Abstract
Introduction
Thyroid cancer (TC) is an important common endocrine malignancy, and its incidence has increased in the past decades. The current TC diagnosis and classification tools are fine-needle aspiration (FNA) and histological examination following thyroidectomy. The metabolite profile alterations of thyroid cells (oncometabolites) can be considered for current TC diagnosis and management protocols.
Methods
This systematic review focuses on metabolite alterations within the plasma, FNA specimens, and tissue of malignant TC contrary to benign, goiter, or healthy TC samples. A systematic search of MEDLINE (PubMed), Scopus, Embase, and Web of Science databases was conducted, and the final 31 studies investigating metabolite biomarkers of TC were included.
Results
A total of 15 targeted studies and 16 untargeted studies revealed several potential metabolite signatures of TC such as glucose, fructose, galactose, mannose, 2-keto-d-gluconic acid and rhamnose, malonic acid and inosine, cholesterol and arachidonic acid, glycosylation (immunoglobulin G [IgG] Fc-glycosylation), outer mitochondrial membrane 20 (TOMM20), monocarboxylate transporter 4 (MCT4), choline, choline derivatives, myo-/scyllo-inositol, lactate, fatty acids, several amino acids, cell membrane phospholipids, estrogen metabolites such as 16 alpha-OH E1/2-OH E1 and catechol estrogens (2-OH E1), and purine and pyrimidine metabolites, which were suggested as the TC oncometabolite.
Conclusion
Citrate was suggested as the first most significant biomarker and lactate as the second one. Further research is needed to confirm these biomarkers as the TC diagnostic oncometabolite.
Introduction
Thyroid cancer (TC) is the most common endocrine-related tumor in the past decades, and its incidence has been increasing all over the world.Citation1–Citation4 The starting point of TC is the thyroid nodule formation detectable by ultrasonography (US) evaluations.Citation5,Citation6 Thyroid nodules are mostly benign, and the current gold standard discriminative tool between TC and benign thyroid nodules (BTNs) is a cytopathologic analysis of percutaneous fine-needle aspiration (FNA) specimens.Citation7 FNA is a simple test that samples a small amount of tissue from the thyroid with a very thin (or “fine”) needle.Citation8–Citation11 Histopathological report of FNA has a weak point of indeterminate results, negative predictive value, and high cost.Citation12,Citation13 Hence, there is an extreme need to find molecular markers either to support FNA or to take the place of FNA.Citation14–Citation17
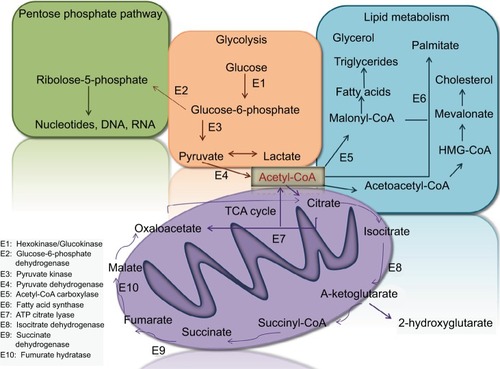
Increasing evidence indicates that tumor-associated mutations represent key factors resulting in different profiles of the cancerous cells’ genomics, epigenomics, transcripttomics, proteomics, and metabolomics.Citation18–Citation20,Citation117,Citation118 Metabolomics is an extensive-scale study of small molecules (>1,000 Da), generally popular as metabolites, within cells, biofluids, tissues, or organisms.Citation21–Citation23 The major dissimilarities between cancerous cells and their counterpart noncancerous cells are their metabolites, which are called “oncometabolites”.Citation24 For the first time, it was revealed in 1927 that tumors display a unique metabolic phenotype, and their glucose level is up to 200 times more than that of normal cells.Citation25 Despite ignorance of oncometabolite impact on cancer diagnosis and management by 1970s, oncometabolites were rediscovered in the past decades.Citation26 Oncometabolites are intrinsic metabolites that either start or continue tumor growth and metastasis. The primary oncometabolite was 2-hydroxyglutarate (2HG), which was recognized as a main metabolite with much higher concentrations in gliomas than normal cells.Citation27 Main oncometabolites can be classified into six hallmarks: 1) those involved in glucose and amino acid uptake, 2) use of adaptable modes of nutrient gaining, 3) use of glycolysis/tricarboxylic acid (TCA) cycle and NADPH production, 4) augmented demand for nitrogen, 5) modifications in metabolite-driven gene regulation, and 6) metabolic contacts with the microenvironment. In fact, limited tumors show all six hallmarks together, and each one can be an indicator of tumor and can guide scientists to the exact tumor classifi-cation and higher efficient tumor management policies.Citation28 There are nine oncometabolites in different types of TCs: 2HG, glucose, fumarate, succinate, sarcosine, glutamine, asparagine, choline, and lactate.Citation29 Recently, some studies on the metabolomics analysis of FNA specimens of thyroid nodules have suggested the benefit of oncometabolites approach as the potential application for the cooperative diagnosis tool of TC.Citation30–Citation33 Several metabolic pathways linking it to the TCA, pentose phosphate pathway, and lipid metabolism are candidate biomarkers to discriminate between normal and cancer cells ().
Figure 1 Several metabolic pathways in normal and cancer cells.
Abbreviation: TCA, tricarboxylic acid.
Here, we present the first meticulous summary of the entire available primary research to evaluate the potential of oncometabolites as the discriminative molecular marker between TC and BTNs.
Research design and methods
Search strategy
The study was conducted according to International prospective register of systematic reviews PROSPERO code: CRD42018088928 (http://www.Crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018088928). All related literature searches from four main databases including MED-LINE (PubMed), Scopus, Embase, and Web of Science for relevant articles were retrieved from January 1, 1998, to end of March 2018 with the key words grouping of “metabolomics”, “metabonomics”, “oncometabolites”, “metabolic profiling”, combined with “Thyroid Neoplasm”, “Thyroid Carcinoma”, “Thyroid Adenoma”, “Thyroid Nodules”, and “Thyroid Cancer” (Supplementary materials). To minimize selection bias, two independent investigators (BA and MS) autonomously checked titles, abstracts, and available full-text articles for application. Further articles were recognized by checking the reference lists from the selected studies. Disagreements were fixed by agreement and discussion with a third researcher (KG).
Eligibility criteria
All nominated studies were reviewed by two authors independently and according to their title and abstract were categorized as the included one or excluded one. The inclusion criteria were as follows: 1) participants included thyroid patients with TC; 2) the control population was specified (eg, patients with BTN, goiter patients, or healthy subjects); 3) all metabolomics detection techniques such as HPLC, ultra performance liquid chromatography (ULC), mass spectrometry (MS), tandem mass spectrometry (TMS), and nuclear magnetic resonance (NMR) spectroscopy were selected; and 4) metabolites were examined in plasma, serum, urine, or FNA specimens. Research studies were excluded if they 1) analyzed metabolite profiles in animals (in vivo studies), 2) analyzed metabolite profiles in cell culture (in vitro studies), or 3) did not contain a suitable control group.
Data extraction and analysis
All data on population distinctiveness and indicative oncometabolites were entered in Excel. FK had performed the data completion steps, which was confirmed by another researcher (MP). Due to the inadequate quantity of studies related to TC and metabolomics, and the extensive method ological heterogeneity and the significant dissimilarities in study population characteristics, an assessable meta-analysis of the data was not applicable.
The quality assessment tools
Here, we used Quality Assessment of Diagnostic Accuracy Assessment (QUADAS) and The Newcastle–Ottawa Scale (NOS) assessment tools to assess the methodological quality of the selected research articles. QUADAS and NOS were used to evaluate quality issues particular for “-omics” (QUADOMICS) more than the quality assessment of studies involving in systematic reviews.Citation34,Citation35 Each research article that scored 12/16 or more on the QUADOMICS tool together with 6/8 or more on NOS were considered as “high quality”, while each research article that scored 11/16, 5/8, or less were considered as “low quality”.
Results
Study selection and characteristics
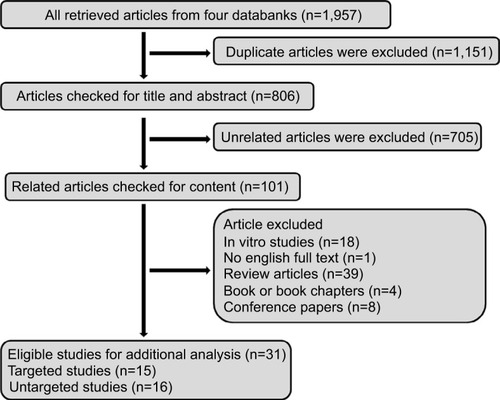
The selection algorithm and results of study selection are presented in . A total of 806 articles were retrieved after duplication deletion, including 374 articles from PubMed, 293 from Scopus, 83 from Web of Science, and 56 from Embase. After deleting the review, in vivo/in vitro studies, and book or conference paper with no available full-text articles, the final 31 articles were chosen for further considerations. A total of 15 studies with targeted metabolites () and 16 studies with untargeted metabolites methods () were selected. Two studies with targeted metabolites were removed because of low quality after quality assessment.
The sample size of the study population was different from one case report that discussed 138 TC cases. Five studies were conducted in USA, 12 in China, five in Korea, two in Poland, two in Italy, one in Japan, one in Nepal, and one in the Nether-lands. Both case/control and case report/series were included in the studies. In most case/control studies, the metabolites were compared between TC as the case group with healthy individuals and BTN or goiter patients as the controls. Exceptionally in two studies, the case/control was based on menopause TC and non-menopause TC. One study in Nepal evaluated the oncometabolites in differentiated thyroid carcinoma (DTC) with an increasing risk of cardiovascular disease. The oncometabolites included amino acids such as isoleucine, leucine, valine, lactate, threonine, alanine, uracil, lysine, glutamate, methionine, aspartate, choline, phosphocholine, glycerophosphocholine, taurine, myo-inositol, glycine, phosphoethanolamine, inosine, thyrosine, hypoxanthine, formate, succinate, and uridine; carboxylic acids such as acetate, citrate, fumarate, and lactate; monosaccharides such as glucose and glycosylation; estrogen metabolites such as 16 alpha-OH E1/2-OH E1 and catechol estrogens (2-OH E1); lipids such as total cholesterol, triglycerides, HDL, LDL, and VLDL; fibrinogen; calcitonin; and carcinoembryonic antigen (CEA). The unique phospholipids of bilayer membrane (phosphatidylcholine, phosphatidylcholine, and sphingomyelin) in addition to thyroid hormones, free triiodothyronine (fT3), free thyroxine (fT4), and thyroid-stimulating hormone (TSH) were also included in the list of metabolites. Some studies used the oncometabolites for TC diagnosis and some for follow-up and management of TC patients.
Amongst all selected studies in this systematic review, two studies were considered metabolite profile of premenopausal women. A targeted study conducted in Manipal Teaching Hospital of Nepal suggested that the hypercoagulable state atherogenic lipid profile is the different metabolite correlated with an increasing risk of cardiovascular disease in DTC patients. Other studies considered the different metabolite profiles as the discriminative tool of thyroid malignancy.
Table 1 Thirteen targeted studies related to the cometabolites in TC
Table 2 The list of 16 untargeted studies related to the cometabolites in TC
Discussion
Cancer studies highlighted the fact that cancer cells are common in biological capabilities such as constant proliferative signaling, growth suppressor’s avoidance, resistance to cell death, replicative immortality, high angiogenesis, reprogrammed energy metabolism, immune-mediated destruction, invasion, and metastasis.Citation11,Citation64,Citation65,Citation119 Metabolic reprogramming orchestrates cancer cell properties, so “cancer metabolism” became an important research topic for cancer management. The first study on cancer metabolism in 1924 suggested that the cancer phenotype for glucose metabolism is unique one and with higher ability of glucose uptake and lactate production is typical in several tumors.Citation66 These pathways are named as “aerobic glycolysis” or the “Warburg effect”, which has the effect on the extracellular fluid around tumor tissue and change it to acidic pH.Citation67–Citation69 Glucose is the critical source of carbon that helps in the maintenance of cancer cell anabolism, TCA anaplerosis, aerobic glycolysis, hexokinase II activation, and modified signal transduction.Citation70,Citation71 Glucose was the most frequent metabolite elevated in most cancersCitation26,Citation72,Citation73 and has been used as the oncometabolite of TCs in both targetedCitation42,Citation44 and untargeted studies.Citation50,Citation54,Citation56 Analysis of the serum metabolic alterations among PTC, benign thyroid tumor, and healthy controls suggested that glucose metabolism cannot be the only important metabolite because metabolism of lipids, amino acids, and nucleic acids is important as well.Citation50 Moreover, it was shown that the mRNA quantity of metabolic enzyme-coding genes resulting in different glucose, fructose, galactose, mannose, 2-keto-d-gluconic acid and rhamnose, malonic acid and inosine, cholesterol and arachidonic acid significantly increased in PTC.Citation56 These studies were confirmed by detecting 31 different metabolites related to amino acid, lipid, glucose, vitamin metabolism, and diet/gut microbiota interaction.Citation74
Metabolome analysis of amino acid profile is under consideration for biomarkers of thyroid malignancy. The plasma-free amino acid (PFAA) profiles of breast cancer, gastric cancer, and TC patients and investigation of their diagnostic potential were shown in the study by Gu et al.Citation75 Carnitine, trimethylamine N-oxide (TMAO), proline, glutamine, and asparagine were known as the most significant metabolites of 392 metabolites in TC.Citation49 In serum specimens of papillary TC patients, the amount of metabolites like valine, leucine, isoleucine, lactic acid, alanine, glutamic acid, lysine, glycine, whereas the lipids, choline, tyrosine decreased.Citation76 Similarly alanine, creatine, glutamine, tyrosine, and valine in both serum and urine of TC patients were diagnosed by H NMR-based method.Citation77
Glycosylation is one of the most frequent posttranslational modification reactions, and almost half of all proteins in eukaryotes are glycosylated.Citation11,Citation78 Some findings revealed the potential of IgG glycosylation as a biomarker for inflammation, metabolic health, and cancers.Citation79,Citation80 In TC, it was suggested that human IgG Fc-glycosylation profiling could be linked with age, sex, female sex hormones, and TC risk.Citation38 IgG glycosylation in addition to glycans, glycome and glycoproteome are important in controlling thyroid cancer development and progression.Citation81,Citation82 The translocase of outer mitochondrial membrane 20 (TOMM20), a marker of oxidative phosphorylation, and monocarboxylate transporter 4 (MCT4), a marker of glycolysis, are candidate metabolites for aggressive behavior of TC.Citation39
High levels of lactate and choline and low levels of citrate, glutamine, and glutamate in malignant thyroid nodules were reported by Ryoo et alCitation33 and suggested them as the discriminative biomarker for determining the preoperative metabolomic profiles of thyroid nodules. Lactate is often augmented in several malignancies including head and neck cancers.Citation30,Citation31,Citation83,Citation84 High lactate level is the sign of glycolytic pathway increasing in response to hypoxia or ischemia in tumor tissues.Citation85–Citation87 Lactogenesis, an important step for the production of lactate, is started and triggered by gene mutations (the Warburg effect), so deregulated lactate metabolism and signaling are the critical elements in carcinogenesis.Citation88 Lactate was established as an important factor in terms of cancer cell mobility and immune suppressor molecule that promote the tumor evasion as well.Citation89,Citation90 Lactate was found to be the most promising metabolite for discrimination of lymph node metastasis from nonmetastatic TC.Citation48 Two studies confirmed that reduced levels of fatty acids and elevated levels of several amino acids (phenylalanine, tyrosine, lactate, serine, cystine, lysine, glutamine/glutamate, taurine, leucine, alanine, isoleucine, and valine) in papillary thyroid micro-carcinoma (PTMC) and rise of phenylalanine, taurine, and lactate and a reduction of choline and choline derivatives, myo- and scyllo-inositol in the malignant tumors vs to the benign ones.Citation59,Citation62
Phospholipids are esters of glycerol, fatty acids, phosphoric acid, and other alcohols. Nearly, all frequent phospholipids are phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidylserine. Evidence showed that phosphatidylcholine, the major phospholipid element of eukaryotic membranes, like choline metabolites resulting from its metabolism, has an important role in cancer proliferation and survival.Citation91 In thyroid malignancies, the increased multiplication and proliferation of cancer cells are linked to the increased choline contents even in FNA specimen.Citation47,Citation57,Citation92 Choline was in the list of discriminative oncometabolites of TC with lymph node metastasis from non-metastatic one.Citation48 These findings are contradictory with a study in which the content of lipids, choline, and tyrosine decreased in malignant TC compared to that in the benign one.Citation31,Citation50
Another metabolite that increased as the result of glycolysis in TC is glycine. It could be one of the essential metabolites in tumorigenesis and mitochondrial synthesis, and consumption of glycine was suggested as a discriminative metabolites triggering the cancer cell growth and development.Citation33 Glycine dehydrogenase enzyme (GLDC), which cleavages glycine and mediates folate cycle charging, is highly expressed in tumor-promoting cells.Citation93–Citation95
Oncometabolomic analysis revealed that citrate uptake largely affected cancer cell metabolism through citrate-dependent metabolic pathways, and the extracellular citrate is provided to cancer cells through a plasma membrane-specific variant of the mitochondrial citrate transporter (pmCiC).Citation96 In the study by Ryoo et al,Citation57 it was suggested that citrate was the most powerful discriminator oncometabolite for diagnosis of TC. Previous studies also indicated that ATP citrate lyase, essential for cell proliferation, is upregulated in some human malignancies such as lung, colorectal, and ovarian cancers.Citation97,Citation98 The inhibition of ATP citrate lyase can block the proliferation of multiple tumor cell lines.Citation99
The role of isocitrate dehydrogenase (IDH) mutations and d-2-HG accumulation in malignancy has increased recently.Citation100 2-HG has been considered as oncometabolites and epigenetic modifiers in different malignancies such as gliomas,Citation101–Citation103 myelogenous leukemia,Citation104 and renal cancer.Citation105 Non-synonymous variants of IDH1 gene have been detected in thyroid carcinomas, and in PTC, the increased levels of 2-HG were reported.Citation106–Citation109 However, it has not been considered as an oncometabolite in TCs.
There are three main forms of estrogen in the human body: estradiol, estrone, and estriol. These forms of estrogen together with estrogen receptor and other estrogen metabolites (16 alpha-OH E1/2-OH E1 and catechol estrogens [2-OH E1]) are more commonly associated with cancer risk.Citation110,Citation111 For checking the possible outcome of estrogens in premenopausal female TC, the concentrations of 14 estrogens were assessed in the urine of patients with PTC preoperatively and postoperatively, and it was confirmed that low mean value of 16α-OH E1/2-OH E1 was observed in preoperative patients, and it was considerably dissimilar to the ratio of postoperative TC cases. The increase of 2-hydroxylation in estrogen metabolism may have a noteworthy relationship with the risk of TC formation in females.Citation46 Moreover, it was shown that higher exposure to estrogens can increase the risk for TC, and 38 urinary estrogen metabolites were checked by Zahid et al, they suggested the unbalanced estrogen metabolism and formation of estrogen-DNA adducts as the role player in the initiation of TC.Citation36 Supporting information indicated to anti-estrogenic dietary supplement function of 3,3′-diindolylmethane (DIM) to help reduce the risk of developing thyroid proliferative disease (TPD).Citation43
In addition, the synthesis of purines and pyrimidine is upregulated in cancer cells, and the catalyzing enzymes of this pathway including thymidylate synthase and inosine synthetase 2 are subjected to Myc-induced upregulation.Citation112,Citation113 Glutamine is a nitrogen source for multiple steps of both purine and pyrimidine synthesis.Citation114 Glutamine is a critical nutrient indispensable for cancer cell growth and is the new therapeutic target in cancers.Citation115,Citation116 In preoperative percutaneous FNA specimens of TC, it was shown that glutamine and glutamate are presented with lower relative concentrations.Citation50,Citation57 These results were generally in agreement with a previous finding obtained using surgical specimens.Citation59 Pathway analysis indicated the “alanine, aspartate and glutamate metabolism” and “inositol phosphate metabolism” as the most relevant pathways in thyroid carcinogenesis.Citation74 However, gastric cancer cells was promoted by cysteine, but inhibited by alanine and glutamic acid because alanine and glutamic acid induced apoptosis of gastric cancer cells.Citation45 Follicular adenomas exhibit a unique metabolic profile with several oncometabolite profiles including glutamine.Citation63
Conclusion
Because of the complexity of thyroid carcinogenesis, a wide range of oncometabolites is suggested as TC diagnostic markers. Potential biomarkers common to all thyroid lesions were mainly fatty acids, amino acids, cell membrane phospholipids, estrogen metabolites (16 alpha-OH E1/2-OH E1 and catechol estrogens(2-OH E1), purine and pyrimidine metabolites, citrate, glucose, mannose, pyruvate, and 3-hydroxybutyrate glycosylation (IgG Fc-glycosylation), TOMM20, MCT4, choline, choline derivatives, myo-/scyllo-inositol, and lactate. Among all metabolites, citrate was suggested as the first most significant oncometabolite and lactate as the second one in thyroid malignancies.
Abbreviations
| BC | = | breast cancer |
| BN | = | benign nodule |
| BTA | = | benign thyroid adenoma |
| BTN | = | benign thyroid nodules |
| CEA | = | carcinoembryogenic antigen |
| CPMG | = | Carr-Pure-Me boom-Gill sequence |
| DESI-MS | = | desorption electrospray ionization mass spectrometry |
| DTC | = | differentiated thyroid carcinoma |
| FA | = | follicular adenoma |
| FTC | = | follicular thyroid cancer |
| GC | = | gastric cancer |
| GC-TOF-MS | = | gas chromatography time-of-flight mass spectrometry |
| HI | = | healthy individual |
| HRMAS | = | high-resolution magic angle spinning |
| LC-DIA-MS | = | liquid chromatography–data independent-mass spectrometry |
| LNMBC | = | lymph node with metastatic breast cancer |
| LNMP | = | lymph node with metastatic PTC |
| MNG | = | multinodular goiters |
| MTC | = | medullary thyroid cancer |
| NAT | = | normal adjacent tissue |
| NLN | = | normal lymph node |
| NMR | = | nuclear magnetic resonance |
| NN | = | non-neoplastic nodule |
| NOEPR | = | nuclear over Hauser effect spectroscopy with P resaturation |
| NTC | = | noncancerous thyroid tissue |
| PTC | = | papillary thyroid carcinoma |
| TCP | = | thyroid cancer patients |
| TMS | = | tandem mass spectrometry |
| TPD | = | thyroid proliferative disease |
| ULC | = | ultra performance liquid chromatography |
| UPLC–QTOFMS | = | ultra-performance liquid chromatography–quadruple time-of-flight mass spectrometry |
| UTC | = | undifferentiated thyroid carcinoma |
Acknowledgments
The authors thank the Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
- EnewoldLZhuKRonERising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005Cancer Epidemiology Biomarkers & Prevention2009183784791
- VergaminiLBFrazierALAbrantesFLRibeiroKBRodriguez-GalindoCIncrease in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based studyJ Pediatr201416461481148524630354
- LarijaniBShirzadMMohagheghiMAEpidemiologic analysis of the Tehran cancer institute data system registry (TCIDSR)Asian Pac J Cancer Prev200451363915075002
- HaghpanahVSoliemanpourBHeshmatREndocrine cancer in Iran: based on cancer registry systemIndian J Cancer20064328016790945
- MoonWJBaekJHJungSLUltrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendationsKorean J Radiol201112111421228935
- LarijaniBMohagheghiMABastanhaghMHPrimary thyroid malignancies in Tehran, IranMed Princ Pract200514639640016220012
- CooperDSDohertyGMHaugenBRRevised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancerThyroid200919111167121419860577
- CibasESAliSZNCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathologyAm J Clin Pathol2009132565866519846805
- PaciniFSchlumbergerMDralleHEuropean consensus for the management of patients with differentiated thyroid carcinoma of the follicular epitheliumEur J Endocrinol2006154678780316728537
- HaghpanahVShooshtarizadehPHeshmatRLarijaniBTavangarSMImmunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinomaAppl Immunohistochem Mol Morphol200614442242517122639
- SaniiSSaffarHTabrizHMQorbaniMHaghpanahVTavangarSMExpression of matrix metalloproteinase-2, but not caspase-3, facilitates distinction between benign and malignant thyroid follicular neoplasmsAsian Pac J Cancer Prev20121352175217822901190
- ProiettiABorrelliNGianniniRMolecular characterization of 54 cases of false-negative fine-needle aspiration among 1347 papillary thyroid carcinomasCancer Cytopathol20141221075175924913568
- Haddadi-NezhadSLarijaniBTavangarSMNouraeiSMComparison of fine-needle-nonaspiration with fine-needle-aspiration technique in the cytologic studies of thyroid nodulesEndocr Pathol200314436937414739493
- KhatamiFLarijaniBTavangarSMCirculating Tumor BRAF Mutation and Personalized Thyroid Cancer TreatmentAsian Pac J Cancer Prev201718229328345323
- KhatamiFTavangarSMLiquid biopsy in thyroid cancer: new insightInt J Hematol Oncol Stem Cell Res2018123234247
- TavangarSMMonajemzadehMLarijaniBHaghpanahVImmunohistochemical study of oestrogen receptors in 351 human thyroid glandsSingapore Med J200748874474717657383
- Mohammadi-AslJLarijaniBKhorgamiZQualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinomaMed Oncol20112841123112820535589
- KildegaardHFBaycin-HizalDLewisNEBetenbaughMJThe emerging CHO systems biology era: harnessing the “omics revolution for biotechnologyCurr Opin Biotechnol20132461102110723523260
- SarmadiSIzadi-MoodNSotoudehKTavangarSMExpressionAPAltered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometriumDiagn Pathol2009414119930726
- GilanyKMinai-TehraniAAminiMAgharezaeeNArjmandBThe challenge of human spermatozoa proteome: a systematic reviewJ Reprod Infertil201718326729062791
- JohnsonCHIvanisevicJSiuzdakGMetabolomics: beyond biomarkers and towards mechanismsNat Rev Mol Cell Biol201617745145926979502
- GilanyKJafarzadehNMani-VarnosfaderaniAMetabolic fingerprinting of seminal plasma from non-obstructive Azoospermia patients: positive versus negative sperm retrievalJ Reprod Infertil201819210911430009145
- AgharezaeeNMarzbaniRRezadoostHKoukhalooSZArjmandBGilanyKMetabolomics: a bird’s eye view of infertile menTehran University Medical Journal20187512860868
- CollinsRRJPatelKPutnamWCKapurPRakhejaDOncometabolites: a new paradigm for oncology, metabolism, and the clinical laboratoryClin Chem201763121812182029038145
- WarburgOWindFNegeleinEThe metabolism of tumors in the bodyJ Gen Physiol19278651953019872213
- ZhouZIbekweEChornenkyyYMetabolic alterations in cancer cells and the emerging role of Oncometabolites as drivers of neoplastic changeAntioxidants20187116
- WardPSPatelJWiseDRThe Common Feature of Cancer-Associated IDH1 and IDH2 Mutations is a Neomorphic Enzyme Activity Converting α-ketoglutarate to the Oncometabolite 2-HydroxyglutarateCancer Cell201017322523420171147
- PavlovaNNThompsonCBThe emerging hallmarks of cancer metabolismCell Metab2016231274726771115
- WishartDSMandalRStanislausARamirez-GaonaMCancer metabolomics and the human metabolome databaseMetabolites20166110
- GuptaNKakarAKChowdhuryVGulatiPShankarLRVindalAMagnetic resonance spectroscopy as a diagnostic modality for carcinoma thyroidEur J Radiol200764341441817462842
- MiccoliPTorregrossaLShintuLMetabolomics approach to thyroid nodules: a high-resolution magic-angle spinning nuclear magnetic resonance-based studySurgery201215261118112423158182
- TianYNieXXuSIntegrative metabonomics as potential method for diagnosis of thyroid malignancySci Rep201551486926486570
- RyooIKwonHKimSCMetabolomic analysis of percutaneous fine-needle aspiration specimens of thyroid nodules: Potential application for the preoperative diagnosis of thyroid cancerSci Rep2016613007527440433
- BaeJMA suggestion for quality assessment in systematic reviews of observational studies in nutritional epidemiologyEpidemiol Health201638e201601427156344
- LumbrerasBPortaMMárquezSPollánMParkerLAHernández-AguadoIQUADOMICS: an adaptation of the Quality Assessment of Diagnostic Accuracy Assessment (QUADAS) for the evaluation of the methodological quality of studies on the diagnostic accuracy of “-omics”-based technologiesClin Biochem20084116–171316132518652812
- ZahidMGoldnerWBeselerCLRoganEGCavalieriELUnbalanced estrogen metabolism in thyroid cancerInt J Cancer201313311n/a9
- VillanuevaJNazarianALawlorKYiSSRobbinsRJTempstPA sequence-specific exopeptidase activity test (SSEAT) for “functional” biomarker discoveryMol Cell Proteomics20087350951817986438
- ChenGWangYQiuLHuman IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancerJ Proteomics201275102824283422365975
- CurryJMTassonePCotziaPMulticompartment metabolism in papillary thyroid cancerLaryngoscope2016126102410241826666958
- de GrootJWKemaIPBreukelmanHBiochemical markers in the follow-up of medullary thyroid cancerThyroid200616111163117017123344
- DoSIKimHSKimKPredictive value of sphingosine kinase 1 expression in papillary thyroid carcinomaAnticancer Res201737105399540528982849
- MittalAPoudelBPandeyaDRGuptaSPSathianBYadavSKMetabolic changes enhance the cardiovascular risk with differentiated thyroid carcinoma – a case control study from Manipal Teaching Hospital of NepalAsian Pac J Cancer Prev20121352335233822901217
- RajoriaSSurianoRParmarPS3,3′-diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: a pilot studyThyroid201121329930421254914
- ShangXZhongXTianXMetabolomics of papillary thyroid carcinoma tissues: potential biomarkers for diagnosis and promising targets for therapyTumour Biol2016378111631117526935059
- GuYChenTFuSPerioperative dynamics and significance of amino acid profiles in patients with cancerJ Transl Med20151313525622826
- LeeSHKimKMJungBHChungWYParkCSChungBCEstrogens in female thyroid cancer: alteration of urinary profiles in pre- and post-operative casesCancer Lett20031891273212445674
- IshikawaSTateyaIHayasakaTIncreased expression of phosphatidylcholine (16:0/18:1) and (16:0/18:2) in thyroid papillary cancerPLoS One2012711e4887323139822
- SeoJWHanKLeeJApplication of metabolomics in prediction of lymph node metastasis in papillary thyroid carcinomaPLoS One2018133e019388329509799
- ZhouJLiYChenXZhongLYinYDevelopment of data-independent acquisition workflows for metabolomic analysis on a quadrupoleorbitrap platformTalanta201716412813628107906
- ZhaoWXWangBZhangLYYanSYYangYHAnalysis on the metabolite composition of serum samples from patients with papillary thyroid carcinoma using nuclear magnetic resonanceInt J Clin Exp Med20158101801326770396
- ZhangJFeiderCLNagiCDetection of metastatic breast and thyroid cancer in lymph nodes by desorption electrospray ionization mass spectrometry imagingJ Am Soc Mass Spectrom20172861166117428247296
- YaoZYinPSuDSerum metabolic profiling and features of papillary thyroid carcinoma and nodular goiterMol Biosyst2011792608261421713270
- XuYZhengXQiuYJiaWWangJYinSDistinct metabolomic profiles of papillary thyroid carcinoma and benign thyroid adenomaJ Proteome Res20151483315332126130307
- WojtowiczWZabekADejaSSerum and urine 1H NMR-based metabolomics in the diagnosis of selected thyroid diseasesSci Rep201771910828831094
- ChoiMHMoonJYChoSHChungBCLeeEJMetabolic alteration of urinary steroids in pre- and post-menopausal women, and men with papillary thyroid carcinomaBMC Cancer201111134221824401
- ChenMShenMLiYGC-MS-based metabolomic analysis of human papillary thyroid carcinoma tissueInt J Mol Med20153661607161426459747
- RyooIKwonHKimSCMetabolomic analysis of percutaneous fine-needle aspiration specimens of thyroid nodules: potential application for the preoperative diagnosis of thyroid cancerSci Rep2016613007527440433
- ShenCTZhangYLiuYMA distinct serum metabolic signature of distant metastatic papillary thyroid carcinomaClin Endocrinol2017876844852
- TorregrossaLShintuLNambiath ChandranJToward the reliable diagnosis of indeterminate thyroid lesions: a HRMAS NMR-based metabolomics case of studyJ Proteome Res20121163317332522509853
- GuoLWangCChiCExhaled breath volatile biomarker analysis for thyroid cancerTransl Res2015166218819525666355
- LiuSZhaoGLiJAssociation of polybrominated diphenylethers (PBDEs) and hydroxylated metabolites (OH-PBDEs) serum levels with thyroid function in thyroid cancer patientsEnviron Res20171591828759783
- LuJHuSMiccoliPNon-invasive diagnosis of papillary thyroid microcarcinoma: a NMR-based metabolomics approachOncotarget20167498176827835583
- DejaSDawiskibaTBalcerzakWFollicular adenomas exhibit a unique metabolic profile. 1H NMR studies of thyroid lesionsPLoS One2013812e8463724376829
- HanahanDWeinbergRAThe hallmarks of cancerCell20001001577010647931
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- WarburgOÜber den Stoffwechsel der CarcinomzelleNaturwissenschaften1924125011311137
- WarburgOOn the origin of cancer cellsScience1956123319130931413298683
- VolkTJähdeEFortmeyerHPGlüsenkampKHRajewskyMFpH in human tumour xenografts: effect of intravenous administration of glucoseBr J Cancer19936834925008353039
- Delli CastelliDFerrautoGCutrinJCTerrenoEAimeSIn vivo maps of extracellular pH in murine melanoma by CEST-MRIMagn Reson Med201471132633223529973
- FadakaAAjiboyeBOjoOAdewaleOOlayideIEmuowhochereRBiology of glucose metabolization in cancer cellsJ Oncol Sci2017324551
- HamanakaRBChandelNSTargeting glucose metabolism for cancer therapyJ Exp Med2012209221121522330683
- LeeASGlucose-regulated proteins in cancer: molecular mechanisms and therapeutic potentialNat Rev Cancer201414426327624658275
- WolfAAgnihotriSMicallefJHexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiformeJ Exp Med2011208231332621242296
- ShenC-TZhangYLiuY-MA distinct serum metabolic signature of distant metastatic papillary thyroid carcinomaClin Endocrinol2017876844852
- GuYChenTFuSPerioperative dynamics and significance of amino acid profiles in patients with cancerJ Transl Med20151313525622826
- ZhaoWXWangBZhangLYYanSYYangYHAnalysis on the metabolite composition of serum samples from patients with papillary thyroid carcinoma using nuclear magnetic resonanceInt J Clin Exp Med2015810180131802226770396
- WojtowiczWZabekADejaSSerum and urine 1H NMR-based metabolomics in the diagnosis of selected thyroid diseasesSci Rep201771910828831094
- MiyoshiEItoYMiyoshiYInvolvement of aberrant glycosylation in thyroid cancerJ Oncol20102010217
- PlompRRuhaakLRUhHWSubclass-specific IgG glycosylation is associated with markers of inflammation and metabolic healthSci Rep2017711232528951559
- ZhangDChenBWangYDisease-specific IgG Fc N-glycosylation as personalized biomarkers to differentiate gastric cancer from benign gastric diseasesSci Rep2016612595727173519
- PinhoSSReisCAGlycosylation in cancer: mechanisms and clinical implicationsNat Rev Cancer201515954055526289314
- ZąbczyńskaMKozłowskaKPochećEGlycosylation in the thyroid gland: vital aspects of glycoprotein function in thyrocyte physiology and thyroid disordersInt J Mol Sci20181992792
- BrizelDMSchroederTScherRLElevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancerInt J Radiat Oncol Biol Phys200151234935311567808
- WalentaSSchroederTMueller-KlieserWLactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncologyCurr Med Chem200411162195220415279558
- KoppenolWHBoundsPLDangCVOtto Warburg’s contributions to current concepts of cancer metabolismNat Rev Cancer201111532533721508971
- RogatzkiMJFergusonBSGoodwinMLGladdenLBLactate is always the end product of glycolysisFront Neurosci201594082225774123
- MichielsCPhysiological and pathological responses to hypoxiaAm J Pathol200416461875188215161623
- San-MillánIBrooksGAReexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg EffectCarcinogenesis201738211913327993896
- Romero-GarciaSMoreno-AltamiranoMMPrado-GarciaHSánchez-GarcíaFJLactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevanceFront Immunol20167Pt 25226909082
- JiangBAerobic glycolysis and high level of lactate in cancer metabolism and microenvironmentGenes Dis201741252730258905
- RidgwayNDThe role of phosphatidylcholine and choline metabolites to cell proliferation and survivalCrit Rev Biochem Mol Biol2013481203823350810
- LiYChenMLiuCMetabolic changes associated with papillary thyroid carcinoma: a nuclear magnetic resonance-based metabolomics studyInt J Mol Med20184153006301429484373
- KwonHOhSJinXAnYJParkSCancer metabolomics in basic science perspectiveArch Pharm Res201538337238025630795
- JainMNilssonRSharmaSMetabolite profiling identifies a key role for glycine in rapid cancer cell proliferationScience201233660841040104422628656
- ZhangWCShyh-ChangNYangHGlycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesisCell20121481–225927222225612
- MycielskaMEDettmerKRümmelePExtracellular citrate affects critical elements of cancer cell metabolism and supports cancer development In VivoCancer Res201878102513252329510993
- HatzivassiliouGZhaoFBauerDEATP citrate lyase inhibition can suppress tumor cell growthCancer Cell20058431132116226706
- ZaidiNSwinnenJVSmansKATP-citrate lyase: a key player in cancer metabolismCancer Res201272153709371422787121
- RenJGSethPYeHCitrate suppresses tumor growth in multiple models through inhibition of glycolysis, the tricarboxylic acid cycle and the IGF-1R pathwaySci Rep201771453728674429
- DangLYenKAttarECIDH mutations in cancer and progress toward development of targeted therapeuticsAnn Oncol201627459960827005468
- YeDGuanKLXiongYMetabolism, activity, and targeting of D- and L-2-hydroxyglutaratesTrends Cancer20184215116529458964
- WardPSCrossJRLuCIdentification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate productionOncogene201231192491249821996744
- ChoiCGanjiSKDeberardinisRJ2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomasNat Med201218462462922281806
- GrossSCairnsRAMindenMDCancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutationsJ Exp Med2010207233934420142433
- ShimEHLiviCBRakhejaDL-2-Hydroxyglutarate: an epi-genetic modifier and putative oncometabolite in renal cancerCancer Discov20144111290129825182153
- RakhejaDBoriackRLMituiMKhokharSHoltSAKapurPPapillary thyroid carcinoma shows elevated levels of 2-hydroxyglutarateTumour Biol201132232533321080253
- HemerlyJPBastosAUCeruttiJMIdentification of several novel non-p.R132 IDH1 variants in thyroid carcinomasEur J Endocrinol2010163574775520702649
- WardPSLuCCrossJRThe potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalizationJ Biol Chem20132886jbc. M112:38043815
- AlimoghaddamKShariftabriziATavangarSMAnti-leukemic and anti-angiogenesis efficacy of arsenic trioxide in new cases of acute promyelocytic leukemiaLeuk Lymphoma2006471818816321832
- MillerVMEstrogen metabolomics: a physiologist’s perspectiveHypertension201056581681820921431
- AlakwaaFMChaudharyKGarmireLXDeep learning accurately predicts estrogen receptor status in breast cancer metabolomics dataJ Proteome Res201817133734729110491
- TongXZhaoFThompsonCBThe molecular determinants of de novo nucleotide biosynthesis in cancer cellsCurr Opin Genet Dev2009191323719201187
- MannavaSGrachtchoukVWheelerLJDirect role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cellsCell Cycle20087152392240018677108
- CoryJGCoryAHCritical roles of glutamine as nitrogen donors in purine and pyrimidine nucleotide synthesis: asparaginase treatment in childhood acute lymphoblastic leukemiaIn Vivo200620558758917091764
- CantorJRSabatiniDMCancer cell metabolism: one hallmark, many facesCancer Discov201221088189823009760
- WiseDRThompsonCBGlutamine addiction: a new therapeutic target in cancerTrends Biochem Sci201035842743320570523
- KhatamiFLarijaniBHeshmatRKeshtkarAMohammadamoliMTeimoori-ToolabiLNasiriSTavangarSM2017Meta-analysis of promoter methylation in eight tumor-suppressor genes and its association with the risk of thyroid cancerPlos One129e018489228926589
- NatanziMMPasalarPKamalinejadMDehpourARTavangarSMSharifiRGhanadianNRahimi-BalaeiMGerayesh-NejadS2012Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in ratsActa Medica Iranica50958959623165807
- OmidfarKMoinfarZSohiANTavangarSMHaghpanahVHeshmatRKashanianSLarijaniBExpression of EGFRvIII in thyroid carcinoma: immunohistochemical study by camel antibodiesImmunological Investigations2009113821658019330625