Abstract
Background
The optimal treatment strategy for patients with non-small-cell lung cancer (NSCLC) with postoperative oligometastases is poorly defined. This two-institution analysis sought to retrospectively compare the efficacy and toxicity of local ablative treatment plus chemotherapy vs local treatment alone in patients with NSCLC who developed oligometastases after surgery.
Patients and methods
Among patients who underwent surgery for stage I–III NSCLC, 163 patients with oligometastases were enrolled between 2005 and 2016 in this study. All patients had ≤5 metachronous metastases with a disease-free interval (DFI) of ≥6 months after surgery. Patients with a second primary cancer, local recurrence, or driver mutations were excluded. Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), failure patterns, and treatment-related toxicities were compared between groups receiving local ablative treatment plus chemotherapy and local treatment alone.
Results
A total of 105 patients who underwent local ablative therapy combined with chemotherapy and 58 patients who received local ablative therapy alone were included in this study. The median follow-up was 19 (range, 1.5–107) months. The combination therapy group had a higher ORR than the local therapy alone group (66.7% vs 46.5%, P=0.012), while the median PFS was 10 vs 7 months (P=0.006) and the median OS was 19 vs 18.5 months (P=0.498), respectively. By multivariate analysis, combination therapy and DFI ≥24 months were associated with superior PFS. Age was the only independent prognostic factor for OS (P<0.001). The incidences of grade ≥3 adverse events were higher in the combination treatment group.
Conclusion
Local ablative therapy plus chemotherapy conferred higher ORR and prolonged PFS but did not improve OS in NSCLC patients with postoperative oligometastases. Further prospective and randomized trials are urgently needed to validate these findings.
Introduction
Non-small-cell lung cancer (NSCLC) remains the leading cause of cancer-related death worldwide, and its prevalence continues to increase in China, which has the highest morbidity and mortality rates. Surgery is the major treatment strategy for most patients with stage I–III NSCLC; however, unfortunately, 19.3%–68.2%Citation1–Citation5 of these patients develop postoperative recurrences. Systemic chemotherapy or targeted molecular therapy is commonly accepted as standard management practice for patients with recurrent NSCLC, based on the evidence of metastatic stage IV disease. Nevertheless, outcomes remain poor for patients with recurrent NSCLC, with postrecurrence survival (PRS) of 8.1–20.7 months.Citation6
Most postoperative recurrence is distant, with half including oligometastases,Citation7 an intermediate state between local lesions and systemic metastases.Citation8 For patients with oligometastatic NSCLC (stage IV, M1b), local ablative therapy combined with chemotherapy can dramatically improve progression-free survival (PFS) without significant toxicities.Citation9 In patients with postoperative oligometastases, the addition of chemotherapy to stereotactic body radiation therapy (SBRT) is also reported to predict significantly superior local control and confer improved overall survival (OS).Citation10 In addition, local radiotherapy combined with systemic treatment conferred a better 2-year survival rate compared with radiotherapy alone,Citation11 demonstrating that the combination of local and systemic treatments may also be promising for the management of postoperative oligometastases.
Nevertheless, postoperative oligometastases do not behave in the same way as stage IV disease detected at original diagnosis.Citation12 As primary lesions are completely resected and since oligometastases have less aggressive biological characteristics than polymetastases,Citation13 local therapy alone has also been attempted for NSCLC patients with postoperative oligometastases. Local therapy alone could successfully control brain,Citation14 adrenal,Citation15 and lungCitation16 oligometastases, leading to long-term survival; moreover, the addition of chemotherapy to local radiotherapy did not improve survival outcomes.Citation17,Citation18 Given the lack of evidence from prospective and randomized clinical trials, the optimal treatment paradigms for postoperative oligometastases remain to be determined. Therefore, we retrospectively compared the efficacy and toxicity of local ablative therapy, with or without chemotherapy, for NSCLC patients with postoperative oligometastases at two centers.
Patients and methods
Patients
This study was approved by the ethics committee of Shan-dong Tumor Hospital, Affiliated to Shandong University and Tai’an Central Hospital. The ethics committee waived the requirement to obtain patient consent because of the retrospective study design. Between January 2005 and January 2016, patients with surgically resected NSCLC and oligometastases who accepted local ablative therapy, with or without chemotherapy, as the first-line treatment was enrolled in this study.
Postoperative pathological stage was based on the seventh edition of the American Joint Committee on Cancer. Chest/abdominal computed tomography (CT) scans, brain CT, magnetic resonance imaging (MRI), bone scintigraphy, or positron emission tomography (PET)/PET-CT scans were used to confirm the number of oligometastases, which was defined as ≤5; mediastinal and supraclavicular lymph nodes in the same radiotherapy field were considered as one lesion. Patients with local recurrence in the surgical margin and ipsilateral hemithorax were excluded. Pathological confirmation was needed if a second primary tumor was suspected. All patients had metachronous metastases, indicating a disease-free interval (DFI) of ≥6 months. Other criteria were age ≥18 years, an Eastern Cooperative Oncology Group (ECOG) performance status score ≤2, complete blood count and blood biochemistry within the normal range, and expected survival ≥3 months. Patients, who harbored EGFR mutations, or ALK or ROS1 rearrangements, were also excluded. Of the 221 patients with postoperative oligometastases, 163 were finally included for further study ().
Treatment regimens
All patients were treated with local ablative therapy. The type of aggressive local therapy was determined by multidisciplinary teams. Patients who underwent surgery were required to have achieved radical (R0) resection. The targeted area of radiotherapy was required to include all metastases. Treatment doses were individualized based on tumor locations and organs at risk. Radiofrequency ablation, particle implantation, and embolization were performed according to the corresponding clinical guidelines. Chemotherapy was delivered either sequentially or concurrently.
Outcome measures
Local tumor response was assessed according to the Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1 and confirmed by imaging. PFS refers to the time from the start of treatment to the first disease progression or death from any cause; OS refers to the time from the treatment date to the last follow-up or to any cause of death; and DFI was defined as the time from surgery to the occurrence of oligometastases. Acute toxicity data were collected using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistics
SPSS 22.0 was used for statistical analyses. Student’s t-test was used to analyze continuous variables. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. Univariate and multivariate analyses of PFS and OS were evaluated using the Cox proportional hazards regression model. Survival curves were constructed by the Kaplan–Meier method. P<0.05 was considered to be statistically significant. All tests were two-sided.
Results
Patients’ characteristics
A total of 163 eligible patients were available for analysis in this study; of whom, 105 received local ablative therapy plus chemotherapy, whereas 58 underwent local ablative therapy alone. The baseline characteristics of all patients are presented in ; no significant differences were detected between the two groups. Among patients with non-squamous carcinoma, adenocarcinoma was the predominant histological subtype, accounting for 80.6%. In the combination therapy group, 100 (95%) patients were treated with platinum-based doublet chemotherapy regimens, including 28 (26.7%), 34 (32.3%), 36 (34.2%), and two (1.9%) patients who received platinum combined with pemetrexed, gemcitabine, docetaxel, and etoposide, respectively. In addition, five (4.7%) patients were administered single-agent regimens with local ablative therapy.
Table 1 Characteristics of patients
Survival and prognostic analyses
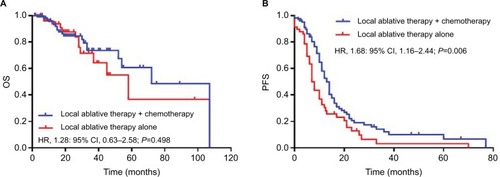
For the entire cohort, the median OS after diagnosis of postoperative oligometastases was 19 months. The median OS for the combination therapy and local treatment groups was 19 (range, 1.5–107) months and 18.5 (range, 2–107) months, respectively (P=0.498; ). The 1-, 3-, and 5-year OS rates for the combination therapy group were 69.5%, 19.0%, and 6.7%, respectively; for the local treatment group, they were 65.5%, 20.7%, and 3.4%, respectively (P=0.599, 0.801, and 0.493, respectively). The median PFS was 10 (range, 0–77) months in the combination therapy group and 7 (range, 0–70) months in the local treatment group, respectively (P=0.006; ). The 1- and 2-year PFS rates were 40.9% vs 25.9% (P=0.054) and 12.4% vs 8.6% (P=0.463) for the combination therapy and local treatment groups, respectively.
Figure 2 Kaplan–Meier curves of OS (A) and PFS (B) for the local ablative therapy with or without chemotherapy in NSCLC patients with postoperative oligometastases.
Abbreviations: NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival.
In univariate analyses, age at diagnosis (P<0.001) and tumor histology (P=0.044) were associated with OS (). In multivariate analyses, age was the only independent prognostic factor for OS (P<0.001, HR 3.91, 95% CI 1.83–8.38). Notably, local ablative treatment with or without chemotherapy was not associated with OS. Both univariate and multivariate analyses identified DFI after surgery of ≤24 months (P=0.017 and 0.012, respectively) and treatment with local ablative treatment alone (P=0.006 and 0.004, respectively) as significant unfavorable prognostic factors for PFS ().
Table 2 Univariate and multivariate analyses of overall survival
Table 3 Univariate and multivariate analyses of progression-free survival
Tumor response and failure patterns
Among the 105 patients in the combination therapy group, 13 complete responses (12.4%), 57 partial responses (54.3%), 29 disease stabilizations (27.6%), and six progressions (5.7%) were recorded, resulting in an objective response rate (ORR) of 66.7%, whereas the ORR for the group receiving local ablative therapy alone was 46.5% (P=0.012; ).
Table 4 ORR and patterns of failure
The failure patterns after treatment of postoperative oligometastases were also further analyzed, and no significant difference between the two groups was detected in local failure, distant failure, or combination failure (P=0.218, 0.149, and 0.991, respectively; ).
Toxicities
Treatment-related acute toxicities are listed in . The most common adverse events in both groups were myelosuppression, nausea/vomiting, fatigue, and radiation esophagitis. The incidences of grade ≥3 myelosuppression and nausea/vomiting were markedly higher in the combination therapy group than in the local treatment group (24.8% and 3.5%, respectively, P=0.001). In addition, in the combination therapy group, one patient developed grade 3 esophagitis and an elderly patient (aged 72 years) died of fungal pneumonia.
Table 5 Treatment toxicities
Discussion
We reported a two-institution retrospective study comparing the efficacy and toxicity of local ablative treatment plus chemotherapy vs local treatment alone in NSCLC patients with postoperative oligometastases. The group who received local treatment combined with chemotherapy had superior PFS and ORR (10 vs 7 months, P=0.006; 66.7% vs 46.5%, P=0.012); however, there was no significant difference in OS between the two groups (19 vs 18.5 months, P=0.498), whereas the incidence of grade ≥3 toxicities was markedly higher in the combination group.
The optimal treatment strategy for NSCLC patients with postoperative oligometastases remains controversial. Emerging evidence indicates that local treatment of postoperative oligometastases predicts a favorable PRS and even cure;Citation19 however, most studies emphasizing the role of local therapy enrolled patients receiving local therapy alone and those receiving local therapy plus systemic treatment, simultaneously,Citation16,Citation20 which complicates evaluation of the value of adding chemotherapy to local therapy. To the best of our knowledge, this is the first study to compare the effects of local ablative treatment plus chemotherapy vs local treatment alone in NSCLC patients with postoperative oligometastases. Although PFS and ORR were superior in patients receiving combination therapy, these did not translate into improved OS. Failure patterns following treatment of postoperative oligometastases also did not differ between the two groups. Considering the finding that oligometastases have not acquired the broad array of genetic changes associated with development of widespread metastases,Citation21 it is unclear whether they are biologically indolent, irrespective of the chemotherapy provided. Further study is needed to compare the efficacy of local therapy vs chemotherapy.
Analysis of prognostic factors for patients with postoperative oligometastatic NSCLC demonstrated that age and DFI could independently predict OS and PFS, respectively, consistent with previous studies.Citation22–Citation24 As younger patients can better tolerate the intensive therapy following failure of first-line treatment, it is not surprising that age ≤65 years may be a marker of superior survival in patients with recurrent NSCLC. DFI, a variable directly describing tumor biology, is an independent factor predicting patient prognosis after recurrent NSCLC. Longer DFI, suggesting relatively slow tumor growth,Citation25 was favorably associated with long-term survival (≥5 years).Citation23 Thus, these factors may be considered when estimating patient prognosis and could be helpful for providing individualized treatment for NSCLC patients with postoperative oligometastases.
Given their prolonged PFS and superior ORR, selected NSCLC patients with postoperative oligometastases would benefit from combination therapy with local ablative treatment plus chemotherapy. MicroRNA markers, which have been determined to successfully distinguish patients with oligometastases-directed SBRT,Citation26 will be useful for identifying subsets of patients who would receive both PFS and OS benefits from local ablative treatment plus chemotherapy. Further development of other markers, such as circulating tumor DNA and circulating tumor cells,Citation27 is also awaited.
This study enrolled patients without EGFR, ALK, or ROS1 mutations, who may not benefit from treatment with tyrosine kinase inhibitors (TKIs). In the new era of targeted therapy, immunotherapy may be an alternative choice for NSCLC patients with postoperative oligometastases. Immunotherapy is a promising therapeutic option for the management of metastatic NSCLC.Citation28–Citation30 Combining immunotherapy with radiotherapy was found to further improve both local and distant diseases through enhanced T-cell activation.Citation31 Nevertheless, many patients did not respond to this type of combination therapy. Further understanding of the biological characteristics of postoperative oligometastases will be valuable for individualized treatment of patients with NSCLC and appropriate selection of patients who would benefit from local radiotherapy alone, local radiotherapy plus immunotherapy, or local radiotherapy plus chemotherapy, which may ultimately expand the survival benefit to more patients.
Like other retrospective studies, this study has some limitations. Bias was unavoidable, since the choice of treatment may be influenced by the physicians’ preferences and experiences. Although patients with oligometastases were identified by analyses of all available imaging findings, PET-CT was not commonly performed at the time of recurrence.
Conclusion
This study is the first to indicate that local therapy combined with chemotherapy improved PFS and ORR for NSCLC patients with postoperative oligometastases, but failed to prolong OS. Considering the increased incidence of grade ≥3 toxicities, this type of combination treatment should be used with caution and limited to specific patients. A prospective and randomized trial of local ablative treatment plus chemotherapy vs local treatment alone is urgently needed to validate these findings.
Ethics approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. For this retrospective study, the ethics committee waived the requirement to obtain patient consent, but all data were kept confidential.
Abbreviations
| ALK | = | anaplastic lymphoma kinase |
| CT | = | computed tomography |
| CTCAE | = | Common Terminology Criteria for Adverse Events |
| DFI | = | disease-free interval |
| ECOG | = | Eastern Cooperative Oncology Group |
| MRI | = | magnetic resonance imaging |
| NSCLC | = | non-small-cell lung cancer |
| ORR | = | objective response rate |
| OS | = | overall survival |
| PET | = | positron emission tomography |
| PFS | = | progression-free survival |
| PRS | = | post recurrence survival |
| RECIST | = | Response Evaluation Criteria for Solid Tumors |
| ROS1 | = | c-ros oncogene 1 kinase |
| SBRT | = | stereotactic body radiation therapy |
| TKIs | = | tyrosine kinase inhibitors |
Acknowledgments
This work was supported by the Project of Postdoctoral Science Foundation of China (Grant No: 2016M590640), the Project of Postdoctoral Innovation of Shandong Province (Grant No: 201501010), National Health and Family Planning Commission of China (201402011), and National Natural Science Foundation of China (81472812).
Disclosure
The authors report no conflicts of interest in this work.
References
- KelseyCRMarksLBHollisDLocal recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patientsCancer2009115225218522719672942
- al-KattanKSepsasEFountainSWTownsendERDisease recurrence after resection for stage I lung cancerEur J Cardiothorac Surg19971233803849332915
- MartiniNBainsMSBurtMEIncidence of local recurrence and second primary tumors in resected stage I lung cancerJ Thorac Cardiovasc Surg199510911201297815787
- MartinJGinsbergRJVenkatramanESLong-term results of combined-modality therapy in resectable non-small-cell lung cancerJ Clin Oncol20022081989199511956257
- DziedzicDARudzinskiPLangfortROrlowskiTPolish Lung Cancer Study Group (PLCSG)Risk factors for local and distant recurrence after surgical treatment in patients with non-small-cell lung cancerClin Lung Cancer2016175e157e16726831834
- PfannschmidtJEditorial on “Long-term survival outcome after postoperative recurrence of non-small cell lung cancer: who is ‘cured’ from postoperative recurrence?”J Thorac Dis201810261061329607121
- TorokJAGuLTandbergDJPatterns of distant metastases after surgical management of non-small-cell lung cancerClin Lung Cancer2017181e57e7027477488
- HellmanSWeichselbaumRROligometastasesJ Clin Oncol19951318107799047
- GomezDRBlumenscheinGRLeeJJLocal consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 studyLancet Oncol201617121672168227789196
- NakamuraMHashimotoNMayaharaHAdditional chemotherapy improved local control and overall survival after stereotactic body radiation therapy for patients with oligo-recurrenceRadiat Oncol20181317529688858
- BaeSHAhnYCNamHHigh dose involved field radiation therapy as salvage for loco-regional recurrence of non-small cell lung cancerYonsei Med J20125361120112723074111
- SekineINokiharaHYamamotoNKunitohHOheYTamuraTComparative chemotherapeutic efficacy in non-small cell lung cancer patients with postoperative recurrence and stage IV diseaseJ Thorac Oncol20094451852119347981
- ShimadaYSajiHKakihanaMKajiwaraNOhiraTIkedaNSurvival outcomes for oligometastasis in resected non-small cell lung cancerAsian Cardiovasc Thorac Ann201523893794426207006
- NiibeYNishimuraTInoueTOligo-recurrence predicts favorable prognosis of brain-only oligometastases in patients with non-small cell lung cancer treated with stereotactic radiosurgery or stereotactic radiotherapy: a multi-institutional study of 61 subjectsBMC Cancer201616165927542716
- De WolfJBellierJLepimpec-BarthesFExhaustive preoperative staging increases survival in resected adrenal oligometastatic non-small-cell lung cancer: a multicentre studyEur J Cardiothorac Surg201752469870329156014
- YamashitaHNiibeYYamamotoTLung stereotactic radiotherapy for oligometastases: comparison of oligo-recurrence and sync-oligo-metastasesJpn J Clin Oncol201646768769127162324
- HishidaTYoshidaJAokageKNagaiKTsuboiMPostoperative oligo-recurrence of non-small-cell lung cancer: clinical features and survivalEur J Cardiothorac Surg201649384785326201958
- KelseyCRCloughRWMarksLBLocal recurrence following initial resection of NSCLC: salvage is possible with radiation therapyCancer J200612428328816925972
- SekiharaKHishidaTYoshidaJLong-term survival outcome after postoperative recurrence of non-small-cell lung cancer: who is ‘cured’ from postoperative recurrence?Eur J Cardiothorac Surg201752352252828482033
- YanoTOkamotoTHaroALocal treatment of oligometastatic recurrence in patients with resected non-small cell lung cancerLung Cancer201382343143524113550
- WeichselbaumRRHellmanSOligometastases revisitedNat Rev Clin Oncol20118637838221423255
- YanoTHaroAYoshidaTPrognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancerJ Surg Oncol2010102785285520886558
- HowellGMCartySEArmstrongMJOutcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasisAnn Surg Oncol201320113491349623793361
- AshworthABSenanSPalmaDAAn individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancerClin Lung Cancer201415534635524894943
- WalshGLO’ConnorMWillisKMIs follow-up of lung cancer patients after resection medically indicated and cost-effective?Ann Thorac Surg1995606156315708787445
- WongACWatsonSPPitrodaSPClinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT)Cancer2016122142242225027206146
- IgnatiadisMLeeMJeffreySSCirculating tumor cells and circulating tumor DNA: Challenges and opportunities on the path to clinical utilityClin Cancer Res201521214786480026527805
- GaronEBRizviNAHuiRKEYNOTE-001 InvestigatorsPembrolizumab for the treatment of non-small-cell lung cancerN Engl J Med2015372212018202825891174
- BrahmerJReckampKLBaasPNivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancerN Engl J Med2015373212313526028407
- BorghaeiHPaz-AresLHornLNivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancerN Engl J Med2015373171627163926412456
- BristowRGAlexanderBBaumannMCombining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation OncologyLancet Oncol2018195e240e25129726389