Abstract
Introduction
In this meta-analysis, we analyzed retrospective cohort studies that assessed the prognostic potential of the pretreatment lymphocyte-to-monocyte ratio (LMR) among patients with ovarian cancer (OC).
Materials and methods
We comprehensively searched electronic databases, including PubMed and Embase, from inception through October 2018. A random-effects model was used to calculate pooled HRs and their 95% CIs for overall survival (OS) and progression-free survival (PFS). The low LMR group was treated as the reference group.
Results
Twelve studies, including 3,346 OC cases at baseline, were included. Overall, our results indicated that LMR was positively associated with both OS (HR: 1.85, 95% CI: 1.50–2.28, P<0.001; I2=76.5%) and PFS (HR: 1.70, 95% CI: 1.49–1.94, P<0.001; I2=24.4%) among OC patients. Stratified analyses indicated that, for OS, the LMR’s protective effect was more evident in studies conducted among younger patients (<55 years) than in those conducted among older patients (≥55 years; P for interaction =0.017), which was confirmed by meta-regression analysis (P=0.004).
Conclusion
This study suggested that a higher pretreatment LMR level was associated with a favorable prognosis among OC patients. Future large-scale prospective clinical trials are needed to confirm the prognostic value of LMR among OC patients.
Introduction
Ovarian cancer (OC) is the fifth most common cause of cancer-related deaths among women, with ~90% of these cases being epithelial ovarian cancer (EOC).Citation1 At the start of 2018, there were an estimated 22,240 newly diagnosed cases and 14,070 deaths due to OC in USA.Citation1 Although OC is less common than other cancers such as breast cancer, OC is attracting increased attention because of its poor prognosis. The 5-year survival rate for OC is only 47.2%. Although great progress has been made in cancer research, the overall prognosis for OC remains poor, because it is often diagnosed late in the disease process and has high recurrence rates after curative resection.Citation2 Therefore, more effective and convenient markers must be identified to estimate the prognosis and select appropriate treatment strategies.
Over the past decades, many theories have been postulated to explain OC’s etiology, and most of them converge on the role of inflammation.Citation3 The systemic inflammatory response is associated with survival in advanced and localized cancers.Citation4 Cancer-related inflammation includes modulating inflammatory cells and mediators such as cytokines and chemokines; however, these markers are not routinely measured despite their direct changes provide a direct surrogate marker of expression (eg, lymphocyte-to-monocyte ratio [LMR]).Citation4 Several recent studies assessed the prognostic effect of pretreatment LMR among patients with OC, but the results were inconsistent. Elevated LMR was shown to increase survival in some,Citation5–Citation7 but not all,Citation8–Citation11 studies. As the statistical power of an individual study may be too weak to identify associations between pretreatment LMR and OC patient survival (sample size of most included studies was <300 OC patients), a meta-analysis combining data from all published studies may be more convincing.
Thus, we conducted a meta-analysis to evaluate the prognostic effect of pretreatment LMR on OC patient survival, which included all eligible publications to date.
Materials and methods
This meta-analysis was conducted in accordance with the PRISMA ().Citation12
Search strategies
A comprehensive literature search of PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI, http://www.cnki.net), and the Wanfang databases (http://www.wanfangdata.com.cn) was conducted from inception through October 2018. The following search terms were used: (lymphocyte-to-monocyte or lymphocyte monocyte or lymphocyte-monocyte or lymphocyte to monocyte or lymphocyte/monocyte or LMR) AND (cancer* or carcinoma* or neoplasm* or malignan* or tumour* or tumor*) AND (ovary or ovarian) without language restriction (Supplementary materials). Related articles generated by Google Scholar (http://scholar.google.com) and PubMed were retrieved. We also scanned the reference lists of related articles to identify all potential useful studies on OC that might have been missed in our database searches.
Study selection
Inclusion criteria were as follows: 1) studies on patients with OC diagnosed histopathologically; 2) studies that assessed the prognostic value of pretreatment LMR among OC patients; 3) studies that reported the LMR cutoff value; 4) studies that reported sufficient information for calculating the HR and its 95% CI; and 5) studies that used overall survival (OS) and/or progression-free survival (PFS) as outcomes. For studies with overlapping data, only the most relevant articles with the largest datasets were included in the final analysis.
Data extraction
Two independent reviewers (X-PG and Y-HL) evaluated all potential articles for inclusion. Disagreements were resolved by discussion among all coauthors. The following information was collected: the first author’s name, publication year, country (region) and ethnicity of the population, publication type, number of OC patients at baseline, age, year of recruitment, time of follow-up, treatment method, tumor stage, histological type, LMR cutoff value, method of obtaining cutoff value, OC diagnostic criteria, survival analysis methods, and prognostic end points (OS or PFS). HRs were extracted from multivariate or univariate analyses or Kaplan–Meier survival curves. If only Kaplan–Meier curves were provided, we extracted data from the survival curves using Engauge Digitizer v.4.1 software.Citation13
Quality assessment
Each study’s methodological quality was assessed as per the Newcastle–Ottawa Scale (NOS),Citation14 which was used to allocate a maximum of nine stars for selection quality of the study population, comparability, and outcome. The studies’ quality scores ranged from 0 to 9, with 7–9 points indicating a high-quality study and 0–6 points indicating a low-quality study.
Statistical analyses
The DerSimonian and Laird random-effects model of inverse variance methods was used to estimate the pooled HRs and 95% CIs. Unless otherwise stated, we used the most fully adjusted RRs from each study, and the low LMR group was treated as the reference group. If the studies used different reference groups to estimate the LMR HR for OS/PFS, we used an Excel macro file to transform the reference group.Citation15
The random-effects model was chosen a priori, because it is considered to be more conservative than the fixed-effects model and it accounts for both within- and between-study heterogeneity.Citation16 Between-study heterogeneity was tested using Cochran’s Q test and Higgins I2 statistic (higher I2 values denote greater heterogeneity).Citation17 We performed subgroup analyses for both OS and PFS to examine the robustness of the results by age (<55 vs ≥55 years), LMR cutoff value (≤3.0 vs >3.0), sample size (≤200 vs >200), and NOS score (<7 and ≥7 points). Influence analysis was also conducted to assess the effect of a single study on the pooled estimates.Citation18 These variables were also analyzed as covariates in the meta-regression analysis. Publication bias was assessed by visually inspecting funnel plots and quantitatively evaluated using Egger’s and Begg’s linear regression asymmetry tests.Citation17 All data were analyzed using Stata software, version 11.0 (StataCorp LP, College Station, TX, USA), and a two-sided P<0.05 was considered statistically significant.
Results
Search results
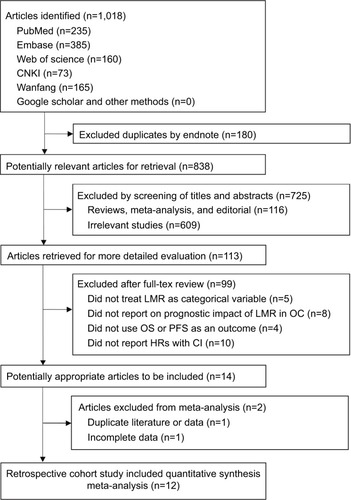
The electronic database searches identified 1,018 articles (), of which 180 duplications were excluded by Endnote. After assessing titles and abstracts and screening full texts, 824 unrelated articles were excluded. For the remaining 14 potentially eligible articles, 2 duplicate studies, 1 duplicate study,Citation19 and 1 study with incomplete dataCitation20 were further excluded. Finally, 12 studies were included.Citation5–Citation11,Citation21–Citation25
Characteristics of the included studies
summarizes the characteristics of the included studies. In total, 3,346 OC patients (weighted age: 55.8 years) were included, with a follow-up period ranging from 23.6 to 58 months. All studies were published in 2016 or later. The number of patients per study ranged from 42 to 672. Eight studies were conducted among Chinese patients, three among Korean patients, and one among American Caucasian patients. The LMR cutoff values ranged from 1.85 to 4.35. The overall NOS scores ranged from 5 to 8 points (). Most cases were EOC, and 76.5% were stage III/IV. Among these studies, three investigated only OS, while nine investigated both OS and PFS.
Table 1 Characteristics of studies included in the meta-analysis
Association between LMR and OS among OC patients
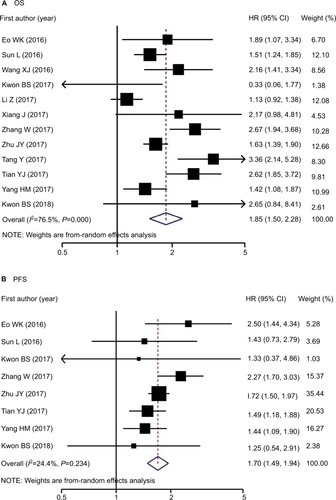
Twelve studies involving 3,346 patients reported LMR and OS data among OC cases.Citation5–Citation11,Citation21–Citation25 Increased LMR was associated with improved OS (pooled HR: 1.85, 95% CI: 1.50–2.28, P<0.001) with significant between-study heterogeneity (P<0.001, I2=76.5%; and ). The association persisted after reanalyzing studies among Asian patients or those with only EOC. Stratified analyses for age, LMR cutoff values, sample size, and NOS score revealed significant interactions for age (P for interaction =0.017) and LMR cutoff values (P for interaction =0.025). The protective effect of elevated LMR was more evident among younger patients than older patients (HR: 2.28 vs 1.47) and among studies using an LMR cutoff of >3.0 than in those using ≤3.0 (HR: 2.09 vs 1.38). Meta-regression analysis further confirmed that age, but not LMR cutoff values, significantly contributed to inter-study heterogeneity (P for regression =0.004 and 0.153; ).
Table 2 Total, stratified, and sensitivity analyses of the associations between pretreatment LMR and survival among OC patients
Association between LMR and PFS in OC patients
Eight studiesCitation5–Citation9,Citation21,Citation23,Citation25 involving 2,114 patients reported data for the association between LMR and PFS among OC patients, and all studies were conducted among Asian patients. Similar to OS, the random-effects combined analysis demonstrated that LMR was positively and significantly associated with PFS (pooled HR: 1.70, 95% CI: 1.49–1.94, P<0.001) but with low between-study heterogeneity (I2=24.4%; P=0.234; and ). The result was similar among studies with only EOC cases. Stratified analyses suggested that the association did not differ among NOS scores, LMR cutoff values, and age strata (P interaction range =0.066–0.987). Meta-regression analysis also revealed that publication year, age, NOS score, sample size, and LMR cutoff value did not significantly contribute to heterogeneity (P for regression range =0.086–0.982).
Sensitivity analysis and bias
The sensitivity analyses indicated that the pooled HRs were not obviously influenced by any single study for either OS or PFS ().
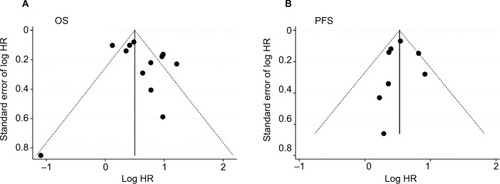
Both Egger’s and Begg’s tests revealed no significant publication bias, and the P-values were 0.732 and 0.272 for OS and 1.000 and 0.887 for PFS. The funnel plots also showed no evidence of publication bias for either OS or PFS ().
Discussion
In this meta-analysis, we first report the prognostic value of pretreatment LMR among OC patients. Our results indicate that higher pretreatment LMR levels are associated with increased OS and PFS among OC patients. Substantial heterogeneity was observed for OS; further subgroup and meta-regression analyses indicated that age contributed to this heterogeneity, and these associations were more evident among younger patients than older populations.
In recent years, several prognostic indicators derived from peripheral blood, such as LMR, have been widely investigated as useful prognostic markers in cancers. LMR has been identified as an independent prognostic factor in patients with various cancers, such as head and neck,Citation26 pancreatic,Citation27 colorectal,Citation28 hepatocellular,Citation29 and breast cancers.Citation30 Our results were consistent with findings from these studies, showing that higher LMR ratios may improve cancer prognoses.
The exact mechanisms by which LMR has some prognostic relevance in OC patients were still unknown. According to the current evidence, lymphopenia might weaken the efficacy of the immune system and be associated with worse prognosis in cancers; cell-mediated cytotoxicity may be attenuated if the level of effector T cells is insufficient.Citation31 Circulating monocytes may contribute to both tumor growth and reduced immunosurveillance through differentiating into macrophages after infiltrating a tumor and then respond to the wide spectrum of chemokines and growth or differentiation factors.Citation31 Thus, the prognostic effect of LMR among OC patients can be assumed to be related to tumor-infiltrating immune cells, such as tumor-infiltrating lymphocytes (TILs), or tumor-associated macrophages. Circulating TILs, as direct measures of intratumoral immunity, may contribute to cancer growth and spread.Citation32 In OC tumor tissue sections, intraepithelial CD8+ TILs correlated with good outcome, and a high ratio of CD8+/FoxP3+ T regulatory cells was beneficial to survival.Citation33 Recent epidemiological studies have also confirmed that the presence of TILs was associated with improved clinical outcomes in OC patients.Citation34–Citation36 Peripheral blood-based parameters (eg, LMR) have been studied as a surrogate measures of intratumoral immunity that reflect a host’s immune response.Citation4 LMR has been shown a statistically significant correlation with CD8+ TILs among patients with breast cancer.Citation37 Tumor-associated macrophages (TAMs) have been suggested to be involved in accelerating angiogenesis, invasion, migration, and metastasis and suppress the body’s autoimmune response against tumor cells.Citation38,Citation39 In addition, LMR had been supposed to reflect the TIL/TAM ratio, as the circulating levels of lymphocytes and monocytes may indicate the formation or the presence of TILs and TAMs, and significant correlation was observed between the LMR and the TIL/TAM ratio.Citation31 Immunotherapy has emerged as one of the most promising approaches for OC treatment,Citation40 and change in the LMR has been supposed to be an early surrogate marker of the efficacy of nivolumab monotherapy.Citation41 Thus, LMR represents the balance between the host’s immune status and the degree of tumor progression, and it may therefore be a prognostic biomarker among OC patients.
Subgroup analyses indicated that the favorable prognostic effect of pretreatment LMR for OS was more evident in studies conducted among younger (<55 years) than older patients (≥55 years; P for interaction =0.017), which was further confirmed by meta-regression analysis (P=0.004). One explanation for our finding is that human aging is characterized by a gradual increase in subclinical chronic inflammation, and older people are more likely to get chronic inflammatory diseases.Citation42 The greater severity of the inflammatory state among older OC patients may weaken the LMR’s protective prognostic effect. In addition, older patients responded more efficiently to immunotherapy, such as programmed death-ligand 1 (nivolumab and pembrolizumab), and PD-L1 (atezolizumab) inhibitors also confirmed this finding.Citation43,Citation44
Some limitations of this meta-analysis should also be considered. First, between-study heterogeneity was significant for OS (I2: 76.5%). Based on subgroup and meta-regression analyses, age was the main source of heterogeneity, and the pooled HR results showed consistent positive relationships. Second, most studies included herein were performed among Asian patients, while only one study examined OS among Caucasian patients,Citation10 and no relevant studies were found for African patients. Thus, the findings of the present study might be limited to Asian patients, and the prognostic effects of LMR for other populations (eg, Caucasian or African) still need further confirmation. Third, the studies included herein differed in how the covariates were adjusted. However, the pooled estimates were similar between the maximal and minimal numbers of covariate adjustment analyses for both OS and PFS, indicating that these confounders were unlikely to significantly bias our findings (data not shown). Fourth, categorical analysis did not allow detecting the best cutoff point, which invites further studies to solve this problem. Fifth, all included studies were retrospective single-center studies, and the bias was unavoidable.
Conclusion
This meta-analysis demonstrated that higher pretreatment LMR values were associated with more favorable outcomes among OC patients, and the associations were stronger for younger patients than older patients. Future large-scale prospective clinical trials are needed to confirm the LMR’s prognostic effect and its cutoff value among OC patients. Therefore, LMR is a readily available, routinely measured, and inexpensive inflammatory biomarker, and if causation and cutoff value of LMR was established, LMR could be easily applied in daily clinical practice.
Author contributions
Study concept and design: F-FZ and SZ. Data extraction and analysis: X-PG and Y-HL. Manuscript drafting: X-PG and Y-HL. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Supplementary materials
Table S1 PRISMA 2009 checklist
Table S2 Methodological quality of all studies based on NOS for assessing the quality of each included study
Table S3 Meta-regression analyses of the associations between pretreatment LMR and survival among OC patients
Detailed search strategies for each database
PubMed (N=215)
#1: lymphocyte-to-monocyte OR lymphocyte monocyte OR lymphocyte-monocyte OR lymphocyte to monocyte OR lymphocyte/monocyte OR LMR
#2: ((cancer* OR carcinoma* OR neoplasm* OR malignan* OR tumour* OR tumor*) AND (ovary OR ovarian)) OR “Ovarian Neoplasms”[mesh]
#3: #1 AND #2
Embase (N=385)
#1: ((cancer* OR carcinoma* OR neoplasm* OR malignan* OR tumour* OR tumor*) AND (ovary OR ovarian)) OR (‘ovarian neoplasms’/exp)
#2: (‘lymphocyte to monocyte’) OR (lymphocyte AND monocyte) OR (‘lymphocyte monocyte’) OR (lymphocyte AND to AND monocyte) OR (LMR) OR (lymphocyte?monocyte)
#3: #1 AND #2
Web of science (N=160)
#1 (Ovarian Neoplasms) OR ((cancer* OR carcinoma* OR neoplasm* OR malignan* OR tumour* OR tumor*) AND (ovary OR ovarian))
#2 (lymphocyte-to-monocyte ratio OR “lymphocyte monocyte ratio” OR “lymphocyte to monocyte ratio” OR LMR)
#3: #1 AND #2
Wanfang (N=165)
#1 摘要:(卵巢癌+卵巢肿瘤)*摘要:(淋巴细胞)*摘 要:(单核细胞))
#2 摘要:(卵巢癌+卵巢肿瘤)*摘要:(LMR) #3 题名或关键词:(卵巢癌+卵巢肿瘤)*题名或关键 词:(淋巴细胞)*题名或关键词:(单核细胞)
#4 题名或关键词:(卵巢癌+卵巢肿瘤)*题名或关键 词:(LMR)
#5 主题:(卵巢癌+卵巢肿瘤)*主题:(淋巴细胞) * 主题:(单核细胞)
#6 主题:(卵巢癌+卵巢肿瘤)*主题:(LMR) #7: #1 OR #2 OR #3 OR #4 OR #5 OR #6
Chinese National Knowledge Infrastructure (CNKI; N=73)
#1 AB=(‘卵巢癌’+’卵巢肿瘤’) and AB=’淋巴 细胞’ and AB=’单核细胞’
#2 AB=(‘卵巢癌’+’卵巢肿瘤’) and AB=’LMR’ #3 TI=(‘卵巢癌’+’卵巢肿瘤’) and TI=’淋巴 细胞’ and TI=’单核细胞’
#4 TI=(‘卵巢癌’+’卵巢肿瘤’) and TI=’LMR’ #5 KY=(‘卵巢癌’+’卵巢肿瘤’) and KY=’淋巴 细胞’ and KY=’单核细胞’
#6 KY=(‘卵巢癌’+’卵巢肿瘤’) and KY=’LMR’ #7 SU=(‘卵巢癌’+’卵巢肿瘤’) and SU=’淋巴 细胞’ and SU=’单核细胞’
#8 SU=(‘卵巢癌’+’卵巢肿瘤’) and SU=’LMR’ #9: #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
References
- EoWKChangHJKwonSHThe lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian cancerJ Cancer20167328929626918042
- SunLSongYEffects of lymphocyte and monocyte ratio on prognosis of epithelial ovarian cancerChinese Clinical Oncology201610909912
- WangXYuanZQiuHThe relationship between preoperative blood lymphocyte-to-monocyte ratio and the prognostic of epithelial ovarian cancerProgi Obstet Gynecol20169654657
- KwonBSLeeHJYangJSongYJSuhDSLeeDHKimKHPrognostic value of preoperative lymphocyte-monocyte ratio in elderly patients with advanced epithelial ovarian cancerObstet Gynecol Sci201760655856429184864
- LiZHongNRobertsonMWangCJiangGPreoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancerSci Rep201774300128223716
- XiangJZhouLLiXPreoperative Monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancerTransl Oncol2017101333927888711
- ZhangWYeBLiangWRenYPreoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancerSci Rep201771954828842710
- ZhuJYLiuCCWangLZhongMTangHLWangHPeripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective studyJ Cancer20178573774328382135
- TangYLiJXuFHuHAssociation between monocyte-to-lymphocyte ratio and prognosis of patients with epithelial ovarian cancerAm J Obstet Gynecol Pediat20175532538
- TianYAnalysis of Prognostic Factors of Epithelial Ovarian CancerHe Bei, ChinaHebei Medical University2017
- YangHMLouGThe relationship of preoperative lymphocyte-monocyte ratio and the clinicopathological characteristics and prognosis of patients with epithelial ovarian cancerZhonghua Zhong Liu Za Zhi201739967668028926896
- KwonBSJeongDHByunJMPrognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinomaJ Cancer2018971127113429675093
Acknowledgments
The study was supported by research grants from National Natural Science Foundation of China (81602853).
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLATrabertBDeSantisCEOvarian cancer statistics, 2018CA Cancer J Clin201868428429629809280
- VergoteITropéCGAmantFEuropean Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials GroupNeoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancerN Engl J Med20103631094395320818904
- MacciòAMadedduCInflammation and ovarian cancerCytokine201258213314722349527
- DupréAMalikHZInflammation and cancer: what a surgical oncologist should knowEur J Surg Oncol201844556657029530345
- ZhangWYeBLiangWRenYPreoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancerSci Rep201771954828842710
- ZhuJYLiuCCWangLZhongMTangHLWangHPeripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective studyJ Cancer20178573774328382135
- EoWKChangHJKwonSHThe lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian cancerJ Cancer20167328929626918042
- KwonBSJeongDHByunJMPrognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinomaJ Cancer2018971127113429675093
- KwonBSLeeHJYangJSongYJSuhDSLeeDHKimKHPrognostic value of preoperative lymphocyte-monocyte ratio in elderly patients with advanced epithelial ovarian cancerObstet Gynecol Sci201760655856429184864
- LiZHongNRobertsonMWangCJiangGPreoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancerSci Rep201774300128223716
- XiangJZhouLLiXPreoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancerTransl Oncol2017101333927888711
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009719621072
- ParmarMKTorriVStewartLExtracting summary statistics to perform meta-analyses of the published literature for survival endpointsStat Med19981724281528349921604
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- HamlingJLeePWeitkunatRAmbühlMFacilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease categoryStat Med200827795497017676579
- RileyRDHigginsJPDeeksJJInterpretation of random effects meta-analysesBMJ2011342d54921310794
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- ManderAClaytonDAssessing the influence of a single study in meta-analysisStata Tech Bull19998108110
- WangXThe Relationship Between Preoperative Blood Lymphocyte-To-Monocyte Ratio and the Prognostic of Epithelial Ovarian CancerZheng Zhou, ChinaThe First Affiliated Hospital of Zheng Zhou University2017
- RomitoAMarchettiCdi SantoGMonocyte-to-lymphocyte ratio as predictor of survival and response to treatment in ovarian cancerInt J Gynecol Cancer1913201727
- SunLSongYEffects of lymphocyte and monocyte ratio on prognosis of epithelial ovarian cancerChinese Clin Oncol201610909912
- TangYLiJXuFHuHAssociation between monocyte-to-lymphocyte ratio and prognosis of patients with epithelial ovarian cancerAm J Obstet Gynecol Pediat20175532538
- TianYAnalysis of Prognostic Factors of Epithelial Ovarian CancerHe Bei, ChinaHebei Medical University2017
- WangXYuanZQiuHThe relationship between preoperative blood lymphocyte-to-monocyte ratio and the prognostic of epithelial ovarian cancerProg Obstet Gynecol20169654657
- YangHMLouGThe relationship of preoperative lymphocyte-monocyte ratio and the clinicopathological characteristics and prognosis of patients with epithelial ovarian cancerZhonghua Zhong Liu Za Zhi201739967668028926896
- ThamTOlsonCKhaymovichJHermanSWCostantinoPDThe lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysisEur Arch Otorhinolaryngol201827571663167029651542
- HuRJMaJYHuGLymphocyte-to-monocyte ratio in pancreatic cancer: prognostic significance and meta-analysisClin Chim Acta201848114214629544747
- SongWWangKZhangRJZouSBPrognostic value of the lymphocyte monocyte ratio in patients with colorectal cancer: a meta-analysisMedicine (Baltimore)20169549e554027930549
- SongWTianCWangKZhangRJZouSBThe pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: a meta-analysisSci Rep201774660128417972
- HuRJLiuQMaJYZhouJLiuGPreoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: a meta-analysisClin Chim Acta20184841629775617
- ZhuYLiMBoCPrognostic significance of the lymphocyte-to-monocyte ratio and the tumor-infiltrating lymphocyte to tumor-associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinomaCancer Immunol Immunother201766334335427915370
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- SatoEOlsonSHAhnJIntraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancerProc Natl Acad Sci U S A200510251185381854316344461
- SatoEOlsonSHAhnJIntraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancerProc Natl Acad Sci U S A200510251185381854316344461
- ZhangLConejo-GarciaJRKatsarosDIntratumoral T cells, recurrence, and survival in epithelial ovarian cancerN Engl J Med2003348320321312529460
- HwangWTAdamsSFTahirovicEHagemannISCoukosGPrognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysisGynecol Oncol2012124219219822040834
- LeeKHKimEYYunJSParkYLDoSIChaeSWParkCHThe prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancerBMC Cancer201818193830285668
- CondeelisJPollardJWMacrophages: obligate partners for tumor cell migration, invasion, and metastasisCell2006124226326616439202
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- McCloskeyCWRodriguezGMGalpinKJCVanderhydenBCOvarian cancer immunotherapy: preclinical models and emerging therapeuticsCancers (Basel)2018108E24430049987
- SekineKKandaSGotoYChange in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancerLung Cancer201812417918830268458
- ElisiaILamVHofsEEffect of age on chronic inflammation and responsiveness to bacterial and viral challengesPLoS One20171211e018888129186188
- KugelCHDouglassSMWebsterMRAge correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populationsClin Cancer Res201824215347535629898988
- EliasRGiobbie-HurderAMcClearyNJOttPHodiFSRahmaOEfficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysisJ Immunother Cancer2018612629618381