Abstract
Purpose
The inflammatory status of patients with cancer appears to affect cancer progression and patient prognosis. We examined the characteristics of cancer-associated systemic and local inflammation and its impact on the overall survival (OS) of patients with locally advanced rectal cancer (LARC) who received neoadjuvant radiotherapy (nRT).
Patients and methods
Seventy-six consecutive LARC patients who received nRT from February 2012 to September 2015 were retrospectively analyzed. The peripheral neutrophil-to-lymphocyte ratio (NLR) was determined at diagnosis, and the CD8+ T-cell count was determined from surgical specimens. Factors associated with OS were identified by univariate and multivariate Cox regression.
Results
The median follow-up time was 23.0 months (range: 2–59), and the overall 5-year OS rate was 68.6% (95% CI =46.06–91.14). Patients with a high NLR (≥2.0) and a low CD8+ T-cell count (<9%) had a significantly worse 5-year OS than those with a low NLR and a high CD8+ T-cell count (P=0.005). NLR was also associated with lymphovascular invasion (P=0.014) and T stage (P=0.047), and the CD8+ T-cell count was associated with mucinous adenocarcinoma (P=0.005) and T stage (P=0.049). An NLR <2.0 was associated with pathological complete regression after nRT (P=0.039). Multivariate Cox regression indicated that NLR (P=0.025), CD8+ T-cell count (P=0.018), age (P=0.020), lymphovascular invasion (P=0.038), and T stage (P=0.011) were independently associated with OS.
Conclusion
A high NLR and a low CD8+ T-cell count were significantly associated with poor survival in our population of patients with LARC. Measurement of markers of systemic and local inflammation might help to predict the prognosis of patients with LARC after nRT.
Introduction
Colorectal cancer (CRC) is the third most common malignancy worldwide, and rectal cancer accounts for 30.7% of all cases of CRC. There will be an estimated 43,030 newly diagnosed cases of rectal cancer in the USA during 2018.Citation1 Neoadjuvant radiotherapy (nRT) and chemoradiotherapy are the standard treatments for locally advanced rectal cancer (LARC).Citation2 However, it remains difficult to predict the short-term efficacy and long-term outcomes following nRT, and this information might help to assist in decisions about subsequent treatment. Therefore, an important clinical priority is the identification of LARC patients who have the greatest risk of poor therapeutic response and poor prognosis.
The cancer-related inflammatory response impacts the development, progression, and clinical outcomes of patients with many different cancers, including CRC, prostate cancer, bladder cancer, and other cancers.Citation3–Citation7 Previous research reported that an increased neutrophil-to-lymphocyte ratio (NLR), a biomarker for systemic inflammation, was associated with poor outcomes and reduced survival in patients with non-metastatic CRC.Citation8,Citation9 Another research indicated that the NLR is an independent prognostic indicator of response to chemotherapy in patients with locally advanced CRC.Citation10 However, the clinical value of NLR in predicting resistance to radiotherapy (RT) and outcomes in patients with LARC is uncertain.
A high level of tumor-infiltrating lymphocytes, especially high CD8+ cytotoxic T-lymphocytes, promotes an antitumor immune response and is associated with favorable outcomes in cancer patients.Citation11–Citation13 Additionally, the presence of CD8+ T-cells within cancer cell nests is associated with more favorable prognosis in patients with CRC.Citation14 Previous research indicated that the peripheral lymphocyte level was associated with response to neoadjuvant treatment in patients with rectal cancer,Citation15,Citation16 esophageal cancer,Citation17 and breast cancer.Citation18,Citation19 Nevertheless, the implications of CD8+ T-cell infiltration into tumors after nRT remain unknown. In addition, little is known about whether the combined use of two inflammation markers (one for systemic inflammation and the other one for local inflammation) has better prognostic value in predicting treatment response and survival.
As far as we know, no previous research has examined the combined use of markers of systemic and local inflammation to predict the clinical outcomes of patients with LARC. Thus, we examined the characteristics of cancer-associated markers of systemic and local inflammation and their association with pathological response and survival in patients with LARC who received nRT.
Patients and methods
Patient selection
Tumor tissue samples were examined retrospectively from 80 consecutive LARC patients who received nRT followed by surgery at our institute from February 2012 to September 2015. The inclusion criteria were pathological confirmation of rectal adenocarcinoma with a clinical diagnosis of T3/4 and/or N+ stage disease, according to the eighth edition of the American Joint Committee on Cancer. All demographic characteristics and laboratory results were recorded. The exclusion criteria were diagnosis with more than one malignancy or Karnofsky performance score <70. In addition, four tissue samples were in poor condition, so these patients were excluded. A total of 76 patients were finally enrolled. This study was approved by the Ethics Committee of Fujian Cancer Hospital, Fuzhou, China (No. KT2018-009-01). The need for individual consent was waived by the ethics committee because patient medical records and tumor specimens were analyzed retrospectively, and no patient-identifiable information was utilized.
Treatments
The RT regimens, consisting of long-course RT (LCRT; 50 Gy in 25 fractions) or short-course RT (25 Gy in 5 fractions), were delivered by intensity-modulated radiation therapy or a conventional two-dimensional technique. The primary tumor and the anorectal, mesorectal, presacral, and internal iliac lymph nodes were part of the clinical target volume for intensity-modulated radiation therapy. Additionally, radiation was delivered to the primary tumor and all nodes that were at risk in the whole pelvic region (prior to the periphery of the sacrum and inferior to the symphysis pubis in the two-dimensional conventional RT). 5-Fluorouracil-based chemotherapy was administered routinely with LCRT. Radical surgery was performed 6–8 weeks after LCRT or 1 week after short-course RT by experienced colorectal surgeons.
Marker of systemic inflammation
The NLR was the primary measure for systemic inflammation. The NLR was available from routine peripheral venous blood samples that were collected within 3 days before nRT, at which time white blood cells, neutrophils, and lymphocyte counts were determined. Blood cell counts were analyzed with an XE-2100 Hematology System (Sysmex Corporation, Kobe, Japan) using whole blood samples collected in EDTA tubes (Changgeng Medical Equipment Co. Ltd, Fuzhou, China).
Marker of local inflammation
The CD8+ T-cell count was the primary measure of local inflammation. These data were obtained from surgical specimens. All paraffin-embedded specimen blocks were sliced into 4 µm sections, xylene was used for deparaffinization, and the sections were then rehydrated by an alcohol gradient. The sections were then rinsed in 0.05% PBS and blocked with hydrogen peroxidase at room temperature. Before immunohistochemistry, heat retrieval with a sodium citrate buffer and EDTA repair were performed. The samples were incubated with the monoclonal anti-CD8 antibody (ab4055, 1:200; Abcam, Southampton, UK) at room temperature for 15 minutes, washed, and then incubated with the secondary antibody for 10 minutes, followed by color development with 3,3′-diaminobenzidine at room temperature in darkness for 10 minutes. All sections were counterstained with hematoxylin. Three random fields, each within the area where the tumor cells were most strongly stained, were chosen from each section for determination of the CD8+ T-cell count. The average count of these three fields was considered the number of CD8+ T-cells per field. The proportion of CD8+ T-cells in all the lymphocytes within the tumor was defined as the density of CD8+ T-cells.
Tumor regression grading (TRG)
The surgical specimens after nRT were independently evaluated by two pathologists using H&E staining. TRG was determined using the five-tiered Mandard system (fibrosis/tumor relation): TRG 1: no residual cancer cells; TRG 2: rare residual cancer cells; TRG 3: dominant fibrosis outgrowing a few residual cancer cells; TRG 4: residual cancer outgrowing fibrosis; and TRG 5: no regressive changes.Citation20
Statistical analyses
The end point of the exploratory analysis was time of overall survival (OS), defined as the time from surgical resection to death. The optimal cutoff values for the NLR and CD8+ T-cell count were determined using the Cutoff Finder (http://molpath.charite.de/cutoff), in which the significance of correlation with a survival variable was determined by fitting Cox proportional hazard models to the dichotomized variable and the survival variable.Citation21 Then, bivariate logistic regression was used to evaluate possible predictors of the NLR and CD8+ T-cell count. A chi-squared test was used to assess the associations between these biomarkers and tumor regression after nRT. Kaplan–Meier method was used to analyze survival, and the log-rank test was used for comparisons of survival curves. Cox regression was performed to identify factors significantly associated with OS. A P-value <0.05 was considered significant. All analyses were conducted using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
We enrolled 76 consecutive patients with LARC who received treatment at our institution (Fujian Cancer Hospital, Fuzhou, China) from February 2012 to September 2015 (). Most patients were younger than 60 years (73.7%) and were male (64.5%), and 49 tumors (64.5%) were in lower rectum (within 5 cm of the anal verge). A total of 45 patients (59.2%) had T2/3 cancer, 31 patients (40.8%) had stage T4 cancer, and 48 patients (63.2%) had lymph node involvement. Fifteen patients (19.7%) had lymphovascular invasion, 14 patients (18.4%) had neural invasion, and 16 patients (21.1%) had tumor nodules.
Table 1 Characteristics of 76 patients with locally advanced rectal cancer
Cutoff values for NLR and CD8+ T-cell count
The Cutoff Finder software identified an optimal NLR cutoff value of 2.0 for our patients. Thus, 35 patients (46.1%) had NLR values below 2.0 and 41 patients (53.9%) had NLR values of 2 or more. This software also indicated the optimal CD8+ T-cell cutoff value was 9%. Thus, 31 patients (40.8%) had low CD8+ T-cell counts (<9%) and 45 patients (59.2%) had high CD8+ T-cell counts (≥9%), as shown in .
Figure 1 Optimization of marker cutoffs in patients with LARC (n=76): Kaplan–Meier curves of OS for patients with low vs high NLR (A) and with low vs high CD8+ T-cell count (C). Waterfall plots showing distributions of the NLR (B) and CD8+ T-cell count (D) among all patients, used for optimal dichotomization by Cutoff Finder software.
Notes: The analysis was conducted by Cutoff Finder software.
Abbreviations: LARC, locally advanced rectal cancer; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival.
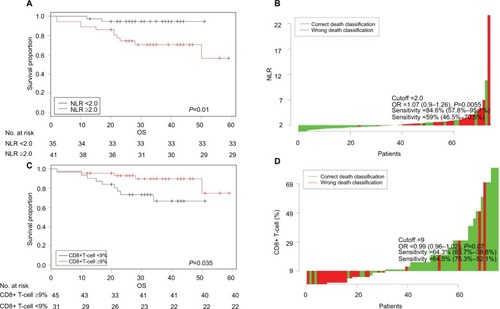
shows the characteristics of the 76 LARC patients categorized by these NLR and CD8+ T-cell count cutoff values. Bivariate logistic analysis indicated that a high NLR was significantly associated with lymphovascular invasion (P=0.014) and stage T4 cancer (P=0.047), but none of the other examined characteristics. Additionally, a low CD8+ T-cell count was significantly associated with mucinous adenocarcinoma (P=0.005) and stage T4 cancer (P=0.049), but none of the other examined characteristics.
Table 2 Clinicopathologic factors associated with NLR and CD8+ T-cells
Association of NLR and CD8+ T-cell count with outcomes
Seven patients (9.2%) had TRG 1, 22 patients (28.9%) had TRG 2, 16 patients (21.1%) had TRG 3, 27 patients (35.5%) had TRG 4, and 4 patients (5.3%) had TRG 5. Based on these data, we classified the patients as having complete (TRG 1) or incomplete (TRG 2–5) tumor regression. We then analyzed the relationship of each inflammation marker with pathological complete response using a chi-squared test. The results show that an NLR <2.0 prior to nRT was associated with pathologic complete regression (pCR; P=0.039), but there was no such association of CD8+ T-cell count with pCR (P=0.396).
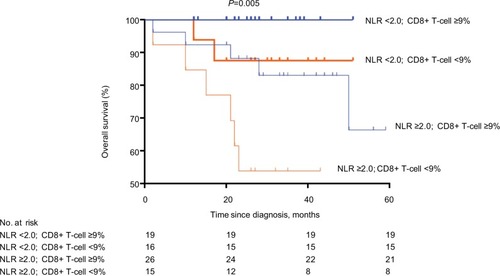
Kaplan–Meier analysis () indicated that patients with a high NLR and a low CD8+ T-cell count had the shortest survival, and patients with a low NLR and a high CD8+ T-cell count had the longest survival (P=0.005; ).
Figure 2 Survival curves of patients with low NLR and high CD8+ T-cell count (n=19), low NLR and low CD8+ T-cell count (n=16), high NLR and high CD8+ T-cell count (n=26), and high NLR and low CD8+ T-cell count (n=15).
Note: The analysis was conducted by Kaplan–Meier method.
Abbreviation: NLR, neutrophil-to-lymphocyte ratio.
shows the results of our univariate and multivariate analyses. Univariate analysis indicated that a low NLR (HR =5.039, 95% CI =1.125–22.573, P=0.035) and a high CD8+ T-cell count (HR =0.322, 95% CI =0.106–0.977, P=0.045) were significantly associated with longer OS. These relationships were also significant in multivariate analysis (NLR: HR =7.707, 95% CI =1.300–45.709, P=0.025; CD8+ T-cell count: HR =0.088, 95% CI =0.012–0.665, P=0.018). Univariate analysis also indicated that age, lymphovascular invasion, mucinous adenocarcinoma, and cancer T stage were significantly associated with OS (P<0.05). Multivariate analysis indicated that age (HR =16.130, 95% CI =1.557–167.151, P=0.020), lymphovascular invasion (HR =7.166, 95% CI =1.115–46.047, P=0.038), and cancer T stage (HR =0.029, 95% CI =0.002–0.449, P=0.011) were independently associated with OS.
Table 3 Correlation of clinicopathologic factors with overall survival
Discussion
To the best of knowledge, this is the first study to evaluate the association of markers of systemic inflammation (NLR) and local inflammation (CD8+ T-cell count), individually and in combination, with the OS of patients with LARC. We found that a lower NLR (<2.0) before nRT was associated with increased tumor regression following nRT. Additionally, a high NLR and a low CD8+ T-cell count were each significantly associated with poor survival. These measures of systemic and local inflammation are easily determined in clinical practice and are promising prognostic indicators for patients with LARC.
Recent studies indicated that several inflammation-based factors were associated with the aggressiveness of several types of tumors. For example, Deng et alCitation22 studied patients with gastric cancer and found that the NLR was significantly associated with the depth of invasion, tumor stage, and lymph node metastasis (P<0.05 for all comparisons). Consistent with previous studies, we found that LARC patients with an NLR of 2.0 or more were more likely to have higher T stage (P=0.047) and lymphovascular invasion (P=0.014). Moreover, a study of patients with breast cancer indicated that a lower CD8+ T-cell count was significantly associated with higher TNM stage (P=0.013), lymph node involvement (P=0.027), and immunopositivity of Ki-67 (a marker of proliferation; P=0.026).Citation23 Similarly, we found that LARC patients with CD8+ T-cell counts <9% after nRT were more likely to have a higher T stage (P=0.049) and mucinous adenocarcinoma (P=0.005).
Increasing evidence suggests that tumor-associated inflammation might affect the induction of chemoradiation resistance of many cancers. In particular, several studies that investigated the associations between systemic inflammation and tumor chemosensitivity found that a decreased NLR was related to a better response to nCT.Citation6,Citation24,Citation25 Additionally, greater intratumoral lymphocytic infiltration was associated with a better response to nCT or nRT.Citation26 Xiao et alCitation27 studied patients with rectal cancer and found a significant correlation of pCR with a low NLR in pretreatment circulating blood (P=0.043), but not with CD8+ T-cell count in biopsy samples (P=0.100). In accordance with these results, we found that a low NLR (<2.0) was associated with favorable tumor regression after nRT (P=0.039), but there was no significant association between CD8+ T-cell count and tumor regression in surgical specimens. Previous research indicated that nRT and nCT can cause immunogenic tumor cell death and induce a T-cell reaction, thereby enhancing systemic antitumor immune effects.Citation28 A study of rectal cancer patients indicated that the CD8+ T-cell counts of surgical specimens collected after neoadjuvant treatment were significantly higher than those from biopsy specimens.Citation29 These findings motivated our investigation of the relationship between CD8+ T-cell counts in biopsy samples before nRT and tumor regression after neoadjuvant treatment.
Previous studies have verified the prognostic significance of inflammation-based biomarkers in cancer patients. For example, a large study found that the NLR was independently associated with outcome in patients with solid tumors (HR =1.58, 95% CI =1.34–1.86).Citation24 Chua et alCitation10 demonstrated that a lower NLR was associated with longer survival of patients with advanced CRC (P=0.009). Another study of patients with nonmetastatic CRC indicated that an NLR of 3.0 or more was independently associated with worse OS (HR =1.64, 95% CI =1.40–1.91).Citation8 A study of patients with CRC indicated that infiltration of cancer cell nests by CD8+ T-cells was a reliable biomarker of longer survival (HR =0.605, 95% CI =0.41–0.89, P=0.0011).Citation14 In accordance with these earlier studies, we found that an NLR of 2.0 or more and CD8+ T-cell count <9% were potential predictors of poor survival rate for patients with LARC. Moreover, our multivariate analysis, which adjusted for sex, age, lymphovascular invasion, neural invasion, mucinous adenocarcinoma, T stage, and N stage, also indicated that NLR (HR =7.71, 95% CI =1.30–45.71, P=0.025) and CD8+ T-cell count (HR =0.09, 95% CI =0.01–0.67, P=0.018) were significantly and independently associated with OS.
We do not yet know why a high NLR and a low CD8+ T-cell count are associated with worse outcomes in patients with LARC. An increased NLR can be due to a high neutrophil count or a low lymphocyte count.Citation30 Neutrophils can produce several cytokines, such as vascular endothelial growth factor and TGF-beta, after recognition and interaction with tumor cells, and this might promote cancer cell proliferation, infiltration, and metastasis.Citation24 On the other hand, tumor-infiltrating lymphocytes, particularly CD8+ T-cells, are considered the hallmark of the local immune response against cancer cell growth.Citation14 Antitumor CD8+ T-cells can infiltrate tumors after RT, and a high dose of radiation can impact the immunosuppressive microenvironment of the tumor.Citation31 Intratumoral CD8+ T-cells can release cytotoxic molecules and cytokines that specifically recognize and kill cancer cells via the major histocompatibility complex class I molecule.Citation32
There were some limitations of this study. First, it had a retrospective design, only examined the records of 76 patients, and did not include patients who did not receive nRT. Second, the CD8+ T-cell count before treatment was unavailable due to the limits of biopsy samples. A future study with a larger sample size is warranted to verify our conclusions.
Conclusion
The NLR and the CD8+ T-cell count are simple and readily available laboratory variables that have potential use in guiding treatment decisions and prediction of prognosis in patients with LARC. A high NLR had a negative impact on tumor radiosensitivity of patients with LARC. In addition, the combined presence of a high NLR and a low CD8+ T-cell count was significantly associated with poor survival. A future study with a larger sample size is warranted to confirm the combined use of these two biomarkers for prediction of prognosis in patients with LARC.
Acknowledgments
The authors would like to express their sincere gratitude to Yanping Chen and Lihua Zhong for help with pathological diagnosis in this study. This research was funded by the Fujian Province Natural Science Foundation, grant numbers 2016J01437 and 2017J01260; the Fujian Medical Innovation Project, grant number 2015-CX-8; the Joint Funds for the Innovation of Science and Technology, Fujian Province, grant number 2017Y9074; the Key Clinical Specialty Discipline Construction Program of Fujian, China, the National Clinical Key Specialty Construction Program; and Fujian Province Finance Department Project (No. (2015) 1249).
This paper has been accepted by ESMO Congress, Munich, Germany, 2018.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- WiltinkLMNoutRAFioccoMNo increased risk of second cancer after radiotherapy in patients treated for rectal or endometrial cancer in the randomized TME, PORTEC-1, and PORTEC-2 trialsJ Clin Oncol201533151640164625534376
- YasuiMHasegawaYKawaharaTBaseline neutrophil-to-lymphocyte ratio predicts the prognosis of castration-resistant prostate cancer treated with abiraterone acetateMol Clin Oncol20188459259429541468
- OjerholmESmithAHwangWTNeutrophil-to-lymphocyte ratio as a bladder cancer biomarker: assessing prognostic and predictive value in SWOG 8710Cancer2017123579480127787873
- Semeniuk-WojtaśALubasAStecRSyryłoTNiemczykSSzczylikCNeutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysisClin Genitourin Cancer2018163e685e69329454639
- SeahJALeibowitz-AmitRAtenafuEGNeutrophil-lymphocyte ratio and pathological response to neoadjuvant chemotherapy in patients with muscle-invasive bladder cancerClin Genitourin Cancer2015134e229e23325777682
- HuBFriedmanGElinavEFlavellRATransmissible inflammation-induced colorectal cancer in inflammasome-deficient miceOncoimmunology2018e981995
- FelicianoEMCKroenkeCHMeyerhardtJAAssociation of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS studyJAMA Oncol2017312e17231928796857
- TempletonAJMcNamaraMGŠerugaBPrognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysisJ Natl Cancer Inst20141066dju12424875653
- ChuaWCharlesKABaracosVEClarkeSJNeutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancerBr J Cancer201110481288129521448173
- HorneZDJackRGrayZTIncreased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancerJ Surg Res201117111521571304
- KilicALandreneauRJLuketichJDPennathurASchuchertMJDensity of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumorsJ Surg Res2011167220721019896677
- OshikiriTMiyamotoMShichinoheTPrognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune responseJ Surg Oncol200384422422814756433
- NaitoYSaitoKShiibaKCD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancerCancer Res19985816349134949721846
- HeoJOhYTNohOKChunMParkJEChoSRNodal tumor response according to the count of peripheral blood lymphocyte sub-populations during preoperative chemoradiotherapy in locally advanced rectal cancerRadiat Oncol J201634430531227927012
- KitayamaJYasudaKKawaiKSunamiENagawaHCirculating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancerRadiat Oncol2010514720525293
- FangPJiangWDavuluriRHigh lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancerRadiother Oncol2018128358459029530432
- LeeHJSeoJYAhnJHAhnSHGongGTumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patientsJ Breast Cancer2013161323923593079
- DenkertCvon MinckwitzGBraseJCTumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancersJ Clin Oncol201533998399125534375
- MandardAMDalibardFMandardJCPathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlationsCancer19947311268026868194005
- BudcziesJKlauschenFSinnBVCutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimizationPLoS One2012712e5186223251644
- DengQHeBLiuXPrognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive modelJ Transl Med20151316625885254
- KimSTJeongHWooOHTumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancerAm J Clin Oncol201336322423122495453
- LiXDaiDChenBTangHXieXWeiWThe value of neutrophil-to-lymphocyte ratio for response and prognostic effect of neoadjuvant chemotherapy in solid tumors: a systematic review and meta-analysisJ Cancer20189586187129581764
- AsanoYKashiwagiSOnodaNPredictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancerAnn Surg Oncol20162341104111026511266
- MatsutaniSShibutaniMMaedaKSignificance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancerCancer Sci2018109496697929464828
- XiaoBPengJZhangRDensity of CD8+ lymphocytes in biopsy samples combined with the circulating lymphocyte ratio predicts pathologic complete response to chemoradiotherapy for rectal cancerCancer Manag Res2017970170829225479
- KalbasiAJuneCHHaasNVapiwalaNRadiation and immunotherapy: a synergistic combinationJ Clin Invest201312372756276323863633
- TengFMuDMengXTumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancerAm J Cancer Res2015562064207426269765
- BrandauSDumitruCALangSProtumor and antitumor functions of neutrophil granulocytesSemin Immunopathol201335216317623007469
- FilatenkovABakerJMuellerAMAblative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissionsClin Cancer Res201521163727373925869387
- KlebanoffCAGattinoniLRestifoNPCD8+ T-cell memory in tumor immunology and immunotherapyImmunol Rev2006211121422416824130
