Abstract
Background
There is a growing recognition that tumor-associated macrophages (TAMs) are recruited to the glioma environment, facilitating tumor proliferation and migration by creating an immunosuppressive microenvironment. CD68 has been widely reported as a specific marker of TAMs in cancer.
Purpose
To clarify the role of CD68 in glioma, we investigated its function at the transcriptome level and relationship with clinical practice.
Patients and methods
In total, 325 RNA-seq data from Chinese Glioma Genome Atlas (CGGA) and 697 RNA-seq data from The Cancer Genome Atlas (TCGA) network were enrolled in this study. CD68-specific findings were further analyzed with R language, and the prognostic impacts were validated through analyzing the overall survival (OS).
Results
CD68 showed a positive correlation with the WHO grade of malignancy in glioma. Meanwhile, CD68 was predominantly expressed in IDH wide type and mesenchymal subtype. Gene ontology (GO) analysis revealed that CD68-related genes were closely related to inflammatory response and immune response. Moreover, seven cultures of metagenes further confirmed that CD68 was a specific marker for macrophages in inflammatory response and played an important role in suppressing T-cell-mediated immunity. The Pearson correlation test suggested that CD68 showed robust correlation with other markers of macrophages and immune checkpoints, including PD-1 and TIM-3. Clinically, a high expression level of CD68 in tumors exhibited a poor survival in glioma patients.
Conclusion
Our results demonstrated that CD68 acted as an immune suppressor and contributed to glioma progression in the tumor microenvironment. These findings may expand our understanding of CD68-specific clinical and immune features in glioma.
Introduction
Glioma accounts for ~50% of all primary brain tumors and is thus the most common intrinsic brain tumors in humans.Citation1 Conventional treatment for gliomas consists of surgical resection followed by radiotherapy and chemotherapy.Citation2 Despite the improvements in standard treatment, patients who suffer from the most aggressive type, glioblastoma (GBM), have a median survival time of only 14–15 months.Citation3 Given these clinical challenges of glioma, there is considerable interest in whether immune mechanisms may have therapeutic value. It has been demonstrated that tumor microenvironment plays a critical role in supporting the malignant growth and progression of glioma.Citation4,Citation5 Tumor microenvironment in the brain is composed of various non-neoplastic cell types, including fibroblasts, infiltrating immune cells, and endothelial cells. These stromal cell types interact with tumor cells to produce cytokines, chemokines, and angiogenic molecules.Citation6,Citation7 Evidence from clinical and experimental studies indicates that tumor-associated macrophages (TAMs) are enriched in glioma and associated with poor prognosis.Citation8 Moreover, TAMs are a critical roadblock to the development of cancer immunotherapies. Therefore, therapies targeted against the TAMs may represent a promising approach for treating glioma.Citation9
Macrophages have been functionally categorized into M1 and M2 polarized cells that correspond to proinflammatory and anti-inflammatory responses, respectively. In line with their functions, TAMs are generally characterized as M2-like macrophage phenotype in tumor microenvironment.Citation10 In GBM, M2 TAMs have been mainly described for their immunosuppressive and tumor-supportive functions.Citation11 Despite that TAMs were reported to play an important role in glioma, the molecular characteristics underlying the pro-tumorigenic functions of TAMs remain to be elucidated.
TAMs are commonly identified by expression of CD68, CD163 or CD11b.Citation12 Recently, a study showed that the accumulation of CD68+/CD11b+/CD163+ M2 TAMs in patients with glioma was closely correlated with poor prognosis.Citation11 Targeting surface markers such as CD11b by using Mac-1 antibodies had been investigated in the tumor mouse model.Citation13 Our previous study also showed that Pseudomonas aeruginosa-mannose-sensitive hemagglutinin re-educated CD163+ M2 macrophages to M1 macrophages in vitro.Citation14 CD68+ TAMs are also associated with decreased survival rates in patients with cancers, including breast cancer and ovarian cancer.Citation15,Citation16 However, there are limited studies about the key molecular characteristics and clinical applications of CD68.
In this study, we aimed to investigate the role of CD68 mRNA expression in whole WHO grade glioma. To explore the expression state of CD68 in glioma, we took advantage of 325 RNA-seq data from Chinese Glioma Genome Atlas (CGGA) dataset. Furthermore, the RNA-seq data of 697 gliomas obtained from The Cancer Genome Atlas (TCGA) network were used to validate our finding. This is the first integrative study characterizing CD68 expression in whole grade glioma molecularly and clinically. A better understanding of the CD68 state and feature in glioma will surely help to further optimize associated cancer therapies.
Methods
Patients and data collection
CD68 expression data of gliomas were obtained from CGGA (http://www.cgga.org.cn/) and TCGA (http://cancergenome.nih.gov/). In CGGA dataset, 325 samples of RNA-seq data were collected into this study. To consolidate the findings that we have revealed in the CGGA dataset, RNA-seq data of 699 glioma samples of all grades from TCGA dataset were used as a validation cohort. Thus, in total, CD68 transcriptional expression data of 1,024 samples were evaluated.
Statistical analyses
The prognostic value of CD68 was estimated by Kaplan– Meier analysis. Other statistical computations and figure drawing were performed using several packages (ggplot2, pheatmap, pROC, and corrgram) in the statistical software environment R, version 3.4.1 (http://www.r-project.org). The main of R script is listed in the “Supplementary materials” section. For all statistical methods, P<0.05 was considered as a significant difference.
Results
CD68 expression was upregulated in GBM and IDH wide-type glioma
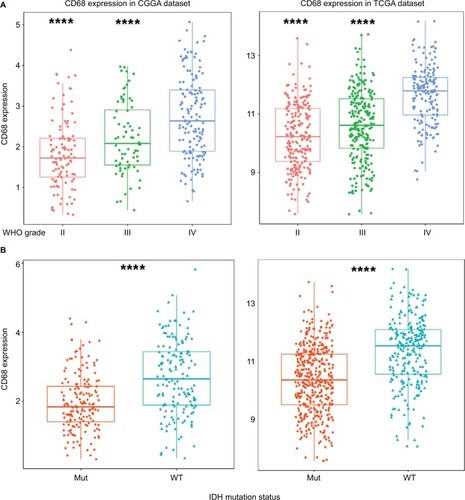
To obtain an overview of CD68 status in glioma, we first examined CD68 expression of RNA-seq data from the CGGA database. It turned out that CD68 was significantly upregulated in GBM (WHO IV) than that in WHO grade II and grade III gliomas (). Additionally, TCGA RNA-seq data were well validated to this result (). These results suggested that a higher CD68 expression was associated with a higher malignancy in gliomas. Emerging studies have demonstrated remarkably different clinical outcomes and oncogenic progression in glioma patients depending on the IDH-mutation status, which was added as a sub-classifier.Citation17 Therefore, we further analyzed the relationship between CD68 expression and IDH-mutation status. We found that IDH wild-type (WT) glioma exhibited a higher level of CD68 than IDH-mutation glioma in the CGGA and TCGA datasets (). These data support the observation that upregulated CD68 may be more related to the neoplastic cell expansion and invasion of IDH WT glioma. Moreover, the performance of the CD68 expression in IDH WT patients was also performed by receiver operating characteristic (ROC) analyses. The areas under the curve (AUCs) were 70.5% and 72.7% in CGGA and TCGA datasets, respectively (Figure S1). Herein, CD68 may serve as a biomarker for IDH-mutant type glioma.
Figure 1 CD68 was highly expressed in GBM and IDH WT glioma.
Notes: (A) The mRNA level of CD68 was upregulated in GBM from CGGA and TCGA datasets. (B) CD68 was significantly increased in IDH WT glioma. ****P<0.0001.
Abbreviations: CGGA, Chinese Glioma Genome Atlas; GBM, glioblastoma; Mut, mutant; TCGA, The Cancer Genome Atlas; WT, wild type.
CD68 expression was highly enriched in mesenchymal molecular subtype glioma
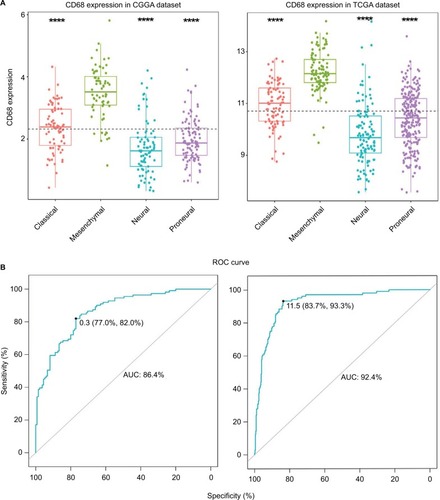
To investigate the molecular expression pattern of CD68, we analyzed the distribution of CD68 in different molecular subtypes of glioma.Citation18 As shown in , CD68 was significantly upregulated in mesenchymal subtype than that in other three subtypes in both CGGA and TCGA cohorts, indicating that CD68 might play an important role in different molecular subtypes. Furthermore, ROC curves for CD68 expression in mesenchymal subtype were performed, and the AUCs were 86.4% and 92.4% in CGGA and TCGA datasets, respectively (). These results showed that CD68 was highly expressed in mesenchymal-subtype glioma, which may play an important biologic function in glioma.
Figure 2 CD68 expression in four subtypes of glioma patients.
Notes: (A) CD68 was highly upregulated in mesenchymal molecular subtype. (B) CD68 could serve as a biomarker to predict mesenchymal subtype in CGGA and TCGA databases. ****P<0.0001.
Abbreviations: AUC, area under the curve; CGGA, Chinese Glioma Genome Atlas; ROC, receiver operating characteristic; TCGA, The Cancer Genome Atlas.
CD68 expression was related to immune-related biological response
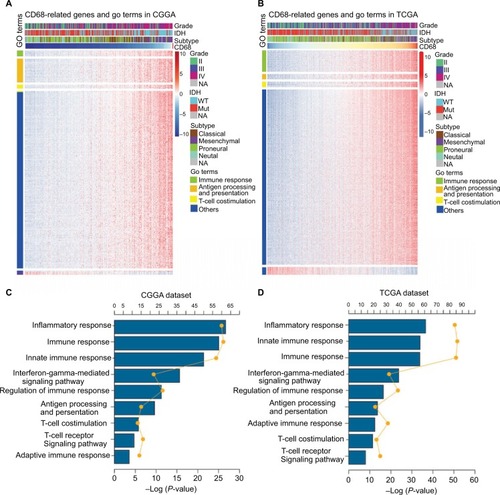
The expression level of CD68 was heterogeneous in both different grades and molecular subtypes of glioma. Next, to gain insights into the contributions of CD68 expression to glioma biology, the gene ontology (GO) analysis in DAVID (https://david.ncifcrf.gov/) was used to screen relevant genes in CGGA and TCGA databases. The Pearson |R| value more than 0.4 was regarded as a significant correlation, and genes are shown in . We found that CD68 positively correlated genes were most involved in immune response, antigen processing and presentation, and T-cell costimulation in both CGGA and TCGA RNA-seq datasets (). The details of CD68 positively related immune process are shown in , which were ranked by P-value. The genes most relevant to CD68 were mostly involved in inflammatory response, immune response, innate immune response, and interferon-gamma-mediated signaling pathway (). Taken together, these results suggested that CD68 played a key role in immune response and inflammatory response of glioma.
Figure 3 CD68 was closely related to immune response and inflammatory response in glioma.
Notes: (A and B) GO analysis exhibited that genes associated with CD68 expression were enriched in immune-related biological process. (C and D) The detail of CD68-related immune process in CGGA and TCGA datasets.
Abbreviations: CGGA, Chinese Glioma Genome Atlas; GO, gene ontology; Mut, mutant; NA, not available; TCGA, The Cancer Genome Atlas; WT, wild type.
Relationship between CD68 and inflammatory response
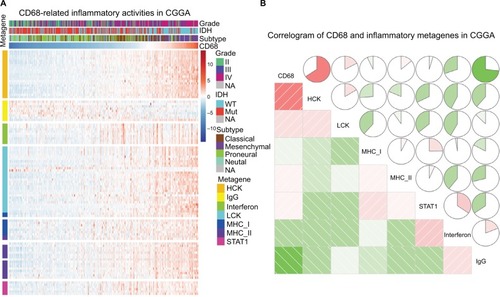
Given the important role of CD68 in mediating inflammatory response, we further identified seven clusters of 104 inflammatory response-related genes, which were generated with results of Gene Set Variation Analysis (GSVA) and defined as metagenes.Citation19 As shown in , in the CGGA dataset, CD68 expression was most correlated with the metagene of HCK, indicating that CD68 was a specific marker for macrophages in glioma and displayed an immunosuppressive function. Meanwhile, CD68 also had a strong correlation with LCK, which was associated with T-cell activity. We observed a similar pattern in glioma of TCGA dataset (Figure S2). These results demonstrated that CD68+ macrophages were upregulated as an immune suppressor when T cells signaling transduction were activated in glioma.
Figure 4 The relationship between CD68 and inflammatory metagenes.
Notes: (A) The heatmap of CD68-related inflammatory metagenes in the CGGA cohort. (B) Correlogram of CD68 and inflammatory metagenes in the CGGA cohort.
Abbreviations: CGGA, Chinese Glioma Genome Atlas; Mut, mutant; NA, not available; WT, wild type.
CD68 was synergistic with other markers of macrophages
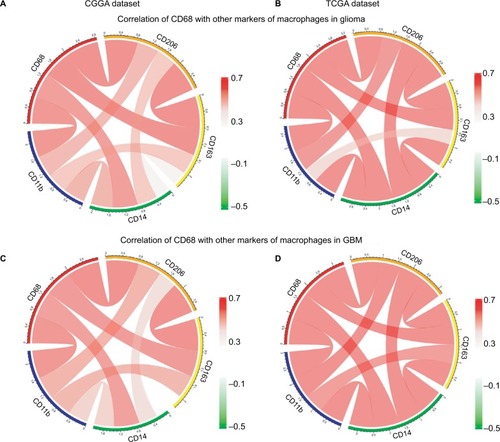
There is strong evidence of tumor progression by TAMs in glioma, and an increased prevalence of TAMs correlates with short survival time.Citation20 Therefore, the surface markers of TAMs have been assessed as promise therapeutic targets. Moreover, TAMs are commonly identified by expression of CD68, CD11b, CD14, CD163, and CD204 in glioma. Herein, we put CD68 into analysis together with other markers. Pearson correlation was performed with these five factors in both CGGA and TCGA datasets. We observed that CD68 expression demonstrated high concordance with CD11b, CD14, CD163, and CD204, suggesting a synergistic relationship of these markers in glioma (). As we have seen, the tumor environment of GBM exhibited higher inflammation and immune response than that of low-grade glioma. To investigate the relationship among these markers of TAMs in GBM, Pearson correlation was also performed. As shown in , CD68 also had a robust correlation with other markers in CGGA and TCGA datasets. These results remind us that for patients who acquired resistance to immunotherapeutic markers based on the expression of CD68, we need to pay attention to the arising of other macrophage makers.
Figure 5 Relationship between CD68 and other markers of macrophages.
Notes: (A and B) Correlation of CD68 and other markers including CD11b, CD14, CD163, and CD206 in glioma from CGGA and TCGA datasets. (C and D) Correlation of CD68 and these makers in GBM.
Abbreviations: CGGA, Chinese Glioma Genome Atlas; GBM, glioblastoma; TCGA, The Cancer Genome Atlas.
Correlation between CD68 and immune checkpoints
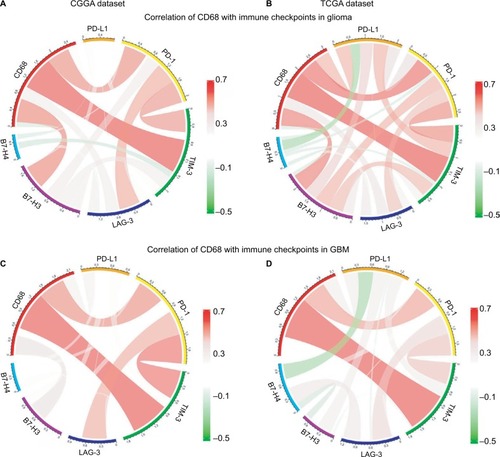
Agents targeting immune checkpoints are being extensively tested in preclinical or clinical trials for multiple solid tumors.Citation21,Citation22 Thus, checkpoint members were enrolled into the analysis, such as B7-H3, B7-H4, PD-1, LAG3, TIM-3, and PD-L1. Surprisingly, CD68 showed strong positive correlations with PD-1, TIM-3, and B7-H3 in CGGA and TCGA datasets (). To our knowledge, PD-1 and TIM-3 seemed to express more in GBM and played almost exactly the same inflammatory activation functions in glioma.Citation23,Citation24 Further analysis revealed that CD68 showed a consistent correlation with PD-1 and TIM-3 in GBM (). It has been reported that PD-L1, B7-H3, and B7-H4 expression levels were associated with malignancy grade of glioma.Citation25–Citation27 Therefore, there was some relationship between CD68 and these immune checkpoints in whole-grade glioma. However, further analysis found that only PD-1 and TIM-3 showed a consistent relationship with CD68 in GBM. These results suggested that immunotherapeutic markers based on the expression of CD68 may be a great addition when patients acquire resistance to PD-1 or TIM-3 inhibitors.
Figure 6 Correlation of CD68 and immune checkpoints.
Notes: (A and B) Relationship between CD68 and immune checkpoints in whole-grade glioma. (C and D) Correlation of CD68 and these immune checkpoints in GBM.
Abbreviations: CGGA, Chinese Glioma Genome Atlas; GBM, glioblastoma; TCGA, The Cancer Genome Atlas.
CD68 expression was associated with poor survival
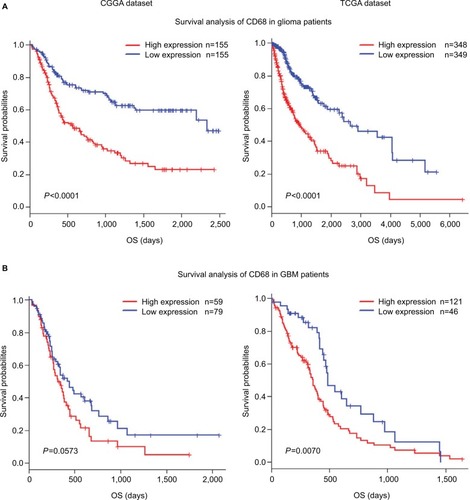
As mentioned earlier, CD68 was an immunosuppressive maker and closely correlated with glioma progression. Thus, to validate the influence of CD68 on prognosis of glioma patients, we performed an overall survival (OS) analysis using the Kaplan–Meier method. Both in CGGA and TCGA datasets, compared with the low expression of CD68 group in whole-grade glioma, patients who had a higher CD68 expression had a significantly shorter survival (). Owing to heterogeneity across different grades of glioma, we further investigated the prognostic value of CD68 expression in GBM of two datasets. Interestingly, we observed the same prognostic tendency of CD68 in GBM patients (). Furthermore, the univariate Cox model of CD68 and other factors were analyzed, which also revealed that CD68 was tightly associated with OS (P<0.001; Table S1). However, the multivariate analysis showed that CD68 expression had no difference in survival (Table S1). These findings suggested that CD68 was a negative prognosticator in glioma due to the immunosuppressive effect of CD68+ macrophages.
Figure 7 Survival analysis for CD68 expression in glioma patients.
Notes: (A and B) Survival analysis for CD68 expression in whole-grade glioma. (C and D) Survival analysis for CD68 in GBM.
Abbreviations: CGGA, Chinese Glioma Genome Atlas; GBM, glioblastoma; OS, overall survival; TCGA, The Cancer Genome Atlas.
Discussion
Glioma is the most common and lethal type of tumor in human brain. Tumor recurrence and treatment resistance are the results of both cancer cell growth and their interaction with tumor microenvironment.Citation28 Recent studies showed that glioma tissues contain tumor cells and glioma-associated non-tumor cells, including stromal cells and immune cells. Notably, as an important type of immune cells, TAMs are abundant in glioma and act as a barrier to build an immunosuppressive microenvironment.Citation29 Therefore, TAMs represent an important therapeutic target for cancer prevention and cure. The surface markers of TAMs such as CD68, CD163, CD11b, and CD206 can be directly targeted in the in vivo and in vitro experiments.Citation10 In this study, we sought to carry out the largest and most comprehensive research of CD68 expression in glioma.
CD68 is a specific marker for M2-type macrophages and has an impact on the tumor progress.Citation29 Previous studies examined the expression of CD68 in various types of cancer such as breast cancer, ovarian cancer, and astrocytomas.Citation16,Citation30,Citation31 However, the landscape of CD68 expression in glioma remains unclear. Herein, the first and biggest integrative study, including 1,024 RNA-seq glioma patients from TCGA and CGGA datasets, characterizing CD68 expression in whole-grade glioma molecularly and clinically was carried out. As expected, CD68 was upregulated in higher malignant glioma, which was similar with the previous finding by Prosniak et alCitation1 reported earlier. Furthermore, we also observed that CD68 was highly enriched in the phenotype of known malignant molecules such as IDH WT state, indicating that the IDH status may be involved in the immune processes of patients with glioma. The four molecular subtypes for glioma were proneural, neural, classical, and mesenchymal. The mesenchymal subtype is associated with poor survival and treatment resistance.Citation32 Interestingly, we found that CD68 expressed significantly higher in mesenchymal subtype than that of other subtypes. Additionally, CD68 can be a biomarker for IDH wide type and mesenchymal phenotype. These results suggested that CD68 was involved in the key step of immunosuppressive process for glioma.
We further investigated the CD68-related biology process in gliomas and found that genes closely related to CD68 were more involved in immune response and inflammatory response. In addition, seven clusters of metagenes were used to analyze the correlation between CD68 expression and inflammatory response. Results showed that CD68 had a strong correlation with HCK and LCK. In other words, CD68 was not only a specific marker for macrophages but also had a negative relationship with T-cell activation. It is well known that inflammation creates a microenvironment that causes neoplastic transformation and potentiates the progression of cancers.Citation33 Thus, these results demonstrated that CD68 expression was tightly associated with tumor-induced immunosuppressive function.
Besides, the expression panel between CD68 and other markers of macrophages is still unknown. In glioma, the analysis of TAMs revealed a predominant population of CD163+ and CD204+ anti-inflammatory M2 macrophages in association with higher tumor grade and a worse patient survival.Citation11 The CD11b+ and CD14+ cells were also accumulated in glioma with associated poor outcome.Citation11 Based on the significance of CD163, CD204, CD11b, and CD14, we next analyzed the relationship between CD68 and these markers. The results showed that CD68 expression had a strong correlation with CD163, CD11b, CD14, and CD204, suggesting that CD68 and these markers played a synergistic role in tumor inflammatory response to promote tumor progression and metastasis. Emerging data showed that the development of monoclonal antibodies to block immune checkpoints is a promising treatment for glioma.Citation34 Moreover, we analyzed the relationship between CD68 and immune checkpoints. Our results first revealed that CD68 showed a consistent synergy with PD-1 and TIM-3 in both TCGA and CGGA datasets. These results suggest that the combination of CD68 and immune checkpoint blockades may be feasible for glioma treatments. A previous study also showed that CD68 expression in glioma tissues may be used for prognosis of survival in these patients.Citation35 Moreover, in both CGGA and TCGA datasets, it was verified that a higher CD68 expression level predicted significantly poor survival for glioma patients. This prognostic tendency was also observed in the patients with GBM. The significant prognostic significance of CD68 gives us more confidence that targeting CD68 significantly improves prognosis of glioma patients, especially GBM patients.
Conclusion
To our knowledge, this is the first study based on a large-scale glioma sample concerning CD68 analyses and its clinical significance. Although the exact mechanisms of CD68 in glioma still require further investigation, our study indicates that targeting CD68 has therapeutic promise for cancer immunotherapy based on its role as a macrophage-mediated immune suppression.
Abbreviations
| TAMs | = | tumor-associated macrophages |
| CGGA | = | Chinese Glioma Genome Atlas |
| TCGA | = | The Cancer Genome Atlas |
| OS | = | overall survival |
| GO | = | gene ontology |
| GBM | = | glioblastoma |
| GSVA | = | Gene Set Variation Analysis |
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2016YFC1303501), Research Grant from the Ministry of Public Health (No. 201501004), and the Major Science and Technology Projects of Henan Province (No. 1611003101000).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- ProsniakMHarshyneLAAndrewsDWGlioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markersClin Cancer Res201319143776378623741072
- KanuOOMehtaADiCGlioblastoma multiforme: a review of therapeutic targetsExpert Opin Ther Targets200913670171819409033
- GrossmanSAYeXPiantadosiSDesideriSNaborsLBRosenfeldMFisherJNABTT CNS ConsortiumSurvival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United StatesClin Cancer Res20101682443244920371685
- QuailDFBowmanRLAkkariLThe tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomasScience20163526288aad301827199435
- QuailDFJoyceJAMicroenvironmental regulation of tumor progression and metastasisNat Med201319111423143724202395
- GolebiewskaABougnaudSStieberDSide population in human glioblastoma is non-tumorigenic and characterizes brain endothelial cellsBrain2013136Pt 51462147523460667
- SilverDJSinyukMVogelbaumMAAhluwaliaMSLathiaJDThe intersection of cancer, cancer stem cells, and the immune system: therapeutic opportunitiesNeuro Oncol201618215315926264894
- PoonCCSarkarSYongVWKellyJJPGlioblastoma-associated microglia and macrophages: targets for therapies to improve prognosisBrain201714061548156028334886
- RuffellBCoussensLMMacrophages and therapeutic resistance in cancerCancer Cell201527446247225858805
- YangLZhangYTumor-associated macrophages: from basic research to clinical applicationJ Hematol Oncol20171015828241846
- Lu-EmersonCSnuderlMKirkpatrickNDIncrease in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastomaNeuro Oncol20131581079108723828240
- HeusinkveldMvan der BurgSHIdentification and manipulation of tumor associated macrophages in human cancersJ Transl Med2011921622176642
- NyweningTMWang-GillamASanfordDETargeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trialLancet Oncol201617565166227055731
- YangLWangFWangLCD163+ tumor-associated macrophage is a prognostic biomarker and is associated with therapeutic effect on malignant pleural effusion of lung cancer patientsOncotarget2015612105921060325871392
- RumneyRMHCoffeltSBNealeTADhayadeSTozerGMMillerGPyMT-Maclow: a novel, inducible, murine model for determining the role of CD68 positive cells in breast tumor developmentPLoS One20171212e018859129220404
- OjalvoLSThompsonEDWangTLTumor-associated macrophages and the tumor immune microenvironment of primary and recurrent epithelial ovarian cancerHum Pathol20187413514729288043
- HuseJTAldapeKDThe evolving role of molecular markers in the diagnosis and management of diffuse gliomaClin Cancer Res201420225601561125398843
- VerhaakRGHoadleyKAPurdomECancer Genome Atlas Research NetworkIntegrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1Cancer Cell20101719811020129251
- RodyAHoltrichUPusztaiLT-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancersBreast Cancer Res2009112R1519272155
- RoeschSRappCDettlingSHerold-MendeCWhen immune cells turn bad-tumor-associated microglia/macrophages in gliomaInt J Mol Sci2018192436
- KimJEPatelMAMangravitiACombination therapy with anti-PD-1, Anti-TIM-3, and focal radiation results in regression of murine gliomasClin Cancer Res201723112413627358487
- HugoWZaretskyJMSunLGenomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanomaCell20161651354426997480
- LiuSWangZWangYPD-1 related transcriptome profile and clinical outcome in diffuse gliomasOncoimmunology201772e138279229308304
- LiGWangZZhangCMolecular and clinical characterization of TIM-3 in glioma through 1,024 samplesOncoimmunology201768e132833928919992
- WangZZhangCLiuXMolecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain gliomaOncoimmunology2016511e119631027999734
- BaralAYeHXJiangPCYaoYMaoYB7-H3 and B7-H1 expression in cerebral spinal fluid and tumor tissue correlates with the malignancy grade of glioma patientsOncol Lett2014831195120125120686
- YaoYYeHQiZB7-H4(B7x)-mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patientsClin Cancer Res201622112778279027001312
- GieryngAPszczolkowskaDWalentynowiczKARajanWDKaminskaBImmune microenvironment of gliomasLab Invest201797549851828287634
- HambardzumyanDGutmannDHKettenmannHThe role of microglia and macrophages in glioma maintenance and progressionNat Neurosci2016191202726713745
- MoritaYZhangRLeslieMPathologic evaluation of tumor-associated macrophage density and vessel inflammation in invasive breast carcinomasOncol Lett20171422111211828789438
- LeenstraSDasPKTroostDde BoerOJBoschDAHuman malignant astrocytes express macrophage phenotypeJ Neuroimmunol199556117257822478
- SharmaABendreAMondalAMuzumdarDGoelNShirasAAngiogenic gene signature derived from subtype specific cell models segregate proneural and mesenchymal glioblastomaFront Oncol2017714628744448
- PollardJWTumour-educated macrophages promote tumour progression and metastasisNat Rev Cancer200441717814708027
- LuksikASMaxwellRGarzon-MuvdiTLimMThe role of immune checkpoint inhibition in the treatment of brain tumorsNeurotherapeutics20171441049106528258545
- StrojnikTKavalarRZajcIDiamandisEPOikonomopoulouKLahTTPrognostic impact of CD68 and kallikrein 6 in human gliomaAnticancer Res20092983269327919661345
