Abstract
Purpose
The aim of this study was to evaluate the prognostic and predictive value of neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (DNLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in soft tissue sarcoma (STS) cases treated with pazopanib.
Materials and methods
The study population included 26 STS cases treated with pazopanib for at least 3 months. NLR, DNLR, LMR, and PLR were evaluated at baseline, and at third month of therapy and also compared with response to pazopanib. Median measurements were taken as cutoff for NLR (4.8), DNLR (3.1), LMR (3.6), and PLR (195). The associations between these cutoff values and survival times (progression-free survival [PFS] and overall survival [OS]) were assessed by Kaplan–Meier curves and Cox proportional models.
Results
Patients with low pretreatment NLR and DNLR had longer OS (P=0.022, P=0.018), but low PLR was found to be associated only with longer OS. Additionally, decrease in NLR and DNLR after 3 months of therapy as compared with pretreatment measurements was found to be associated with an advantage for OS (P=0.021, P=0.010, respectively) and PFS (P=0.005, P=0.001, respectively). Response to pazopanib; changes in NLR, DNLR, LMR, and PLR; and >3 metastatic sites were found to be independent risk factors in univariate analysis, but NLR was the only independent risk factor in multivariate analysis.
Conclusion
Low pretreatment and decrease in NLR and DNLR values, and regression/stable disease after 3 months of pazopanib are predictive factors for longer OS and PFS.
Introduction
Soft tissue sarcomas (STSs) are a heterogenous group of mesenchymal neoplasias accounting for 1%–3% of the malignant tumors.Citation1,Citation2 Surgery is the mainstay of therapy in STSs, but radiotherapy and/or chemotherapy are other therapeutic options according to the subtype of the disease, margin status, and stage of the disease.Citation3 About 50% of the cases have metastases at the beginning or during follow-up period. The primary aim is to provide palliation in patients with metastatic disease, and median OS was found to be 12–18 months.Citation4 In STSs, an essential mechanism for tumor progression and metastasis is angiogenesis, as seen in various malignant tumors, and anti-angiogenic agents are frequently used in daily practice. Pazopanib is a multi-tyrosine kinase inhibitor (TKI) with anti-angiogenic effect targeting vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and c-KİT.Citation5 Advantage for progression-free survival (PFS) and decrease in mortality rate have been shown with pazopanib in PALETTE study covering different types of STSs except liposarcoma (4.6 vs 1.6 months).Citation6 However, it is important to find predictive factors for better responders in STS cases treated with pazopanib. Although TP53 and FGFR3 have been found to be predictive factors in these cases, these markers are not practical in daily practice.Citation7,Citation8
Inflammation has a key role in cancer development, progression, and metastatic events.Citation9 Proangiogenic factors such as growth hormones, VEGF, and cytokines secreted by tumors increase angiogenesis and have essential role for causing inflammation in STSs, as in other malignant diseases.Citation10 High neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (DNLR), platelet-to-lymphocyte ratio (PLR), and low lymphocyte-to-monocyte ratio (LMR) are practical indexes for indicating systemic inflammation and poor prognosis in malignancies including STSs.Citation11–Citation14 There is limited data about the association between inflammatory indexes and response to new therapies including TKIs. The aim of this study was to evaluate the predictive value of NLR, DNLR, LMR, and PLR in STS cases treated with pazopanib.
Materials and methods
We retrospectively reviewed 26 STS cases treated with pazopanib after progression following 1–3 lines of chemotherapy. NLR, DNLR, LMR, and PLR were calculated with standard formulas before and after 3 months of pazopanib treatment. Median values were taken as cutoff for NLR, DNLR, LMR, and PLR. Cutoff values were 4.8 for NLR, 3.1 for DNLR, 3.6 for LMR, and 195 for PLR. Response Evaluation Criteria in Solid Tumors (version 1.1) was used to detect tumor response to pazopanib at the third month of therapy. PFS and overall survival (OS) were defined as the period from the start of pazopanib treatment until the first instance of disease progression, death, or the last clinical evaluation. Data were censored at the last evaluation if the patient was still alive.
Statistical analyses
Kaplan–Meier curves and the log-rank test were used to analyze the association between patient-related clinical parameters and survival times, and Pearson’s chi-squared test was used to analyze the associations between the clinical parameters and inflammation indexes including NLR, DNLR, PLR, and DNLR. Univariate and multivariate Cox regression analyses were used to evaluate the potential prognostic factors including age, sex, grade of the tumor, initial response to pazopanib treatment, number of metastases, treatment line for pazopanib use and inflammation indexes, and changes before and after treatment: NLR, DNLR, LMR, PLR, ΔNLR, ΔDNLR, ΔLMR, and ΔPLR. HRs and 95% CIs were estimated based on the Cox analysis. All analyses were performed using SPSS software (version 21), and a P-value <0.05 was considered statistically significant.
Ethical approval
Ethical approval was not required based on the law and the national ethical guidelines of our country and written informed consent was not required for individual patient because of retrosprective nature.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Female/male ratio was 14/12 and median age was 47.5 (range 19–76) years. Sarcoma subtypes included leiomyosarcoma in eight cases, undifferentiated pleomorphic sarcoma in six cases, malignant peripheral nerve sheet tumor in four cases, synovial sarcoma in three cases, fibrosarcoma in two cases, and liposarcoma, rhabdomyosarcoma, and Ewing’s sarcoma one case in each subtype. Histopathologic grade was II and III in nine and 17 cases, respectively. Initial treatments included mesna, doxorubicin, ifosfamide, and dacarbazine in 21 cases (80.8%). Second-line therapy comprised gemcitabine–docetaxel combination in 19 cases. Pazopanib had been used as second-line therapy in six cases, third-line therapy in 14 cases, and fourth-line therapy in six cases.
Pretreatment median NLR, DNLR, LMR, and PLR were 4.74, 3.04, 3.58, and 194.11, respectively. Clinical and demographic parameters and pretreatment median NLR, DNLR, LMR, and PLR values are presented in . The association between clinicopathological variables and inflammatory markers including NLR, DNLR, LMR, and PLR are summarized in . NLR, DNLR, and PLR were not found to be associated with sex, age, and grade, but LMR was found to be associated with the grade of the disease (P=0.046). However, LMR was not found to be related to age and gender (P=1.000, P=0.248) like other indexes. Only DNLR was found to be associated with initial response to pazopanib and disease progression (P=0.041, P=0.023). Median follow-up was 8.1 months (range 3.1–51.7 months); 19 patients died and seven patients were alive when this analysis was done in March 2018. Mean and median OS were 19.2 and 14.7 months and PFS were 11.3 and 7.2 months, respectively. There was no association between survival times and age, grade, sex, and the number of pazopanib treatment line (PFS: P=0.606, P=0.727, P= 0.290, P=0.331 and OS: P=0.623, P=0.61; P=0.285, P=0.142, respectively). However, OS and PFS were found to be longer in cases detected with stable disease or regression after 3 months of pazopanib treatment: median OS was 23.13 months in cases without progression, while it was 5.03 months in cases with progression (P=0.001). Median PFS was 12.03 months in cases without progression, while it was 4.33 months in cases with progression (P=0.000). shows the survival times in cases with and without progression. OS and PFS times according to the clinical parameters and inflammation indexes, including NLR, DNLR, LMR, PLR, and changes of these parameters after therapy, are summarized in .
Figure 1 OS (A) and PFS (B) curves according to response to 3 months of pazopanib treatment.
Abbreviations: OS, overall survival; PFS, progression-free survival.
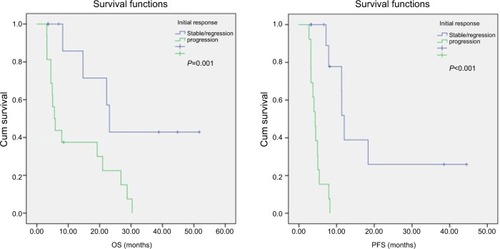
Table 2 The association between pretreatment NLR, DNLR, LMR, and PLR values and clinicopathological parameters (n=26)
Table 3 Overall and progression-free survival times according to clinical parameters and NLR, DNLR, LMR, PLR and changes of these parameters after therapy (n=26)
NLR and DNLR values according to the determined cutoffs were found to be predictive of OS (P=0.022, P=0.018, respectively), but only DNLR was predictive of PFS (P=0.007). Median OS and PFS were found to be 23.13 and 7.2 months in cases with lower NLR (<4.8), while they were 5.87 and 5.4 months in cases with higher NLR (>4.8), respectively. Median OS and PFS were found to be 23.13 and 11.37 months in cases with lower DNLR (<3.1), while they were 5.03 and 4.47 months in cases with higher DNLR (>3.1), respectively. Median PFS and OS according to the NLR and DNLR are shown in . PLR was found to be predictive of only OS but not PFS: median OS was 22.33 months in cases with PLR <195, while it was 4.93 months in cases with PLR >195 (P=0.031). There was no difference in OS and PFS values according to the LMR cutoffs: 22.23 vs 7.93 months and 7.9 vs 7.2 months for LMR >3.6 and LMR <3.6, respectively.
Figure 2 OS and PFS times according to inflammatory markers: NLR (A, B), DNLR (C, D), LMR (E, F), and PLR (G, H).
Abbreviations: OS, overall survival; PFS, progression-free survival; NLR, neutrophil-to-lymhocyte ratio; DNLR, derived neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; PLR, platelet-to-lymphocyte ratio.
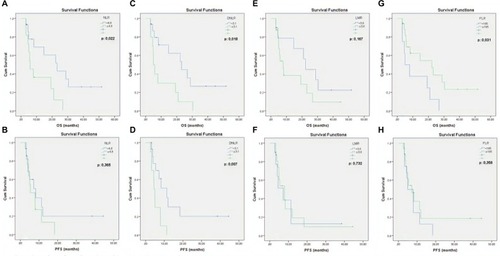
Table 1 Patient Demographics and Clinical Characteristics (n: 26)
shows the association between survival times and changes after 3 months of pazopanib treatment for NLR, DNLR, LMR, and PLR. Significantly longer OS and PFS were detected in cases with decreased NLR and DNLR after pazopanib therapy. Median OS was 23.13 months in cases with decreased NLR, while it was 5.6 months in cases with increased NLR (P=0.021). Median PFS was 12.03 months in cases with decreased NLR, while it was 4.47 months in cases with increased NLR (P=0.005). Similarly, median OS was 23.13 months and 5.87 months in cases with decreased and increased DNLR, respectively (P=0.010). Median PFS was 12.03 months and 4.33 months in cases with decreased and increased DNLR, respectively (P=0.001). There were no significant differences in OS and PFS times between cases showing changes in LMR and/or PLR (P=0.887, P=0.557, P=0.204, P=0.061; ).
Figure 3 OS and PFS times according to changes (Δ) in inflammatory markers after pazopanib treatment: ΔNLR (A, B), ΔDNLR (C, D), ΔLMR (E, F), and ΔPLR (G, H).
Abbreviations: OS, overall survival; PFS, progression-free survival; NLR, neutrophil-to-lymhocyte ratio; DNLR, derived neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; PLR, platelet-to-lymphocyte ratio.

A Cox proportional hazards model was used to evaluate the potential predictors and the results are shown in . Univariate analysis revealed that OS was significantly associated with response to pazopanib (HR: 4.492, 95% CI: 1.668–12.096; P=0.003), NLR (HR: 0.327, 95% CI: 0.120–0.892; P=0.029), DNLR (HR: 0.344, 95% CI: 0.137–0.865; P=0.023), PLR (HR: 2.763, 95% CI: 1.053–7.252; P=0.039), NLR decrease (HR: 3.178, 95% CI: 1.127–8.960; P=0.029), and DNLR decrease (HR: 3.377, 95% CI: 1.267–9.020, P=0.015). In multivariate analysis, only NLR (HR: 1.680, 95% CI: 1.201–2.348; P=0.002) was found to be the independent prognostic factor for OS.
Table 4 Univariate and multivariate analyses of potential prognostic factors for overall survival
Discussion
Local therapies are curative in cases with localized STSs. However, 5-year survival rates are as low as 8% in cases with metastatic disease, and there is no significant improvement in survival times despite novel drugs including TKIs.Citation15 TKIs are expensive drugs and are associated with some side effects, some of which may be life-threatening. For this reason, it is important to identify who will benefit from TKIs in early treatment period, and in this study we wanted to explore the predictive markers in STS cases treated with pazopanib. For this purpose, we looked for the predictive value of some inflammation indexes, and found that some inflammatory markers could act as predictors. Lower pretreatment NLR and DNLR as well as decrease in these index values after 3 months of therapy were found to be associated with longer OS and PFS. Despite the low number of cases in our study group, these results are interesting and are simple guide for the clinicians to identify the patients who will benefit from pazopanib treatment for STSs.
It is very well known that pazopanib is an anti-angiogenic TKI targeting VEGFR, PDGFR, fibroblast growth factor receptors, and c-KIT.Citation16 Recent reports have shown the ability of anti-angiogenic TKIs with modulation of immune system through direct effects on tumor vasculature as well as indirect effects on both the tumor cells and immune cells.Citation17
This may suggest that inflammation is associated with angiogenesis and sarcomagenesis.
Prognostic factors for metastatic STSs are age, duration of symptoms, tumor size, anatomical sites, compartment/deep location of the tumor, radiotherapy, tumor size, grade, histologic subtype, and response to chemotherapy.Citation18,Citation19 The lack of prognostic significance of age, sex, grade, and histologic subtype is due to the low number of cases included in our study group.
Pazopanib has been approved in April 2012 after Phase III PALETTE study which showed PFS but not OS advantage (4.6 vs 1.6 months for PFS and 12.5 vs 10.7 months for OS).Citation20 Similar to heterogeneity in all studies, PALETTE study included highly heterogenous subtypes of STSs except liposarcoma, and complexity is valid in this study as reported in other studies and also in our study. Tumor response was assessed after 6 months of pazopanib therapy in PALETTE study and progression was reported in 61% of the cases.Citation21 We measured response to therapy at the third month of therapy, which is relatively early response assessment for TKI, and detected progression in 50% of the cases. We found significantly shorter PFS and OS times in cases showing progression after 3 months of pazopanib therapy. This is important due to the earlier evaluation of response to pazopanib and to select which patients benefitted from pazopanib therapy and to stop the drug in nonresponsive cases after 3 months. However, this finding must be confirmed with larger studies.
The role of angiogenesis in STSs has been shown previously and involves the expression of angiogenesis inducers such as VEGF A-B-C-D, FGF1-2, PDGF, angiogenin, TGF α-β, TNF-α, and ILs 1–8. Synergistic association between inflammation and angiogenesis has been confirmed, and pathogenetic role of these pathways in STSs has been defined more clearly in recent years.Citation22 It is well-known that inflammatory locus is hypoxic, and hypoxia is an important pro-angiogenic signal activating the hypoxia-inducible factor signaling pathway. Inflammation is associated with the recruitment of circulating leukocytes and platelets, and the activation of resident macrophages, mast cells, and also fibroblasts. This activation produces large quantities of pro-angiogenic factors; however, lymphocytes in peripheral blood also show anti-tumor effect.Citation23,Citation24 It has been shown many times in various tumors that increase in NLR, DNLR, and PLR and decrease in LMR levels cause inflammation and predict poor prognosis. However, the association between inflammation indexes and prognosis is challenging and controversial in STSs. Idowa et al reported that NLR >5 indicates poor prognosis in STSs.Citation25 Li et al showed poor prognostic property of NLR but not LMR and PLR in Ewing sarcoma.Citation26 NLR has been found to be a more powerful prognostic indicator as compared with carcinoembryonic antigen (CEA) in uterine sarcoma but no difference was found in PFS and OS according to NLR 2.12 cutoff.Citation27 On the other hand, Yi Que et al showed shorter OS and PFS in cases with high NLR and PLR in univariate analysis but shorter OS in cases with only high PLR in multivariate analysis.Citation28 Szakandera et al studied 340 cases with STS and showed shorter DFS and OS in cases with DNLR ≥2.39.Citation29 We found shorter OS and PFS in cases with DNLR ≥3.1 and only shorter OS in cases with NLR ≥4.8 and PLR ≥195, but did not find difference in OS and PFS according to the LMR. In addition, NLR, DNLR, and PLR were found to be significant for OS in univariate analysis but only NLR in multivariate analysis in our study.
Yi Que et al found an association between PLR and NLR with grade and PLR with sex.Citation28 It is clear that higher grade tumor is associated with more aggressive tumor behavior. We did not find an association between inflammatory parameters and clinicopathological variables including age, grade, and sex. Although median OS and PFS were found to be longer in our cases with grade II as compared with grade III tumor (20.967 vs 15.468 months and 7.90 vs 4.33, respectively), the lack of statistically significant association between grade and inflammation indexes is due to the low number of cases. It has been found that plasma VEGF and basic FGF levels are elevated by 10- to 13-folds in sarcoma patients as compared to controls, and markedly elevated expression of matrix metalloproteinase 2 and PDGFR-α has been found in sarcoma tissues as compared with non-malignant tissues.Citation30 For this reason, angiogenesis is one of the most important mechanisms of sarcoma progression in STS cases with metastatic disease. It has been proposed that inflammation regresses as angiogenesis decreases. According to this proposal, inflammation will decrease with effective treatment when a patient responds to anti-angiogenic drugs like pazopanib. Kobayashi et al found longer PFS and OS in cases showing a decrease in NLR after 1 month of pazopanib treatment.Citation31 We found longer survival times in cases with lower NLR and DNLR before pazopanib treatment, and there was a decrease in these indexes after 3 months of pazopanib treatment. This is important due to the prediction of response to expensive and relatively toxic anti-angiogenic drug treatment by a simple, practical, and inexpensive method.
Limitations
Our study has three limitations: short follow-up, retrospective nature, and low number of the patients. This retrospective study presents no big claim, and is not a biomarker study as well. We have not performed power analysis, and we evaluated only 26 cases treated with pazopanib and do not have sufficient clinical data. Another important concern of this study is related to the utility of these indexes for evaluations of response to pazopanib, but it is not associated with survival benefit for pazopanib. However, confirmation and/or validation of this study with a large sample will be more informative and useful.
Conclusion
Low pretreatment NLR and DNLR values are predictive of longer PFS and OS in cases with STS and decrease in the levels of these parameters after 3 months of treatment is predictive of response to pazopanib in these cases.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
- CoindreJ-MTerrierPGuillouLPredictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomasCancer200191101914192611346874
- ItoMBarysLO’ReillyTComprehensive mapping of p53 pathway alterations reveals an apparent role for both SNP309 and MDM2 amplification in sarcomagenesisClin Cancer Res201117341642621159888
- CragoAMBrennanMFPrinciples in management of soft tissue sarcomaAdv Surg201549110712226299493
- KoliouPKaravasilisVTheochariMPollackSMJonesRLThwayKAdvances in the treatment of soft tissue sarcoma: focus on eribulinCancer Manag Res20181020721629440930
- ChellappanDKChellianJNgZYThe role of pazopanib on tumour angiogenesis and in the management of cancers: a reviewBiomed Pharmacother20179676878129054093
- HeudelPCassierPDerbelOPazopanib for the treatment of soft-tissue sarcomaClin Pharmacol201246523204874
- KoehlerKLiebnerDChenJLTP53 mutational status is predictive of pazopanib response in advanced sarcomasAnn Oncol201627353954326646755
- PalmaNMorrisJCAliSMRossJSPalSKExceptional response to pazopanib in a patient with urothelial carcinoma harboring FGFR3 activating mutation and amplificationEur Urol201568116817025766722
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- SzadeAGrochot-PrzeczekAFlorczykUJozkowiczADulakJCellular and molecular mechanisms of inflammation-induced angiogenesisIUBMB Life201567314515925899846
- TempletonAJMcnamaraMGŠerugaBPrognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysisJ Natl Cancer Inst20141066dju12424875653
- SongSLiCLiSGaoHLanXXueYDerived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancerOnco Targets Ther2017103145315428706446
- NishijimaTFMussHBShacharSSTamuraKTakamatsuYPrognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysisCancer Treat Rev2015411097197826481060
- ProctorMJMorrisonDSTalwarDA comparison of inflammation-based prognostic scores in patients with cancer. a Glasgow Inflammation Outcome StudyEur J Cancer201147172633264121724383
- ItalianoAMathoulin-PelissierSCesneALTrends in survival for patients with metastatic soft-tissue sarcomaCancer201111751049105420945333
- SchöffskiPPazopanib in the treatment of soft tissue sarcomaExpert Rev Anticancer Ther201212671172322716487
- KwilasARDonahueRNTsangKYHodgeJWImmune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapyCancer Cell Microenviron201521e67726005708
- VraaSKellerJNielsenOSSneppenOJurikAGJensenOMPrognostic factors in soft tissue sarcomas: the Aarhus experienceEur J Cancer199834121876188210023309
- ChoongPFRüdigerHAPrognostic factors in soft-tissue sarcomas: what have we learnt?Expert Rev Anticancer Ther20088213914618279053
- WilkyBAMeyerCFTrentJCPazopanib in sarcomas: expanding the PALETTECurr Opin Oncol201325437323666473
- van der GraafWTABlayJ-YChawlaSPPazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trialLancet201237998291879188622595799
- RocchiLCaraffiSPerrisRMangieriDThe angiogenic asset of soft tissue sarcomas: a new tool to discover new therapeutic targetsBiosci Rep2014346e0014725236925
- ChenLEndlerAShibasakiFHypoxia and angiogenesis: regulation of hypoxia-inducible factors via novel binding factorsExp Mol Med200941128495719942820
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- IdowuOKDingQTaktakAFGChandrasekarCRYinQClinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcomaBiomarkers201217653954422793493
- LiY-JYangXZhangW-BYiCWangFLiPClinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcomaCancer Manag Res2017944345129033609
- KimHSHanKHChungHHNeutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: a case-matched comparisonEur J Surg Oncol201036769169820570475
- QueYQiuHLiYPreoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcomaBMC Cancer201515164826432433
- SzkanderaJGergerALiegl-AtzwangerBThe derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patientsAm J Surg2015210111111625586599
- YoonSSSegalNHParkPJAngiogenic profile of soft tissue sarcomas based on analysis of circulating factors and microarray gene expressionJ Surg Res2006135228229016603191
- KobayashiHOkumaTOkaHNeutrophil-to-lymphocyte ratio after pazopanib treatment predicts response in patients with advanced soft-tissue sarcomaInt J Clin Oncol201823236837429086877
