Abstract
Background
Bladder cancer (BC) is the most common cancer of the urinary tract and invariably predicts a poor prognosis. In this study, we found a reliable gene signature and potential biomarker for predicting clinical prognosis.
Methods
The gene expression profiles were obtained from the GEO database. By performing GEO2R analysis, numerous differentially expressed genes (DEGs) were found. Three different microarray datasets were integrated in order to more precisely identify up-expression genes. Functional analysis revealed that these genes were mainly involved in cell cycle, DNA replication and metabolic pathways.
Results
Based on protein-protein interactome (PPI) networks that were identified in the current study and previous studies, we focused on KIF15 for further study. The results showed that KIF15 promotes BC cell proliferation via the MEK -ERK pathway, and Kaplan‐Meier survival analysis revealed that KIF15 expression was an independent prognostic risk factor in BC patients.
Conclusion
KIF15 may represent a promising prognostic biomarker and a potential therapeutic option for BC.
Keywords:
Introduction
Globally, bladder cancer (BC) is considered one of the most serious cancers, it is the ninth most common cancer and has the 13th highest rate of mortality when compared with 81,190 newly diagnosed cases, and 17,240 BC-related deaths was estimated in the USA in 2018.Citation1 In China, the annual incidence rate is 80.5/100,000, and the estimated mortality rate is 32.9/100,000.Citation2 Although treatment for BC has improved in recent decades, there are still areas of unmet need, such as the short survival time of patients with invasive stage tumors or metastatic diseases. Many studies have shown that the progression of BC is associated with proliferation and invasion.Citation3 The development of BC is a complex process initiated by accumulation of genetic and epigenetic alterations. Therefore, it is necessary to investigate the fundamental mechanisms of BC development to find effective therapeutic strategies leading to improved survival.
Gene expression profiling has been recognized as a useful tool to reveal the mechanism of tumor progression and for biomarker prediction.Citation4,Citation5 As the body of expression data in public databases grows, reanalysis and integration of these available data are more likely to provide novel clues for other researches. We screened for genes with abnormal overexpression in BC using public databases with three gene expression profile microarrays. In addition, we used a network and pathway-based approach to find novel molecular biomarkers of BC. Using this approach, we found that KIF15, a gene encoding a member of the kinesin family of proteins, may play a crucial role in BC proliferation.
The kinesin-12 family member KIF15 is a plus-end directed kinesin that localizes in a mitosis-specific manner to both spindle microtubules and chromosomes.Citation6–Citation9 Most proteins of KIF family own ATP-dependent activity and can drive microtubule-dependent plus-end movement.Citation10 Kinesins participate in the transport of macromolecules in several essential cellular processes, such as mitosis and meiosis.Citation11 Studies have demonstrated that kinesin proteins play critical roles in the genesis and development of human cancers.Citation12,Citation13 Some kinesin proteins are associated with drug resistance in malignancy.Citation14 Thus, KIF15 may be a potential anticancer biomarker.
In this study, we demonstrate that KIF15 promotes BC proliferation by the MEK–ERK signaling pathway. Our results suggest that KIF15 plays an important role in BC progression and is a novel therapeutic strategy for BC.
Materials and methods
Microarray data
The gene expression profiles of GSE27448, GSE40355, and GSE42089 were obtained from the National Center of Biotechnology Information GEO database (GEO, http://www.ncbi.nlm.nih.gov/geo/). The GSE27448 expression profile, which was based on GPL2895 GE Healthcare/Amersham Biosciences CodeLink Human Whole Genome Bioarray, was submitted by Zaravinos et al. The GSE40355 profile, submitted by Hecker et al, was based on GPL8227 Agilent-019118 Human miRNA Microarray 2.0 G4470B and GPL13497 Agilent-026652 Whole Human Genome Microarray 4 × 44K v2. The GSE42089 expression profile, which was based on GPL9828 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array, was submitted by Sherman et al.
Differential gene expression analysis
The gene expression data were analyzed with GCBI online software GEO2R (https://www.gcbi.com.cn/gclib/html/index) and R software (version 3.4.0; https://www.r-project. org/). Data on upexpression genes were used to construct a customized Venn diagram. Differential gene expression showing ≥2-fold change was tested for statistical significance using t-test and a defined P-value cutoff of <0.05.
Functional and pathway enrichment analysis of upexpression differentially expressed genes (DEGs)
In order to investigate upexpression DEGs at the molecular and functional level, we used an online database. The Database for Annotation Visualization and Integrated Discovery (DAVID) is a method to carry out analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG); DAVID has been previously described.Citation15–Citation17 We used the human genome as the background, with P<0.05 considered statistically significant.
Protein–protein interactome (PPI) network construction and modules selection
Search Tool for the Retrieval of Interacting Genes (STRING) is an online database (http://www.string-db.org) that was used to evaluate the interactions between different proteins. In order to identify interaction functions between upexpression DEGs, we entered the genes into STRING for further analysis. The upexpression DEGs showing significant upregulation were imported into the Cytoscape plugin for network visualizations. Next, we performed module analysis via the MCODE plugin with the following default parameters: degree cutoff ≥2, node score cutoff ≥2, K-core ≥2, and max depth =100.
Cell culture and reagents
BC cell lines were purchased from the American Type Q6 Culture Collection (Manassas VA, USA). The cell lines T24 and 253J were maintained in Roswell Park Memorial Institute 1640 medium supplemented with 10% FBS (Sigma-Aldrich Co., St. Louis, MO, USA). The cells were incubated at 37°C and 5% CO2.
Synthesis of si RNA and transfection of cells
Bladder cells T24 and 253J were transfected with specific siRNAs targeting KIF15 (si-KIF15). The sequences of siR-NAs were
siRNA1: 5′-GCGGUUAUAAUGGUACCAUTT-3′
siRNA2: 5′-GCUGGAAAGAGUUUCCUUUTT-3′
siRNA3: 5′-GGAUUUAGCAGGAUCUGAATT-3′
The siRNA were purchased from GenePharma (Suzhou, China). Untreated BC cells were cultured for 24 hours before transfection. Cells were transfected with siRNA (100 nM) complexes using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Cell proliferation assay
Briefly, 5×103 cells/well were seeded into a 96-well plate and transfected with siRNA. Cell proliferation was assessed by using cell counting kit-8 (CCK-8) (Beyotime Institute of Biotechnology, Shanghai, China) after 24, 48, 72, 96, and 120 hours; absorbance was measured by ELISA microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Ethynyl deoxyuridine (Edu) assay was performed by using an Edu Kit (Ribobio, Guangzhou, China) following the manufacturer’s instructions. Experiments were repeated three times.
Colony formation assay
Approximately 1,500 BC cells were seeded into 6-well dishes with medium. One week later, the cells were transfected with siRNA, and after 2 weeks, viable colonies >0.1 mm in diameter were stained with crystal violet and scored. Data are presented as the mean ± SD, and experiments were performed in triplicate.
Patients and immunohistochemical analysis
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Committee Board of Shandong University Qilu Hospital (Jinan, China). Written informed consent was obtained from all 106 patients. The patients underwent radical cystectomy for BC, which was diagnosed by imageological and pathology examination at our institution from 2009 to 2014. Patients with missing information were excluded from this study. Patients with autoimmune disease or cancer in other systems or who received neoadjuvant chemotherapy and radiotherapy were also excluded from study. The specimens were used after obtaining informed consent from the patients and were stained according to the manufacturer’s protocol (Immunostain SP Kit, Dako-Cytomation). For immunohistochemistry staining, briefly, paraffin-embedded sections of BC tissues were deparaffinized and then heated in a pressure pot for 3 minutes to retrieve the antigens. Then, the sections were incubated with primary antibodies against KIF15 (Proteintech, Rosemont, IL, USA) overnight at 4°C. Antibody binding was detected using a peroxidase-conjugated secondary antibody at 37°C for 30 minutes. A 3,3′-diaminobenzidine substrate kit was used to perform the chromogenic reaction. The intensity of the staining was rated using the following criteria: 0 = negative, 1 = low, 2 = medium, and 3 = high. The extent of staining was scored as 0 = 0% stained, 1 = 1%–25% stained, 2 = 26%–50% stained, and 3 = 51%–100% stained. Final scores were calculated by multiplying the score for intensity by the score for extent and dividing the samples by one of the four grades: 0 = negative, 1–2 = low staining, 3–4 = medium staining, and 4–6 = high staining. The following criteria were used to quantify the expression levels of KIF15 in BC tissues: high expression = final scores of 4–6 and low expression = final scores of 0–3.
Immunoblotting
Western blot analysis was performed as previously described.Citation18 Briefly, 100 µg of protein was separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk for 2 hours and were incubated at 4°C overnight with primary antibodies. The primary antibody used for immunoblotting was the same anti-KIF15 antibody (Proteintech) that was used for IHC. Other primary antibodies used for immunoblotting were antibodies against GAPDH (Affinity, Cincinnati, OH, USA), P-c-Raf, P-MEK, P-p90Rsk, P-ERK1/2, ERK1/2, CyclinD1, CyclinE2, CDK2, and CDK4 purchased from CST (Cell Signaling Technology, Danvers, MA, USA). Horseradish peroxidase-conjugated secondary antibodies were used to detect the primary antibodies, and protein bands were visualized using an Odyssey scanner (Li-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics Program. Each experiment was performed in triplicate, and the values are presented as the mean ± SD, unless otherwise stated. The variance between groups was statistically compared. Student’s t-test was used to compare the mean values. Kaplan–Meier curves were analyzed for relevant variables. The log-rank test was used to analyze differences in survival times among the patient subgroups. The risk factors associated with the prognoses of these patients were evaluated using Cox’s proportional hazard regression model. All probability values had a statistical power level of 90%, and a two-sided level of 5%; P<0.05 was considered to be significant.
Results
Identification of DEGs and aberrantly expressed mRNAs in BC
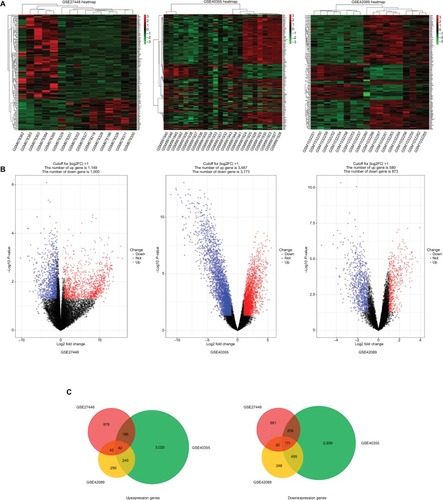
GEO is a free database that has various microarray profiles and next-generation sequencing data. In order to acquire a more reliable gene signature, we integrated the datasets GSE27448, GSE40355, and GSE42089 into the merged gene expression dataset. Using the criteria of P<0.05 and ≥2-fold change in expression over control , a total of 153 genes were identified after the analysis carried out by GEO2R and Venn diagram, which included 42 upexpression genes and 111 downexpression genes. The DEGs heat map, volcano plot, and integrated Venn diagram are presented in . Then we analyzed the upexpression genes.
Figure 1 The analysis result of GSE27448, GSE40355, and GSE42089.
Notes: (A) Heatmap overview of the differentially expressed genes. Red: upregulation; green: downregulation. (B) Volcano plot of the differentially expressed genes. (C) The result of Venn diagram. DEGs were divided into two groups: upexpression and downexpression. Different color areas meant different datasets and the cross areas represented the commonly changed DEGs.
Abbreviation: DEGs, differentially expressed genes.
GO and KEGG analysis of upexpression genes
The 42 upexpression genes were inputted into the online biological tool DAVID.Citation19 GO analysis included three aspects: molecular function (MF), biological process (BP), and cellular component (CC) ().Citation20 The GO analysis results indicated that upexpression genes were enriched in 24 BP terms, 11 CC terms, and 6 MF terms. Further analysis showed that these genes were significantly enriched in cell division, DNA replication, mitotic nuclear division, and nucleoplasm (). In order to reveal the potential functions, we performed KEGG pathway enrichment analysis ().Citation16 As shown in , the enriched pathways were cell cycle, DNA replication, and metabolic pathways.
Figure 2 The functional analysis of integrated upexpression genes.
Notes: (A) GO analysis and significant enriched GO terms of upexpression genes in BC tissues. (B) Significantly enriched pathway terms of upexpression genes.
Abbreviation: ATP, adensosine triphosphate; BC, bladder cancer; GO, gene ontology.
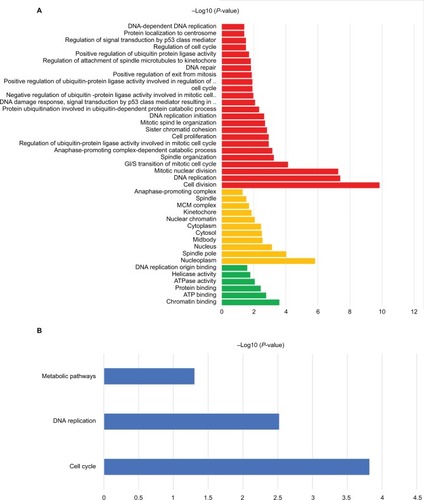
Table 1 The significant enriched GO analysis of integrated upexpression genes in BC
Table 2 Signaling pathway analysis of integrated upexpression genes in BC
PPI networks construction and module analysis
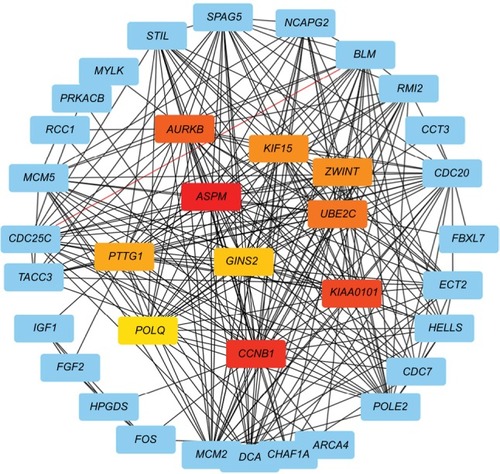
In order to analyze the different gene interactions and find hub-genes, PPIs were constructed by using Cytoscape. According to the PPI networks (), the top ten genes with highest interaction degrees were KIF15, ASPM, CCNB1, KIAA0101, AURKB, ZWINT, UBE2C, PTTG1, POLQ, and GINS2. Kinesin proteins play critical roles in the development of human cancers,Citation12,Citation13 and KIF15 is involved with the progression of many solid tumors,Citation21 so we decided to study whether the abnormal expression of KIF15 was related to bladder carcinogenesis.
Correlation of KIF15 expression with clinicopathological characteristics and prognosis of BC patients
We examined the expression pattern of KIF15 protein by IHC staining in a retrospective cohort of 106 specimens from patients with BC to explore the clinical significance of KIF15 in BC (). The clinicopathologic characteristics of the 106 patients are shown in . In detail, higher level KIF15 expression was detected in 58 (54.7%) tumor tissue specimens, whereas 48 (45.3%) specimens exhibited low/absent level. Regarding the correlation between KIF15 expression level and clinicopathological parameter in patients, patients with higher expression of KIF15 (with a cutoff at median) presented advanced TNM stage. Kaplan– Meier survival analysis revealed that patients with low KIF15 expression had more favorable overall survival compared with high KIF15 expression (P=0.01; ).The results of univariate analysis and multivariate analysis by Cox proportional hazards models suggested that KIF15expression was an independent prognostic risk factor (P=0.012 and 0.003, respectively; ).
Figure 4 Correlation of KIF15 expression with clinicopathological characteristics and prognosis of bladder cancer patients.
Notes: (A) IHC staining of KIF15 proteins in bladder cancer samples. (B) BC patients with high expression of KIF15 presented have worse overall survival, and low expression was opposite (P=0.01).
Abbreviations: BC, bladder cancer; IHC, immunohistochemistry.
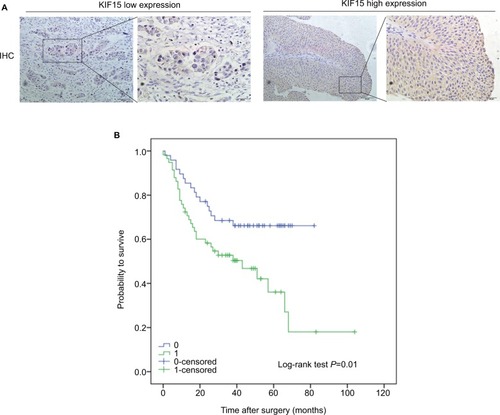
Table 3 Association between KIF15 expression and clinicopathological features of 106 patients with bladder cancer
Table 4 Cox proportional hazard model analysis of prognostic factors in patients with bladder cancer
KIF15 promotes BC cells proliferation
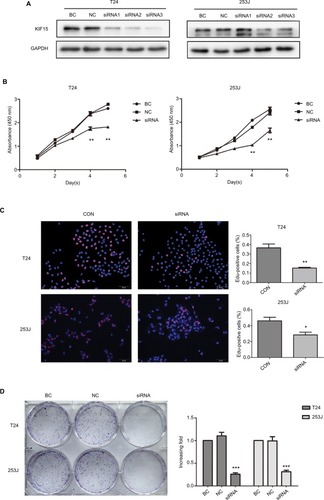
We interrupted the expression of KIF15 by using siRNA, and we confirmed downregulation of KIF15 protein in T24 and 253J BC cells (). Compared with the negative control (NC) group, KIF15 expression knockdown suppressed BC cell proliferation, as measured by the CCK-8 assay, Edu assay, and colony formation assay ().
Figure 5 KIF15 promotes BC cells proliferation.
Notes: (A) T24 and 253 J cells transfected with siRNA, respectively, were subject to Western blotting. (B–D) CCK-8 assay, Edu assay, and colony formation assay comparing proliferation ability of KIF15 knockdown and NC group. Adjustments of brightness, contrast, and size are applied to the whole images of Western blot-based analyses without elimination of any information present in the original, including backgrounds. Data are presented as mean ± SD. All experiments are performed in triplicate, and mean values are shown. *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: BC, bladder cancer; CCK-8, cell counting kit-8; CON, control; Edu, ethynyl deoxyuridine; NC, negative control.
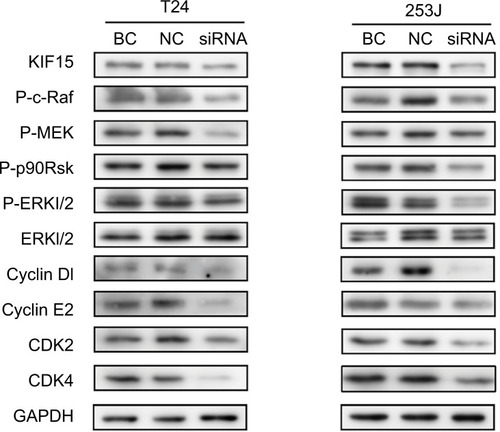
KIF15 promotes BC cells proliferation via the MEK–ERK pathway
To further elucidate the mechanisms underlying KIF15 promotion of BC proliferation, we used KIF15 knockdown cells and NC cells. Previous studies showed that KIF15 is associated with the MEK–ERK signaling pathway.Citation21 We therefore investigated whether changes in KIF15 expression could affect activation of the MEK–ERK signaling pathway in BC cells. Western blot analysis showed that KIF15 knockdown inhibited p-ERK expression in BC cells (). Furthermore, KIF15 knockdown downregulated the expression of P-c-Raf, P-MEK, P-p90Rsk, CyclinD1, CyclinE2, CDK2, and CDK4, which promotes cell cycle progression in BC cells.
Figure 6 KIF15 promotes BC cells proliferation via the MEK–ERK pathway.
Notes: Western blot analysis showed that KIF15 knockdown inhibited p-ERK, P-c-Raf, P-MEK, P-p90Rsk, CyclinD1, CyclinE2, CDK2, and CDK4 expression in BC cells. Adjustments of brightness, contrast, and size are applied to the whole images of western blot-based analyses without elimination of any information present in the original, including backgrounds.
Abbreviations: BC, bladder cancer; NC, negative control.
Discussion
In the past decade, great efforts have been made to explore the molecular mechanism of proliferation and metastasis for cancer cells. Nevertheless, critical details regarding these mechanisms in BC remain incompletely understood. Hence, we undertook a detailed investigation using the GEO database and acquired microarray data regarding DEGs in tumor and normal tissues. A Venn diagram was constructed and 42 upexpression genes were found to be strongly related to the development of BC. Both KEGG and GO analysis were performed to illuminate the potential biological functions and pathways involved in bladder tumorigenesis.
GO analysis showed that these genes were mainly enriched in cell division, DNA replication, mitotic nuclear division, and nucleoplasm. As shown in previous studies, cell division and DNA replication played an important role in the proliferation of malignant BC cells.Citation22 Furthermore, KEGG enrichment analysis revealed that upexpression genes were enriched in cell cycle, DNA replication, and metabolic pathways. According to our research, some hub genes, especially KIF15, have been classified by constructing the PPI network based on the degree, which may suggest a strategy for therapeutic interventions in BC.
Although KIF15 has been studied for 10 years, the role of KIF15 in regulating the behavior of cancer cells has not yet been elucidated. Klejnot et al reported that kinesins are a superfamily of proteins with important roles in eukaryotic intracellular trafficking and cell division.Citation23 Some proteins participate in molecular intracellular transport, and others play an important role in the process of mitosis and cytokinesis.Citation24
Drechsler et al showed that KIF15 is a second tetrameric spindle motor (in addition to kinesin-5, Eg5) and described the mechanisms by which KIF15 and its inhibitor hTpx2 modulate spindle microtubule architecture.Citation9,Citation25 The interaction of KIF15 with microtubules has also been described.Citation23 Another study demonstrated that KIF15 drove centrosome separation during bipolar spindle assembly.Citation26
Studies of KIF15 have shown that it is important for several tumors. For example, KIF15 is critical for K5I resistance in HeLa cells.Citation27 KIF15 is considering as a prognostic marker and new therapeutic target for endocrine therapy-resistant breast cancer.Citation28 KIF15 is also overexpressed in lung cancer and may be important in the cell cycle.Citation29 Meanwhile, it has been found that inhibitors of Aurora A and KIF11 overcome KIF15-dependent drugresistance.Citation30 In this study, we found that KIF15 promotes BC cell proliferation via the MEK–ERK pathway.
In the current study, a number of patients with higher expression of KIF15 were found to be more susceptible to BC progression, though no significant clinicopathological difference was observed. Cox proportional hazard model analysis showed that significant poor prognosis was associated with a higher level of KIF15 expression. This contradiction may be attributable to the patients in the study population, most of whom had muscle-invasive BC, as BC patients reportedly suffer poor survival despite receiving the standard therapy.Citation31 Indeed, more BC patients should be included to validate this conclusion clinically.
The MEK–ERK pathway plays an important role in cell proliferation and participates in the genesis of many epithelial cancers. The MEK–ERK pathway participates in several cellular processes such as proliferation, differentiation, and motility and plays an important role in BC prognosis.Citation32,Citation33 This pathway is often upregulated in cancer tissues and plays an vital role in anticancer therapy.Citation34 Currently, the efficacy of MEK inhibitors targeting the MEK–ERK pathway has been put in clinical trials for study,Citation35 and according to the previous research, MEK–ERK pathway can regulate cyclinD1 transcription and influence the cell cycle process.Citation36 Furthermore, from our studies, ERK also regulates the formation of the cyclinE/CDK2 complex. Thus, MEK–ERK pathway plays an important role in KIF15 regulation of BC cells proliferation.
However, clinical features were not distinctly different between patients with higher vs lower expression of KIF15, and detailed mechanisms are not yet understood. Larger sample studies and more extensive investigation of these mechanisms are needed in future investigations of KIF15 in BC.
Conclusion
This study used an online public database to find DEGs, identified a novel role for KIF15 in promoting BC cell proliferation, and demonstrated a potential link between KIF15 and the MEK–ERK signaling pathway. In conclusion, our study provided evidence supporting KIF15 as a key regulator and correlated KIF15 expression and the clinical prognosis of BC patients. Therefore, KIF15 may be a potential therapeutic target for BC treatment in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 81470987 and 81170702 to B Shi), the Tai Shan Scholar Foundation to B Shi, the Key Research and Development Plan of Shandong Province (Grant 2017GSF18105 to B Shi), the Science and Technology Development Project of Shandong Province (Grant 2014GSF118054 to B Shi), and Science Foundation of Qilu Hospital of Shan-dong University (Grant 2015QLMS28 to B Shi).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- BlackPCDinneyCPBladder cancer angiogenesis and metastasis – translation from murine model to clinical trialCancer Metastasis Rev2007263–462363417726580
- ChenYQiCXiaLLiGIdentification of novel genetic etiology and key molecular pathways for seminoma via network-based studiesInt J Oncol20175141280129028791339
- PengJWuYTianXHigh-throughput sequencing and co-expression network analysis of lncRNAs and mRNAs in early brain injury following experimental subarachnoid haemorrhageSci Rep201774657728417961
- MitchisonTJMaddoxPGaetzJRoles of polymerization dynamics, opposed motors, and a tensile element in governing the length of Xenopus extract meiotic spindlesMol Biol Cell20051663064307615788560
- TanenbaumMEMacůrekLGaljartNMedemaRHDynein, LIS1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assemblyEmbo J200827243235324519020519
- VannesteDTakagiMImamotoNVernosIThe role of Hklp2 in the stabilization and maintenance of spindle bipolarityCurr Biol200919201712171719818619
- TanenbaumMEMacůrekLJanssenAGeersEFAlvarez-FernándezMMedemaRHKif15 cooperates with Eg5 to promote bipolar spindle assemblyCurr Biol200919201703171119818618
- FlorianSMayerTUModulated microtubule dynamics enable Hklp2/Kif15 to assemble bipolar spindlesCell Cycle201110203533354422024925
- LiuMNadarVCKozielskiFKozlowskaMYuWBaasPWKinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branchingJ Neurosci20103044148961490621048148
- YokotaKSasakiHOkudaKKIF5B/RET fusion gene in surgically-treated adenocarcinoma of the lungOncol Rep20122841187119222797671
- MinakawaYKasamatsuAKoikeHKinesin family member 4A: a potential predictor for progression of human oral cancerPLoS One2013812e8595124386490
- BusterDWBairdDHYuWExpression of the mitotic kinesin Kif15 in postmitotic neurons: implications for neuronal migration and developmentJ Neurocytol2003321799614618103
- Gene Ontology ConsortiumThe gene ontology (Go) project in 2006Nucleic Acids Res200634Database issueD322D32616381878
- OgataHGotoSSatoKFujibuchiWBonoHKanehisaMKEGG: Kyoto encyclopedia of genes and genomesNucleic Acids Res199927129349847135
- ShermanBTDawHuangTanQDAVID knowledgebase: a gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysisBMC Bioinfo20078426
- ZhaoJLiPFengHCadherin-12 contributes to tumorigenicity in colorectal cancer by promoting migration, invasion, adhersion and angiogenesisJ Transl Med201311128824237488
- DawHuangShermanBTLempickiRASystematic and integrative analysis of large gene lists using DAVID bioinformatics resourcesNat Protoc200941445719131956
- AshburnerMBallCABlakeJAGene ontology: tool for the unification of biology. The gene ontology ConsortiumNat Genet2000251252910802651
- WangJGuoXXieCJiangJKif15 promotes pancreatic cancer proliferation via the MEK-ERK signalling pathwayBr J Cancer2017117224525528595260
- ZhouNSinghKMirMCThe investigational Aurora kinase A inhibitor MLN8237 induces defects in cell viability and cell-cycle progression in malignant bladder cancer cells in vitro and in vivoClin Cancer Res20131971717172823403633
- KlejnotMFalnikarAUlaganathanVCrossRABaasPWKozielskiFThe crystal structure and biochemical characterization of Kif15: a bifunctional molecular motor involved in bipolar spindle formation and neuronal developmentActa Crystallogr D Biol Crystallogr201470Pt 112313324419385
- MessinLJMillarJBRole and regulation of Kinesin-8 motors through the cell cycleSyst Synth Biol20148320521325136382
- DrechslerHMcHughTSingletonMRCarterNJMcAinshADThe Kinesin-12 Kif15 is a processive track-switching tetramerElife20143e0172424668168
- EskovaAKnappBMatelskaDAn RNAi screen identifies Kif15 as a novel regulator of the endocytic trafficking of integrinJ Cell Sci2014127Pt 112433244724659801
- SturgillEGNorrisSRGuoYOhiRKinesin-5 inhibitor resistance is driven by Kinesin-12J Cell Biol2016213221322727091450
- ZouJXDuanZWangJKinesin family deregulation coordinated by bromodomain protein ANCCA and histone methyltransferase MLL for breast cancer cell growth, survival, and tamoxifen resistanceMol Cancer Res201412453954924391143
- BidkhoriGNarimaniZHosseini AshtianiSMoeiniANowzari-DaliniAMasoudi-NejadAReconstruction of an integrated genome-scale co-expression network reveals key modules involved in lung adenocarcinomaPLoS One201387e6755223874428
- MaHTErdalSHuangSPoonRYSynergism between inhibitors of Aurora A and KIF11 overcomes KIF15-dependent drug resistanceMol Oncol2014881404141824950801
- GrossmanHBNataleRBTangenCMNeoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancerN Engl J Med2003349985986612944571
- VajraveluBNHongKUAl-MaqtariTC-kit promotes growth and migration of human cardiac progenitor cells via the PI3K-Akt and MEK-ERK pathwaysPLoS One20151010e014079826474484
- NingXDengYIdentification of key pathways and genes influencing prognosis in bladder urothelial carcinomaOnco Targets Ther2017101673168628356754
- HayashidoYKitanoHSakaueTOverexpression of integrin αv facilitates proliferation and invasion of oral squamous cell carcinoma cells via MEK/ERK signaling pathway that is activated by interaction of integrin αvβ8 with type Ⅰ collagenInt J Oncol20144551875188225190218
- JohnsonGLStuhlmillerTJAngusSPZawistowskiJSGravesLMMolecular pathways: adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancerClin Cancer Res201420102516252224664307
- DaksisJILuRYFacchiniLMMarhinWWPennLJMyc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycleOncogene1994912363536457526316