Abstract
Background
Giant cell tumor of bone (GCTB) is a locally aggressive tumor, and its postoperative recurrence remains a problem. The present meta-analysis aimed to analyze the effect of bisphosphonates (BPs) on local recurrence of GCTB.
Methods
Seven case–control studies were included by computerized searches of bibliographic databases (PubMed, AMED, EMBASE, the Cochrane library, ISI Web of Science, and China National Knowledge Infrastructure). The pooled adjusted ORs were calculated to evaluate the local recurrence of GCTB.
Results
The BP group presented significantly lower total local recurrence rate than the control group in GCTB (P<0.01). Subgroup analysis shows BP group presented significantly lower local recurrence than the control group in GCTB with different tumor grades (P<0.05). In patients who underwent intralesional curettage, a significantly lower local recurrence rate was found in the BP group compared with the control group (P<0.01), but no significance was found for patients who underwent wide resection (P=0.16). None of the included studies described severe adverse effects related to BPs.
Conclusion
The results confirmed the effect of BPs on reducing the local recurrence of GCTB, and the effect is not influenced by the tumor grades. BPs are benefit for the patients who underwent intralesional curettage but not recommended for those who underwent wide resection.
Background
Giant cell tumor of bone (GCTB) is a common and locally aggressive bone tumor in East and Southeast Asian patients, which usually involves the end of long bones and comprises approximately one-fifth of all benign and potentially malignant bone tumors.Citation1 Although surgery remains the preferred treatment for GCTB, it is limited by a high recurrence rate regardless of wide resection or intralesional curettage. GCTBs are classified by Campanacci et al into three grades: stage I, latent; stage II, active; and stage III, aggressive according to their radiological appearance.Citation2 Wide resection may lead to severe restricted movement which is usually performed in stage III patient.Citation3 For tumors classified as stage I or II, intralesional curettage is often performed first. It was reported that the local recurrence rates range from 19% to 50% in the first 2 years for both surgical procedures.Citation4–Citation7
A multitude of studies revealed that chemical cauterization, such as hypertonic saline, phenol, alcohol, and liquid nitrogen, and other physical treatments, such as blurring, cryotherapy, and argon laser after intralesional curettage may reduce the risk of recurrence.Citation8–Citation11 These methods could cause many complications, such as infections, pathologic fractures, and damage to soft tissues. Recent studies reported the potential of bone metabolism drugs for GCTB, including bisphosphonates (BPs) and denosumab.Citation12–Citation14 Recent studies reveal that denosumab failed to show a benefit on local recurrence in the adjuvant setting in patients with GCTB treated with surgery.Citation15–Citation18 BPs are inhibitors of bone resorption, which promote bone mineralization and inhibit farnesyl pyrophosphate synthase.Citation19 Some studies showed that BPs could promote apoptosis of the stromal cell component, and reduce the recurrence rate after surgery in GCT.Citation20–Citation22
Recently, there were some studies evaluating the effect of BPs on preventing postoperative recurrence of GCTB.Citation23–Citation29 However, to the best of our knowledge, there is no definite direction or consensus on the application of BPs in GCTB. The present meta-analysis of six case–control trials aimed to confirm the effect of BPs on the local recurrence of GCTB and analyze the influence of different tumor stages and different procedures of surgery on this effect.
Methods
Study registration
The present meta-analysis was conducted according to the PRISMA ().Citation30 The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO).Citation31
Literature search
The literature search was conducted in duplicate by two independent investigators. An electronic systematic search of four databases including PubMed, AMED, EMBASE, the Cochrane Library, ISI Web of Science, and China National Knowledge Infrastructure was performed for relevant articles from the inception dates to October 28, 2018. The search consisted of the following keywords and Boolean operators: (alendronate OR pamidronate OR etidronate OR zoledronate OR clodronate OR bisphosphonate) AND (giant cell tumor). To include more additional eligible studies, a manual search was carried out on the bibliographies of related reviews and reference lists of all selected articles. If necessary, the authors of studies were contacted to provide additional information.
Selection criteria
The studies selected for inclusion were required to contain the following criteria: 1) the participants were diagnosed as GCTB and underwent surgical treatment; 2) the intervention was oral, intramuscular, or intravenous BPs after surgery; 3) the outcomes must include the local recurrence rate of GCTB; and 4) the design was case–control study. The exclusion criteria were as follows: 1) studies with no reported follow-up time or those with <6 months of follow-up time, 2) data of local recurrence were unavailable, and 3) the same participants reported in a previous article with a short follow-up.
Data extraction
The data were extracted by two independent investigators. If any disagreement was found, a third reviewer was consulted. For each eligible study, basic information was extracted onto a data collection form including the following parameters: the first author name, publication year, sample size, intervention protocol, control protocol, similarities between BP group and control group, surgical procedures, follow-up duration, and outcome measurements. If outcome data were not described as text, it was extrapolated from the accompanying figures, tables, or other supplementary material. Finally, the characteristics of the seven included studies were shown in . The primary outcome measurement was the local recurrence rate of GCTB. And the second measurement was the local recurrence rate in different subgroups, including different tumor grades (stage I–II and stage III) and different surgical procedures (intralesional curettage and wide resection). A sensitivity analysis was performed for the effect size by omitting the studies for which risk of bias and heterogeneity was imputed.
Table 1 Characteristics of the included studies
Methodological quality assessment
In the included studies, the methodological quality was independently assessed by two reviewers with the New-castle–Ottawa scale for risk of bias, in which assessing factors included selection, comparability, and exposure. The weighted kappa for the agreement on the trial quality between reviewers was 0.86 (95% CI, 0.80–0.92). The criteria of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system were used to evaluate the quality of evidence.
Statistical analysis
Extracted results were pooled in a meta-analysis. The meta-analysis was performed by computing ORs and their 95% CIs weighted by the inverse of a variance in Review Manager 5.3.5 software (The Cochrane Collaboration, Oxford, UK). The statistical heterogeneity was tested with I2 and the chi-squared test.Citation32 The value of I2 >50% was considered as high statistical heterogeneity and <50% as low statistical heterogeneity, respectively.Citation33 When there was no statistical evidence of heterogeneity, a fixed effects model was used; otherwise, a random-effect model was chosen.
Sensitivity analyses were performed by omitting each of the individual study. The heterogeneity P-value <0.05 was considered as statistically significant. The sensitivity analysis was performed only if there were three or more studies in comparison. Publication bias was assessed by visually inspecting the funnel plot asymmetry.
Results
Studies selection
A flow diagram illustrating the study identification is shown in . Literature search initially yielded 307 relevant articles, and 173 articles were excluded because they were duplicated. Of the 134 remaining articles, 86 articles were excluded by screening the title and abstract. Another 41 of the qualifying studies were excluded after their full texts were retrieved because they were laboratory studies, non-case–control study, reviews, unavailable data, or not relevant to the topic.
Figure 1 Flowchart of the selection strategy and inclusion/exclusion criteria for the present meta-analysis.
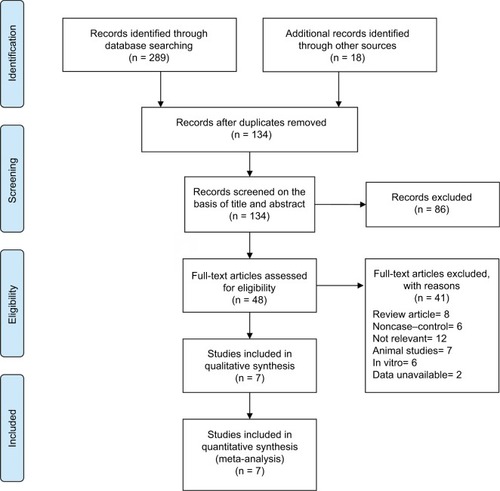
Finally, seven case–control studies involving 332 participants were included in our meta-analysis.Citation23–Citation29 The weighted kappa for agreement on eligibility between reviewers was 0.87 (95% CI, 0.79–0.95). The characteristics of the included trials are summarized in .
Methodological quality
The quality of included studies was assessed according to the Newcastle–Ottawa scale ().Citation34,Citation35 According to the Newcastle–Ottawa scale, the risk of bias within the included studies reached 6.43 stars on average, which is also acceptable as there is no study with a high risk of bias. Three studies were judged to have a low risk of bias (more than seven stars),Citation24,Citation26,Citation27 and three studies were found to have a moderate risk of bias (five or six stars).Citation23,Citation25,Citation28,Citation29 The reviewers achieved excellent agreement in the quality assessment of studies (intraclass correlation: 0.88; 95% CI, 0.84–0.92).
Table 2 Newcastle–Ottawa scale assessment of the quality of the studies
Recurrence rate of GCTB
It was illustrated that the BP group presented significantly lower local recurrence rate than the control group in GCTB (seven studies, OR, 0.20; 95% CI, 0.11–0.38; P<0.01) (). The BP group presented significantly lower local recurrence rate than the control group in GCTB in both of the subgroups with different tumor grades. For patients with stage I–II GCTB, a significant difference in local recurrence rate was found between the BP group and the control group (six studies, OR, 0.29; 95% CI, 0.11–0.76; P<0.05) (). In patients with stage III GCTB, a significant difference in local recurrence rate was found between the BP group and the control group (six studies, OR, 0.16; 95% CI, 0.07–0.39; P<0.01) (). Subgroup analysis based on different surgical procedures reveals a different result. In patients who underwent intralesional curettage, a significant difference in local recurrence rate was found between the BP group and the control group (five studies, OR, 0.19; 95% CI, 0.08–0.49; P<0.01) (). In patients who underwent wide resection, there was no significant difference in local recurrence rate between the BP group and the control group (four studies, OR, 0.35; 95% CI, 0.08–0.1.51; P=0.16) ().
Figure 2 Forest plots for the effect of BPs on total postoperative recurrence in patients with GCTB.
Abbreviations: BPs, bisphosphonates; GCTB, giant cell tumor of bone.
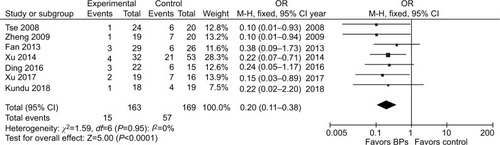
Figure 3 Forest plots for subgroup analysis for the effect of BPs on postoperative recurrence in patients with GCTB with different tumor grades.
Notes: (A) For patients with stage I–II GCTB, a significant difference in local recurrence rate was found between the BP group and the control group (P<0.05). (B) For patients with stage III GCTB, a significant difference in local recurrence rate was found between the BP group and the control group.
Abbreviations: BPs, bisphosphonates; GCTB, giant cell tumor of bone.
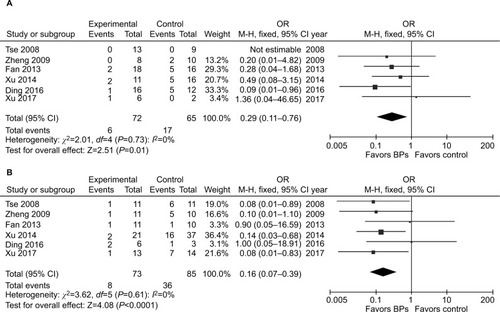
Figure 4 Forest plots for subgroup analysis for the effect of BPs on postoperative recurrence in patients with GCTB with different surgical procedures.
Notes: (A) For patients who underwent intralesional curettage, a significant difference in local recurrence rate was found between the BP group and the control group (P<0.01). (B) For patients who underwent wide resection, there was no significant difference in local recurrence rate between the BP group and the control group (P=0.16).
Abbreviations: BPs, bisphosphonates; GCTB, giant cell tumor of bone.
Risk of bias across studies
Publication bias assessments using funnel plots () indicated that there was no significant asymmetry and no significant evidence of bias among the included studies of the five meta-analyses.
Heterogeneity and sensitivity analysis
There was no statistical heterogeneity in these five analyses (total recurrence: χ2=1.59, P=0.95, I2=0%; subgroup of stage I–II GCTB: χ2=2.01, P=0.73, I2=0%; subgroup of stage III GCTB: χ2=3.62, P=0.61, I2=0%; subgroup of intralesional curettage: χ2=0.67, P=0.95, I2=0%; subgroup of wide resection: χ2=1.86, P=0.60, I2=0%). The heterogeneity and overall effect were not significantly altered by omitting any study.
Strength of evidence
According to the criteria of the GRADE system for evidence quality,Citation36 all the included trials in the present meta-analysis began as high-quality or moderate-quality evidence, which was downgraded by five categories of limitations (). Inadequate case definition, substantial loss to follow-up, and inconsistent reporting of outcomes in some studies might raise the risk of bias. The number of included patients <150 is considered to be small and may cause imprecision and effect size >0.05 is considered to be large and strengthen the evidence.
Adverse acute reaction
No serious or fatal acute adverse effect was reported related to BPs in all the included studies. The most common acute side effects were fever and gastric dyspepsia mentioned in three studies: 15 patients in Zheng et al;Citation24 six patients in Fan;Citation25 seven patients in Ding et al.Citation27 There was no major adverse effect on renal function or stress fractures.
Discussion
GCTB usually does not remain latent and tends to develop progressive destruction of the affected bone.Citation37 Therefore, surgical treatment should be performed as early as possible. Wide resection has an advantage of lower recurrence rate which is 0%–12%,Citation38,Citation39 as it removes the tumor entirely. Wide resection has been used in Campanacci stage III tumors or some cases without marked functional impairment, such as the ulna, fibula, and other small bones.Citation40,Citation41 However, wide resection may lead to restricting movement. Intralesional curettage with adjuvant methods is the preferred treatment for most cases of GCTB. This surgical procedure shows a better functional outcome but is associated with a higher risk of local recurrence.Citation42,Citation43 With developed surgical procedures and local adjuvants, the local recurrence rate of GCTB remains to be >20% in the recent studies.Citation4–Citation7,Citation43
Nowadays denosumab, a monoclonal antibody to RANK ligand, is approved for the most common use for this type of pathology. Due to its efficacy, denosumab is recommended as the first option in inoperable or metastatic GCT. However, some clinical studies showed that denosumab might have no effect on reducing the risk of recurrence in patients with GCTB following curettageCitation17,Citation18 or even might increase the risk of recurrence.Citation15,Citation16 In vitro studies found that the inhibitory effect of denosumab on neoplastic cells and osteoclast survival were not observed, whereas BPs inhibited the growth of neoplastic cells and osteoclast survival.
Experimental studies confirmed the cytotoxic effect of BPs on neoplastic stromal cells of GCTB, and clinical studies showed that administration of BPs could reduce the recurrence rate of GCTB after surgery. The present meta-analysis of case–control studies verifies that BPs could reduce the postoperative recurrence of GCTB from 33.7% to 9.2% (57 of 169 vs 15 of 163), with no statistical heterogeneity. Subgroup analysis showed that BP group presented significantly lower local recurrence rate than the control group, regardless of the tumors’ Campanacci stages. The antitumor mechanism of BPs is not clear. It was reported that BPs could induce the apoptosis of neoplastic stromal cells by blocking the mevalonate pathway. Moreover, BPs have been proved to inhibit the proteolytic activity of tumor cell-derived matrix metalloproteinase-2 (MMP-2) and MMP-9 by inhibiting the zinc-dependent proteolytic activity of matrix MMPs, which are essential for the degradation of extracellular matrix proteins, invasion, and migration.Citation44
Subgroup analysis of different surgical procedures showed that the recurrence of GCTB was significantly lower in BP group for patients who underwent intralesional curettage, but there was no difference between BP group and control group for patients who underwent wide resection. One possible interpretation of the different result is that wide resection avoids the marginal positive of bone in curettage and decreases the recurrence rate to a low level. Another interpretation is that recurrence is associated with soft tissue infiltration and wide resection removes all the infiltrate soft tissue.Citation45 These results suggest that BPs are benefit for the patients who underwent intralesional curettage but not necessary for those who underwent wide resection.
In this meta-analysis, six of the seven included studies reported preoperative application of BPs. Preoperative application of BPs could reduce tumor size and prevent surgical dissemination, but the frequency should be restricted because delayed surgery may lead to progression of tumor lesions. The duration of postoperative application of BPs was from 3 months to 2 years. Prolonged postoperative application of BPs was considered important because most instances of recurrence occur in the first 2 years after surgery.Citation46 In these studies, the main adverse reactions of BPs are mild and nonfatal in patients without renal dysfunction or stress fractures and include fever and digestive upset. However, some studies found that long-term and large-dose systemic administration of BPs might induce osteonecrosis of the jaw and atypical fracture of long bones.Citation47,Citation48 Above all, this meta-analysis confirmed the effect of BPs on local recurrence of GCTB but did not conclude that BPs can substitute the role of denosumab. It is reported that denosumab is very efficient in unresectable or metastatic GCTB as a neoadjuvant setting.Citation49 In fact, denosumab was associated with tumor responses and reduced the need for morbid surgery in patients with GCTB.Citation50 In the present meta-analysis, no evidence showed the role of BPs on these two parameters, tumor responses and the need for morbid surgery in patients with GCTB.
The strength of the present meta-analysis consists of being rigorously conducted according to PRISMA guidelines and using a robust systematic review and meta-analysis procedures. Moreover, the advantage of this meta-analysis over individual studies is a convincing clinical recommendation of postoperative application of BPs for GCTB to practitioners.
The present meta-analysis has several limitations. First, the limited number of studies and the small sample size in some studies might reduce the precision of the pooled estimates. For subgroup analysis, the total number of patients who underwent wide resection was only 51, which would lead to large bias in the overall effect. Further investigation with big sample size is required to confirm the different effect of BPs on the recurrence of GCTB in patient who underwent different surgical procedures. Second, all the included studies were case–control, which would downgrade the strength of evidence. Finally, the presented study analyzed the middle-term effect of BPs on local recurrence of GCTB, and the long-term effect remained unknown and required more clinical studies.
Conclusion
In the present meta-analysis of case–control, comparing controlled treatment, the use of BPs as an adjuvant therapy decrease the local recurrence rate of GCTB. This effect is not influenced by different Campanacci stages of GCTB. BPs are benefit for the patients who underwent intralesional curettage but not recommended for those who underwent wide resection.
Acknowledgments
This research was supported by Zhejiang Province Natural Science Foundation of China under Grant No. LQ16H060002, Medical and Health Science and Technology Project of Zhejiang Province under Grant No. 2016KYB120, and China Postdoctoral Science Foundation under Grant No. 2017M612012.
Supplementary materials
Figure S1 The funnel plots asymmetry for the outcome showed the evidence of publication bias on the meta-analyses for (A) total postoperative recurrence, (B) subgroup of stage I–II GCTB, (C) subgroup of stage III GCTB, (D) subgroup of intralesional curettage, and (E) subgroup of wide resection.
Abbreviation: GCTB, giant cell tumor of bone.
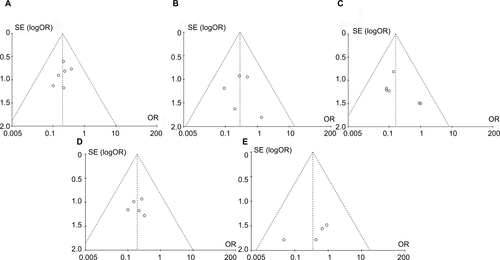
Table S1 PRISMA 2009 checklist
Table S2 GRADE evidence profile for effect of BPs on recurrence giant cell tumor of bone
Disclosure
The authors report no conflicts of interest in this work.
References
- ThangarajRGrimerRJCarterSRStirlingAJSpilsburyJSpoonerDGiant cell tumour of the sacrum: a suggested algorithm for treatmentEur Spine J20101971189119420076978
- CampanacciMBaldiniNBorianiSSudaneseAGiant-cell tumor of boneJ Bone Joint Surg Am19876911061143805057
- KamalAFSimbolonELPrabowoYHutagalungEUWide resection versus curettage with adjuvant therapy for giant cell tumour of boneJ Orthop Surg2016242228231
- GastonCLBhumbraRWatanukiMDoes the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone?J Bone Joint Surg Br201193-B1216651669
- BalkeMSchremperLGebertCGiant cell tumor of bone: treatment and outcome of 214 casesJ Cancer Res Clin Oncol2008134996997818322700
- BeneveniaJRiveroSMMooreJSupplemental bone grafting in giant cell tumor of the extremity reduces nononcologic complicationsClin Orthop Relat Res2017475377678326932739
- ChanCMAdlerZReithJDGibbsCPRisk factors for pulmonary metastases from giant cell tumor of boneJ Bone Joint Surg Am201597542042825740033
- van der HeijdenLDijkstraPDvan de SandeMAThe clinical approach toward giant cell tumor of boneOncologist201419555056124718514
- NithyananthMPriscillaAJBoopalanPVTitusVTLeeVNTime required for effective action of phenol against giant cell tumour cellsJ Orthop Surg2014221104107
- van der HeijdenLvan der GeestICSchreuderHWvan de SandeMADijkstraPDLiquid nitrogen or phenolization for giant cell tumor of bone? A comparative cohort study of various standard treatments at two tertiary referral centersJ Bone Joint Surg Am2014965e3524599207
- ChaudharyPKhadimHGajraADamronTShahCBisphosphonate therapy is effective in the treatment of sacral giant cell tumorOnkologie2011341270270422156450
- BorkowskaAGoryńTPieńkowskiADenosumab treatment of inoperable or locally advanced giant cell tumor of boneOncol Lett20161264312431828101196
- BalkeMCampanacciLGebertCBisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of boneBMC Cancer201010146220799989
- LauCPHuangLWongKCKumtaSMComparison of the anti-tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of boneConnect Tissue Res201354643944924060052
- MüllerDABeltramiGScocciantiGCampanacciDAFranchiACapannaRRisks and benefits of combining denosumab and surgery in giant cell tumor of bone – a case seriesWorld J Surg Oncol201614128127809843
- ErraniCTsukamotoSLeoneGDenosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettageJ Bone Joint Surg Am2018100649650429557866
- MakIWEvaniewNPopovicSTozerRGhertMA translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumabJ Bone Joint Surg Am20149615e12725100780
- ShibuyaITakamiMMiyamotoAIn vitro study of the effects of denosumab on giant cell tumor of bone: comparison with zoledronic acidPathol Oncol Res Epub20171120
- YokoyamaTMizuguchiMOstermannAProtonation state and hydration of bisphosphonate bound to farnesyl pyrophosphate synthaseJ Med Chem201558187549755626314394
- ChengYYHuangLKumtaSMLeeKMLaiFMTamJSCytochemical and ultrastructural changes in the osteoclast-like giant cells of giant cell tumor of bone following bisphosphonate administrationUltrastruct Pathol200327638539114660277
- ChangSSSuratwalaSJJungKMBisphosphonates may reduce recurrence in giant cell tumor by inducing apoptosisClin Orthop Relat Res2004426103109
- ChenKHWuPKChenCFChenWMZoledronic acid-loaded bone cement as a local adjuvant therapy for giant cell tumor of the sacrum after intralesional curettageEur Spine J201524102182218825940568
- TseLFWongKCKumtaSMHuangLChowTCGriffithJFBisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case-control studyBone2008421687317962092
- ZhengXYinQKumtaSMHuangHZhangYZhangTClinical study of bisphosphonates reducing local recurrence in extremity giant cell tumor of boneChinese Clin Oncol20091411001104
- FanJAnalysis of Zoledronic Acid to Assist Clinical Effect Surgical Treatment for Giant Cell Tumor of Bone [master thesis]Changsha, Hunan, ChinaCentral South University2013
- XuWXuLLiLPrognostic factors of giant cell tumor in mobile spineChin J Orthop201434487493
- DingLHanXHuangTZhangHAdjuvant administration of bisphosphonates decreases local recurrence rate of extremity giant cell tumor of boneChin J Heal Care Med2016186769
- XuWWangYWangJLong-term administration of bisphosphonate to reduce local recurrence of sacral giant cell tumor after nerve-sparing surgeryJ Neurosurg Spine201782204716721
- KunduZSSenRDhimanASharmaPSiwachRRanaPEffect of intravenous zoledronic acid on histopathology and recurrence after extended curettage in giant cell tumors of bone: a comparative prospective studyIndian J Orthop2018521455029416169
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaborationBMJ2009339jul21 1b270019622552
- ShiMChenLWangYWangWYanSEffect of bisphosphonates on local recurrence of giant cell tumor. PROSPERO International prospective register of systematic reviews web Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018087636Accessed September 8, 2018
- BeckerLCochrane Handbook for Systematic Reviews of Interventions Version 5.1.4Chichester, EnglandWiley-Blackwell2011
- HigginsJPTThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- WellsGSheaBO’ConnellDThe Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-AnalysesOttawa, Ontario, CanadaOttawa Hospital Research Institute2013
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- SchünemannHJOxmanADBrozekJGrading quality of evidence and strength of recommendations for diagnostic tests and strategiesBMJ200833676531106111018483053
- McgoughRLRutledgeJLewisVOLinPPYaskoAWImpact severity of local recurrence in giant cell tumor of boneClin Orthop Relat Res200543811612216131879
- Arbeitsgemeinschaft KnochentumorenBeckerWTDohleJLocal recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapyJ Bone Joint Surg Am20089051060106718451399
- ErraniCRuggieriPAsenzioMAGiant cell tumor of the extremity: a review of 349 cases from a single institutionCancer Treat Rev20103611719879054
- KlenkeFMWengerDEInwardsCYRosePSSimFHGiant cell tumor of bone: risk factors for recurrenceClin Orthop Relat Res2011469259159920706812
- MozaffarianKModjallalMVosoughiARTreatment of giant cell tumor of distal radius with limited soft tissue invasion: curettage and cementing versus wide excisionJ Orthop Sci201823117417929110910
- PazionisTJAlradwanHDeheshiBMTurcotteRFarrokhyarFGhertMA systematic review and meta-analysis of en-bloc vs intralesional resection for giant cell tumor of bone of the distal radiusOpen Orthop J20137110310823730371
- GortzakYKandelRDeheshiBThe efficacy of chemical adjuvants on giant-cell tumour of bone. An in vitro studyJ Bone Joint Surg Br201092101475147921089702
- BoissierSFerrerasMPeyruchaudOBisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastasesCancer Res200060112949295410850442
- XuLJinJHuASoft tissue recurrence of giant cell tumor of the bone: prevalence and radiographic featuresJ Bone Oncol20179101429018768
- KarpikMGiant cell tumor (tumor gigantocellularis, osteoclastoma)--epidemiology, diagnosis, treatmentOrtop Traumatol Rehabil201012320721520675862
- MiglioratiCAWooSBHewsonIA systematic review of bisphosphonate osteonecrosis (BON) in cancerSupport Care Cancer20101881099110620411279
- SellmeyerDEAtypical fractures as a potential complication of long-term bisphosphonate therapyJAMA2010304131480148420924014
- RutkowskiPGastonLBorkowskaADenosumab treatment of inoperable or locally advanced giant cell tumor of bone--Multicenter analysis outside clinical trialEur J Surg Oncol20184491384139029650420
- ChawlaSHenshawRSeegerLSafety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 studyLancet Oncol201314990190823867211
