardiothorac Surg2013442e120e12523657547
- HeJHuangYShiSHuYZengZComparison of effects between central and peripheral stage I lung cancer using image-guided stereo-tactic body radiotherapy via helical tomotherapyTechnol Cancer Res Treat201514670170725911646
- TrottiAColevasADSetserACTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatmentSemin Radiat Oncol200313317618112903007
- ZhengXSchipperMKidwellKSurvival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysisInt J Radiat Oncol Biol Phys201490360361125052562
- SunBBrooksEDKomakiRU7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trialCancer2017123163031303928346656
- StellaFLucianoGDell’AmoreAPulmonary Metastases from NSCLC and MPLC (Multiple Primary Lung Cancers): Management and Outcome in a Single Centre ExperienceHeart Lung Circ201625219119526525847
- CreachKMBradleyJDMahasittiwatPRobinsonCGStereotactic body radiation therapy in the treatment of multiple primary lung cancersRadiother Oncol20121041192222248508
- AraiJTsuchiyaTOikawaMClinical and molecular analysis of synchronous double lung cancersLung Cancer201277228128722560922
- OnoKSugioKUramotoHDiscrimination of multiple primary lung cancers from intrapulmonary metastasis based on the expression of four cancer-related proteinsCancer2009115153489350019452548
- VerstegenNELagerwaardFJHaasbeekCJSlotmanBJSenanSOutcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven diseaseRadiother Oncol2011101225025422056535
- HorneZDDohopolskiMJClumpDABurtonSAHeronDEThoracic reirradiation with SBRT for residual/recurrent and new primary NSCLC within or immediately adjacent to a prior high-dose radiation fieldPract Radiat Oncol201883e117e12329724402
- VideticGMMDoningtonJGiulianiMStereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based GuidelinePract Radiat Oncol20177529530128596092
- MatthiesenCThompsonJSDe La Fuente HermanTAhmadSHermanTUse of stereotactic body radiation therapy for medically inoperable multiple primary lung cancerJ Med Imaging Radiat Oncol201256556156623043577
- BeyreutherHMultiplicität von Carcinomen bei einem Fall von sog. “Schneeberger” [Multiplicity of carcinomas in a case of so-called “Schneeberger” lung cancer with tuberculosis]. Lungenkrebs mit Tuber-kuloseVirchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin19242501–2230243 German
- MartiniNMelamedMRMultiple primary lung cancersJ Thorac Cardiovasc Surg1975704606612170482
- ShenKRMeyersBFLarnerJMJonesDRAmerican College of Chest PhysiciansSpecial treatment issues in lung cancer: ACCP Evidence-based clinical practice guidelines (2nd Edition)Chest20071323 Suppl290S305S17873175
- MatsunagaTSuzukiKTakamochiKOhSNew simple radiological criteria proposed for multiple primary lung cancersJpn J Clin Oncol201747111073107728973259
- KlementRJBelderbosJGrillsIPrediction of early death in patients with early-stage NSCLC-Can we select patients without a potential benefit of SBRT as a curative treatment approach?J Thorac Oncol20161171132113927060654
- GuckenbergerMAllgäuerMAppoldSSafety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysisJ Thorac Oncol2013881050105823817193
